Continuous-flow ventricular assist devices (VADs) are the standard of care for implantable mechanical circulatory support [1]. However, some doubts have been raised based on the experience with cardiopulmonary bypass about possible adverse effects of non-pulsatile flow on organ function [2]. Pulsatile perfusion might have a beneficial effect on peripheral organs probably through an action on systemic vascular resistance and on microcirculation, as a result of less endothelial damage and normalization of nitric oxide (NO) release. However, long-term use of newer generation continuous-flow devices has resulted in similar improvements in organ function [3]. Actually, blood flow through continuous-flow VADs is not really continuous, since it depends on the differential pressure between the left ventricle and the ascending aorta at a certain VAD speed [4]. During support, the failing native heart continues to function and it generates a variation in intracardiac pressures along the cardiac cycle: during systole an increase in left ventricular pressure will be transmitted to the pump and will transiently increase the VAD flow, generating some degree of arterial pulsatility. Potapov et al. [5] first detected a pulsatile flow in patients implanted with DeBakey continuous-flow device. Moreover, intermittent aortic valve opening, either spontaneous or generated by periodical reduction of VAD speed, has a major role in maintenance of pulsatility. Recently, Potapov et al. [6] also demonstrated that long-term mechanical circulatory support with continuous-flow devices does not adversely influence arterial wall properties of the end-organ vasculature: in this histological study, no differences in arterial wall characteristics were found between tissue samples from liver, kidney, coronary arteries, and brain between patients treated with continuous-flow devices and patients with pulsatile-flow (PF) devices. No data is available whether these concepts apply differently to rotary and centrifugal pumps. In order to evaluate the degree of arterial pulsatility in patients implanted with newer generation continuous-flow VAD, we performed Doppler measurements of flow parameters in two patients, one patient implanted with HeartMate II (Thoratec, Pleasanton, CA) axial pump (patient A) and one implanted with HeartWare HVAD (HeartWare Inc, Miami Lakes, FL) centrifugal pump (patient B). Doppler studies were performed 3 months after implantation in both patients, with a comprehensive examination of both central and peripheral vascular vessels (common carotid arteries, middle cerebral arteries, upper and lower limb arteries). For each Doppler measurement pulsation index (PI) was calculated (PI: [Vmax-Vmin]/Vmean). All data were retrospectively collected by chart review after local ethical committee approval, and treated anonymously. In both patients we found some degree of pulsatility, which was higher in the peripheral vascular vessels (mean PI 1,15 in omeral and femoral arteries in patient A and mean PI 0,86 in patient B) than in the central vessels (mean PI 0,4 in internal carotid and middle cerebral arteries and mean PI 0,43 in patient B) (Figure 1, Video 1, available at the URL: <span>http://www.hsrproceedings.org/allegati/video/hsrp-04-268-s001.mpg</span>).
Figure 1.
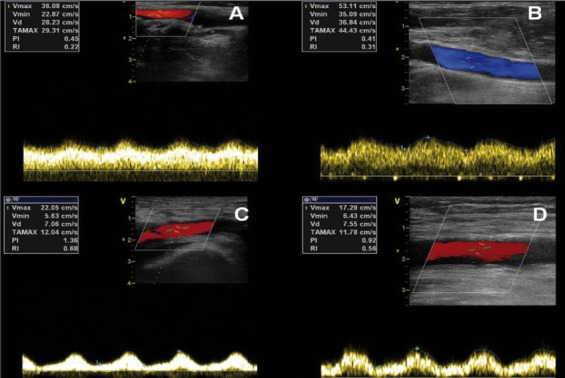
Pulsatile flow in internal carotid artery in patient A (A) and in patient B (B) and in common femoral artery in patient A (C) and B (D).
In both patients simultaneous echocardiographic imaging of the aortic valve showed no systolic valve opening (Figure 2, Video 2, available at the URL: <span>http://www.hsrproceedings.org/allegati/video/hsrp-04-268-s002.mpg</span>), associated with a severe reduction of left ventricular ejection fraction (20% both in patient A and patient B).
Figure 2.
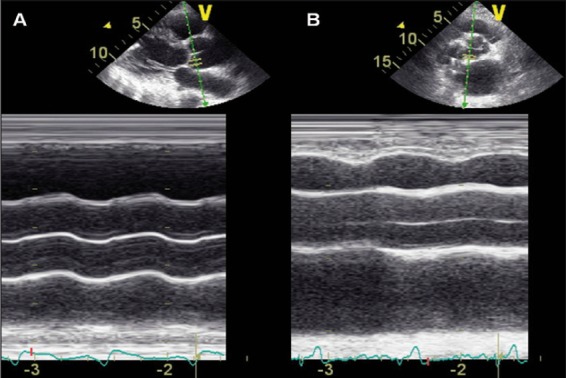
M-mode imaging of aortic valve in patient A (A) and patient B (B) showing any systolic valve opening during the cardiac cycle.
The examination was performed in both patients at 90 mmHg mean systolic blood pressure; the HeartMate II was running at 9400 revolutions per minute (rpm) and HeartWare HVAD at 2700 rpm. This clinical experience highlights for the first time the presence of flow pulsatility in both central and peripheral vessels in patients implanted with last generation continuous-flow VADs, with similar parameters in axial and centrifugal pumps. These data add a piece of information on the physiological adaptation of circulation after continuous flow pump implantation.
Footnotes
Source of Support Nil.
Conflict of interest None declared.
Cite as: Melisurgo G, De Bonis M, Pieri M, Nisi T, Silvetti S, Zangrillo A, Pappalardo F. Is flow really continuous in last generation continuous flow Ventricular Assist Devices? A comparison between HeartMate II and HeartWare HVAD. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2012; 4 (4): 268-269
References
- Slaughter M S, Rogers J G, Milano C A. et al. Advanced heart failure treated with continuous-flow left ventricular assist device. . N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- Ji B, Undar A. An evaluation of the benefits of pulsatile versus nonpulsatile perfusion during cardiopulmonary bypass procedures in pediatric and adult cardiac patients. ASAIO J. 2006;52:357–361. doi: 10.1097/01.mat.0000225266.80021.9b. [DOI] [PubMed] [Google Scholar]
- Kamdar F, Boyle A, Liao K. et al. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28:352–359. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Slaughter M S. Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg. 2012;25:490–494. doi: 10.1111/j.1540-8191.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- Potapov E V, Loebe M, Nasseri B A. et al. Pulsatile flow in patients with a novel nonpulsatile implantable ventricular assist device. Circulation. 2000;102:183–187. doi: 10.1161/01.cir.102.suppl_3.iii-183. [(Suppl. 3)] [DOI] [PubMed] [Google Scholar]
- Potapov E V, Dranishnikov N, Morawietz L. et al. Arterial wall histology in chronic pulsatile-flow and continuous-flow device circulatory support. J Heart Lung Transplant. 2012;31:1171–1176. doi: 10.1016/j.healun.2012.08.013. [DOI] [PubMed] [Google Scholar]


