Abstract
Background
The growing concern about cannabis use, the most commonly used illicit drug worldwide, has led to a significant increase in the number of human studies using neuroimaging techniques to determine the effect of cannabis on brain structure and function. We conducted a systematic review to assess the evidence of the impact of chronic cannabis use on brain structure and function in adults and adolescents.
Methods
Papers published until August 2012 were included from EMBASE, Medline, PubMed and LILACS databases following a comprehensive search strategy and pre-determined set of criteria for article selection. Only neuroimaging studies involving chronic cannabis users with a matched control group were considered.
Results
One hundred and forty-two studies were identified, of which 43 met the established criteria. Eight studies were in adolescent population. Neuroimaging studies provide evidence of morphological brain alterations in both population groups, particularly in the medial temporal and frontal cortices, as well as the cerebellum. These effects may be related to the amount of cannabis exposure. Functional neuroimaging studies suggest different patterns of resting global and brain activity during the performance of several cognitive tasks both in adolescents and adults, which may indicate compensatory effects in response to chronic cannabis exposure.
Limitations
However, the results pointed out methodological limitations of the work conducted to date and considerable heterogeneity in the findings.
Conclusion
Chronic cannabis use may alter brain structure and function in adult and adolescent population. Further studies should consider the use of convergent methodology, prospective large samples involving adolescent to adulthood subjects, and data-sharing initiatives.
Introduction
Cannabis is the illicit drug most widely available and used worldwide [1], [2], consumed by between 125 and 203 million people, largely younger age group (15–34 years), which corresponds to an annual prevalence rate of 2.8%–4.5% [1], [2]. Despite the fact that many individuals tend to discontinue cannabis use after their initial experimentation with the drug [1] and the percentage of individuals who develop dependence is lower than that associated with alcohol (15%) or tobacco (32%) use, around 9% of cannabis users develop dependence in the long term [3], [4]. Cannabis use has been associated with a range of acute and chronic mental health problems, such as anxiety, depression, neurocognitive alterations and deficits as well as increased risk of psychotic symptoms and disorders, the severity of these effects being dependent on frequency of use, age of onset and genetic vulnerability [5]–[15]. These effects are probably related to effects on the endocannabinoid system, which can modulate the neuronal activity of other neurotransmitter systems, such as dopamine, through its action on the most abundant cannabinoid receptor in brain, the cannabinoid receptor 1 (CB1) [16], [17]. CB1 receptors mature slowly, reaching maximal levels during adolescence [18], and are particularly concentrated in brain regions that are critical for executive functioning, reward processing and memory, such as the prefrontal cortex, anterior cingulate cortex, basal ganglia, medial temporal areas (e.g., hippocampus and amygdala) and cerebellum [19].
Animal studies have consistently demonstrated that delta-9-tetrahydrocannabinol (THC), the main psychoactive component of cannabis [20], is able to disrupt the regulatory role of the endogenous cannabinoid system [21], inducing neurotoxic changes in brain regions rich with cannabinoid receptors that might dramatically affect the process of maturational refinement of cortical neuronal networks [22]–[24] and lastly promote changes in brain structure and alter emotional and cognitive performance [25], particularly if the exposure has been during the adolescent period [26], [27]. In contrast to animal literature, results from human studies investigating chronic cannabis users are often inconsistent. These discrepancies may be due to heterogeneity in socio-demographic characteristics of the population studied, imaging techniques employed, as well as differences in drug usage patterns and psychiatric comorbidities that may not always be apparent or result in contact with mental health services and hence may not be appropriately controlled for in studies where participants are screened for presence of co-morbid psychiatric disorder merely by enquiring about previous contact with mental health services [28]–[30]. However, overall the results suggest that long-term cannabis use may result in persistent alterations in brain function and morphology that would extend beyond the period of intoxication [28], [31], and that earlier onset of use may be associated with greater detrimental effects [32], [33].
It is remarkable to note that although the onset of cannabis use is typically during adolescence, a few imaging studies have been conducted with adolescent users [28], [34]. Since brain development continues up to young adulthood [35], adolescence may be a critical period during which chronic cannabis exposure may have far-reaching consequences [36]. Although brain size is thought to stabilize around the age of five years [37], important neurodevelopmental processes continue throughout adolescence, including myelinization [38], synaptic refinement [39] and gray matter volume reduction [40]. While the long-term effects of cannabis use may potentially have major implications for social and family life, education and occupational functioning, its effects on brain structure and function have not been well determined.
The growing concern about cannabis use has led to a significant increase in the number of human studies using neuroimaging techniques to determine the effect of the substance on brain structure and function, as well as to several recent reviews examining this topic [28], [29], [34], [41]–[46]. However, some authors have only reviewed studies investigating the acute effects of cannabis [45], [46] or those published over the last decade [41], [44], while others did not adequately specify criteria for selecting studies [41], [43] or included those studies that investigated only adult population [29], [42]. In the present review, we have conducted a systematic literature search to assess and integrate the evidence of the impact of chronic cannabis use on brain structure and function, focusing on studies in the adolescent and adult population. Papers published until August 2012 have been included following a comprehensive search strategy and pre-determined set of criteria for article selection [29].
Methods
Data for this systematic review was collected with an advanced document protocol in accordance with the PRISMA guidelines [47]. This protocol provided a checklist for reporting systematic reviews (see Table S1).
Search strategy
Electronic searches were performed using EMBASE (1980-August 2012), Medline (1966-August 2012), PubMed (1966-August 2012) and LILACS (1982-August 2012) databases. The following key words were used: cannabis; marijuana; marihuana; delta-9-tetrahydrocannabinol; THC; cannabidiol, CBD; neuroimaging; brain imaging; computerized tomography, CT; magnetic resonance, MRI; single photon emission tomography, SPECT; functional magnetic resonance, fMRI; positron emission tomography, PET; diffusion tensor MRI, DTI-MRI; spectroscopy, MRS. All the studies published up to August 2012 were included without language restriction.
Selection criteria
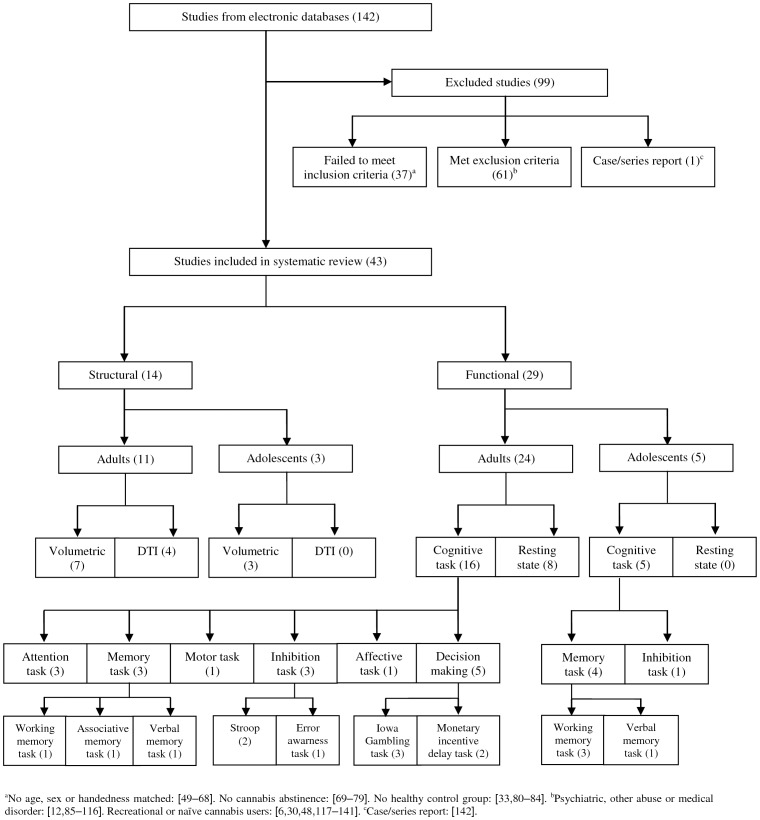
A general review of all neuroimaging studies investigating brain structure or function was initially performed. We obtained a total of 142 published papers (Figure 1). Studies were included or excluded if they expressly stated the following criteria. Inclusion criteria were: (i) use of structural or functional neuroimaging techniques involving chronic cannabis users; (ii) inclusion of a control group of healthy volunteers matched by age, gender and handedness; and (iii) users had to be abstinent for at least 12 hours before brain scanning. Exclusion criteria were: (i) non-neuroimaging studies of cannabis use; (ii) neuroimaging studies that involved participants who had other neurological or psychiatric disorders, or individuals who met criteria for alcohol dependence or other substance use disorders (abuse or dependence) different from cannabis and nicotine, or participants who were not abstinent or who tested positive for drugs other than cannabis on urine screening test; and (iii) neuroimaging studies with recreational or naïve cannabis users.
Figure 1. Flow diagram of included neuroimaging studies in chronic cannabis users.
We defined chronic cannabis users as persons who used cannabis several times a week and who had done so for at least two years. Recreational (or occasional) cannabis users were defined as persons who had used cannabis sporadically (less than four times a month), and naïve users or healthy controls were persons who had used cannabis less than 15 times in their lifetime, according to standardized strict criteria [29], [48].
Any publication that reported data using two different neuroimaging techniques from the same subjects (e.g., structural MRI and functional MRI) or a study examining the same subjects with two different cognitive tasks (e.g., verbal working memory and visual attention task) was considered as two studies in this review.
Data extraction
Data was independently extracted by two reviewers. In case of disagreement, opinion from a third senior researcher was sought to assess whether study criteria were fulfilled. From the articles included we recorded names of authors, year of publication, socio-demographic (e.g., sample size, gender, age, handedness) and cannabis use characteristics (e.g., duration, age of onset, frequency of cannabis use), imaging type and design, exclusion criteria (for neurological, psychiatric or drug history), confirmation of abstinence from other drugs (whether checked by urine test), rest/active condition (for functional imaging studies), type of cognitive task performed during functional imaging and psychopathological variables assessed (e.g., psychotic or depressive symptoms). With regard to alcohol use, we assessed if subjects met criteria for alcohol abuse or for excessive alcohol consumption (more than 21 or 14 standard alcohol units per week for males or females, respectively) based on the reported data. For structural and functional imaging data, the primary measures of interest were global and regional volume, and global and regional activity [cerebral blood flow (CBF), regional CBF (rCBF) or blood oxygen level dependent signal BOLD)]. The secondary outcome was its correlation with clinical variables. We collected the statistically significant results of each outcome variable, and recorded whether a multiple comparison correction was done to prevent bias towards false positives.
Results
Of the 142 studies identified, thirty-six did not meet the a priori selection criteria [33], [49]–[84] and sixty-two met the exclusion criteria [6], [12], [30], [48], [85]–[141] or were case/series reports [142] (for more detailed information, see Figure 1). The remaining 43 studies were classified according to the neuroimaging technique used (structural/functional), age of the participants [adolescents (≤ 18 years) and adults (> 18 years] and testing conditions (resting state/cognitive task) (Figure 1). The studies included comprised: 14 structural neuroimaging studies [11 in adult users and 3 in adolescent users; 10 volumetric studies and 4 diffusion tensor imaging studies (DTI)] and 29 functional neuroimaging studies on the chronic effects of cannabis (24 in adult users and 5 in adolescent users; 8 in the resting state and 21 during a cognitive task).
1. Structural neuroimaging studies in adult chronic cannabis users
We identified 11 structural MRI studies that examined adult chronic cannabis users and met our selection criteria (Table 1). Structural differences were obtained in seven of them in terms of global brain measures [143] or gray/white matter changes [144]–[149]. Four studies did not find any significant structural alterations when comparing chronic cannabis users with healthy controls [150]–[153]. The abstinence period for all participants before they underwent the structural MRI was between 12 and 24 hours, apart from two studies [145], [152] (for details see Table 1).
Table 1. Structural neuroimaging studies in chronic cannabis users.
| Author (yr.) | Method | CU M/F | HCM/F | Mean (SD) age CU/HC | Image analysis | Abstinence (Mean days) | Results* | ||||
| ADULTS | Global measures | Regionalmeasures(GM) | Regional measures (WM) | Detailed results | Correlations with clinical variables | ||||||
| GM-WM CSF-TIV | FL-PL-TL-OL BG-Cb | ||||||||||
| Block et al. (2000) [143] | MRI 1.5T | 9/9 | 7/6 | 22.3 (0.5)/ 22.6 (0.5) | Whole brainROI | ≤ 1 | ○ ○ • ○ | ○ ○ ○ ○ ○ ○ | ○ | ↓ CSF | |
| Tzilos et al. (2005) [153] | MRI 1.5T | 16/6 | 19/7 | 38.1 (6.2)/ 29.5 (8.5) | ROI | ≤ 1 | ○ ○ ○ ○ | ○ | |||
| Matochik et al. (2005) [148] | MRI 1.5T | 11/0 | 8/0 | 25.4 (5)/ 29.7 (4.7) | Whole brainROI† | ≤ 1 | • ○ • ○ • ○ | • | GMd: ↓ R parahippocampus; ↑ precentral gyrus and R thalamus. Hippocampus (ROI): ↓ GMdWMd: ↓ L PL; ↑ parahippocampus and fusiform gyrus, lentiform nucleus and pons | ↑ WMd L precentral gyrus with duration of use (yr.) | |
| Gruber et al. (2005) [151] | DTI 3T | 8/1 | 8/1 | 26 (3.6)/ 26.2 (3.1) | Whole brainROI† | NE | ○ | ||||
| DeLisi et al. (2006)** [150] | DTI 1.5T | 9/1 | 9/1 | 21.1 (2.9)/ 23 (4.4) | Whole brainROI | ≤ 1 | ○ ○ ○ ○ | ○ | ○ | ||
| Jager et al. (2007) [152] | MRI 1.5T | 13/7 | 13/7 | 24.5 (5.2)/ 23.6 (3.9) | Whole brainROI† | 7 | ○ | ○ | |||
| Yücel et al. (2008) [149] | MRI 3T | 15/0 | 16/0 | 38.8 (8.9)/ 36.4 (9.8) | ROI | ≤ 1 | ○ ○ ○ | • | Hippocampus: ↓ L/RAmygdala: ↓ L/R | ↓ L hippocampus with cumulative exposure (yr.) and higher positive psychotic symptoms | |
| Arnone et al. (2008) [144] | DTI 1.5T | 11/0 | 11/0 | 25.0 (2.9)/ 23.3 (2.9) | ROI | ≤ 1 | • | Corpus callosum: ↑ MD | |||
| Ashtari et al. (2011)*** [145] | MRI 1.5T | 14/0 | 14/0 | 19.3 (0.8)/ 18.5 (1.4) | ROI | 201 | ○ ○ ○ ○ | • | Hippocampus: ↓ L/R | ↑ hippocampus with verbal learning and memory scores in HC; ↓ hippocampus with amount of cannabis use | |
| Gruber et al. (2011) [147] | DTI 3T | 14/1 | 14/1 | 25.0 (8.7)/ 25.2 (8.4) | ROI | ≤ 1 | • | R Genu: ↑ MDL FL: ↓ FA | ↑ FA L frontal with higher BIS total and motor subscale score; ↑ FA and ↓ MD in FL with later age of onset | ||
| Cousijn et al. (2011) [146] | MRI 3T | 21/12 | 26/16 | 21.3 (2.4)/ 21.9 (2.4) | Whole brainROI† | ≤ 1 | ○ ○ ○ ○ | ○ ○ • ○ ○ • | ○ | GM: ↑ anterior cerebellum | ↓ R amygdala and ↓ L/R hippocampus with amount of cannabis use (weekly) or severity of cannabis dependence |
| ADOLESCENTS | |||||||||||
| Medina et al. (2009)† [154] | MRI 1.5T | 12/4 | 10/6 | 18.1/ 17.9 (16–18.9) | ROI | 28 | ○ | • | ○ | PFC: ↑ in F CUPFC: ↓ in M CU | ↓ PFC in CU and ↑ PFC in HC with better executive functioning |
| Medina et al. (2010)† [155] | MRI 1.5T | 12/4 | 10/6 | 18 (0.7)/ 18 (0.9) | ROI | 28 | ○ | • | Cerebellum: ↑ inferior posterior (lobules VIII–X) vermis | ↑ Vermis with poorer executive functioning | |
| McQueeny et al. (2011) [156] | MRI 3T | 27/8 | 36/11 | 18.0/ 17.7 | ROI | 28 | ○ | • | R amygdala: ↑ in F CU | ↑ R amygdala with internalizing symptoms in F CU | |
Note: Yr. = Years; CU = Cannabis users; HC = Healthy controls; M = Male; F = Female; SD = Standard deviation; GM = Grey matter; GMd = Grey matter density; WM = White matter; WMd = White matter density; CSF = Cerebral spinal fluid; TIV = Total intracranial volume; FL = Frontal lobe; PL = Parietal lobe; TL = Temporal lobe; OL = Occipital lobe; BG = Basal ganglia; Cb = Cerebellum; L = Left hemisphere; R = Right hemisphere; T = Tesla; MRI = Magnetic resonance imaging; DTI = Diffusion tensor imaging; ROI = Region of interest; NE = Not specified; MD = Mean diffusivity; FA = Fractional anisotropy; PFC = Prefrontal cortex; BIS = Barrat Impulsivity scale.
If not otherwise specified, results are presented in terms of chronic cannabis users.
• = Significant differences; ○ = Non-significant differences; = Not examined.
Two subjects in the marijuana group met criteria for excessive alcohol consumption.
Five subjects in the marijuana group met criteria for alcohol abuse.
Subjects with symptoms of alcohol abuse or dependence were included.
Multiple comparison correction.
1.1. Volumetric studies
Of the seven studies comparing global brain volume measures between chronic cannabis users and healthy controls, there was only one study reporting significant differences [143], namely reduced ventricular cerebral spinal fluid (CSF) in cannabis users. Another study [145] reported total brain volume difference between groups which was no longer significant when the authors covaried for confounding factors such as premorbid intelligence.
Among the six studies employing a whole-brain analysis approach [143], [146], [148], [150]–[152], two further studies described differences between chronic cannabis users and controls [146], [148]. Matochik et al. (2005) [148] found lower grey matter density in the right parahippocampus and greater grey matter density in the precentral gyrus and right thalamus in cannabis users, while Cousjin et al. (2011) [146] found a larger anterior cerebellum in cannabis users. Matochik et al. (2005) [148] also reported differences in white matter density, such as lower density in the left parietal lobe and higher in parahippocampus, fusiform gyrus, lentiform nucleus and pons.
With regard to the three studies that focused on specific regions of interest, all studies reported bilateral volumetric reductions in the hippocampus [145], [148], [149] and one reported volume reductions in the right amygdala [149]. Some studies have also reported correlations between regional brain volume measures and cannabis use parameters, clinical and neuropsychological measures. For instance, a smaller hippocampal volume has been related to a greater exposure to cannabis [145], [146], [149], severity of cannabis dependence [146] and more severe positive psychotic symptoms [149]. Ashtari et al. (2011) [145] described a positive association between larger hippocampus volumes and higher verbal learning and memory scores in healthy controls but not in cannabis users [145]. It is remarkable to note that these findings were in patients with an average of 6.7 months of abstinence, which appears to support of the idea that cannabis use may cause long-term brain alterations.
With respect to other brain regions, Cousijn et al. (2011) [146] reported a negative correlation between amygdala volume and the amount of cannabis use or dependence, while Matochik et al. (2005) [148] found an association between increased white matter density in left precentral gyrus and longer duration of cannabis use.
1.2. Diffusion tensor imaging (DTI) studies
Four studies have used DTI to examine the integrity of white matter tracts in chronic cannabis users [144], [147], [150], [151], of which half have reported positive results [144], [147]. Arnone et al. (2008) [144] found increased mean diffusivity (MD) in the corpus callosum while Gruber et al. (2011) [147] found increased MD in the right genu as well as reductions in left frontal fractional anisotropy (FA). Gruber et al. (2011) [147] also reported a positive association between left frontal FA and impulsivity scores, and higher FA and lower MD in the frontal lobes being associated with a later age of initiation of cannabis use.
2. Structural neuroimaging studies in adolescent chronic cannabis users
Three volumetric studies in adolescent chronic cannabis users were included, two of which consist of the same sample [154], [155]. As an exception, these two studies [154], [155] were included despite involving participants with symptoms of alcohol dependence given the modest number of studies included in this population (for details see Table 1). The MRI scans, focused on specific regions of interest and were obtained following 28 days of abstinence from cannabis use. Medina et al. (2009, 2010) [154], [155] reported significantly larger volumes of the inferior posterior vermis, as well as a marginal group-by-gender interaction in the prefrontal cortex, in which female and male cannabis users demonstrated, respectively, larger and smaller prefrontal cortex volumes compared to the same-gender controls. McQueeny et al. (2011) [156] also described an effect of gender in which female cannabis users but not males, exhibited a larger right amygdala volume.
In terms of correlations, Medina et al. (2010) [155] found that larger volumes of the vermis were associated with poorer executive functioning while McQueeny et al. (2011) [156] found that larger right amygdala volume was associated with more internalizing symptoms (e.g., anxiety/depression). Lastly, Medina et al. (2009) [154] also found that increased volume in the prefrontal cortex was associated with poorer executive functioning among cannabis users while the opposite pattern was observed in controls, suggesting that female users may be at increased risk for cannabis-induced prefrontal abnormalities.
3. Functional neuroimaging studies in adult chronic cannabis users
3.1. Resting state
We included eight case-control studies comparing resting rCBF in adult chronic cannabis users and non cannabis using healthy controls (Table 2). The imaging methods used were as follows: H215O-PET [157], 133Xe-SPECT [158], 18F-FDG-PET [159], [11C]- raclopride-PET [159]–[162] and [18F]FMPEP-d2 [163]. Functional differences between groups were found in all studies, except for the four [11C]-raclopride-PET studies [159]–[162]. Abstinence periods ranged from 12 hours to 542 days (for details see Table 2). Block et al. (2000) [157] described reduced bilateral rCBF in the posterior cerebellum and ventral prefrontal cortex but also increased rCBF in the anterior cingulate cortex in cannabis users. Lundqvist et al. (2001) [158] found a trend of lower global CBF in cannabis users, as well as reduced rCBF in the right prefrontal and superior frontal cortex. Sevy et al. (2008) [159] reported lower glucose metabolism in the right orbitofrontal cortex, putamen bilaterally and precuneus in chronic cannabis users. However, there were no significant differences between the groups in striatal D2/D3 receptor availability and no correlation between striatal [11C]-raclopride-PET binding potential and glucose metabolism [159]. Consistent with these results, three other [11C]- raclopride-PET studies [160]–[162] failed to find any differences between groups in dopamine D2/D3 receptor availability in the striatum as a whole or it functional subdivisions. However, while Stokes et al. (2012) [160] also failed to find any association between lifetime frequency of cannabis use and binding potential values, Albrecht et al. (2012) [161] described a negative correlation with both urine levels of cannabis metabolites and self-report of recent cannabis consumption. Finally, Hirvonen et al. (2011) [163] demonstrated a reversible and regionally selective downregulation of CB1 receptors. At baseline, current users had approximately 20% less CB1 receptor density in the neocortex and limbic regions, which was negatively correlated with years of cannabis exposure. After four weeks of abstinence from cannabis use, CB1 receptor density returned to normal levels in all brain regions, except for the hippocampus [163].
Table 2. Functional neuroimaging studies in chronic cannabis users.
| Author (yr.) | Method | CUM/F | HCM/F | Mean (SD) age CU/HC | Image analysis | Condition | Abstinence (Mean days) | Results* | ||
| ADULTS | Brain area | Detailed results | Correlations with clinical variables | |||||||
| Functional (resting state) | FL-PL-TL-OL I-BG-Cb | |||||||||
| Block et al. (2000) [157] | H2 15O-PET | 8/9 | 6/6 | 22.4 (0.5)/22.6 (0.5) | Whole brain | Resting state | ≤ 1 | • ○ ○ ○ ○ ○ • | ↓ rCBF L/R cerebellum and VMPFC↑ rCBF R ACC | |
| Lundqvist et al. (2001) [158] | 133Xe-SPECT | 12/0 | 14/0 | 29.8 (5)/ 27.8 (5.2) | Whole brain | Resting state | 1.6 | • ○ ○ ○ ○ ○ ○ | ↓ global CBF (trend)↓ rCBF R superior PFC, superior frontal | |
| Sevy et al. (2008) [159] | 18F-FDG-PET | 6/0 | 6/0 | 20.0 (1.0)/20.0 (1.0) | Whole brain | Resting state | 105 | • • ○ ○ ○ • ○ | ↓ rCBF R OFC and R posterior parietal cortex and L/R putamen | |
| Sevy et al. (2008) [159] | [11C]-raclopride-PET | 6/0 | 6/0 | 20.0 (1.0)/20.0 (1.0) | ROI | Resting state | 105 | ○ | ||
| Hirvonen et al. (2011) [163] | [18F]FMPEP-d2 | 30/0 | 28/0 | 28 (8)/ 32 (10) | Whole brainROI | Resting state | 1 and 26 | • • • • ○ ○ ○ | 1 day: ↓ VT neocortex and limbic cortex26 days: ↑ VT neocortex and limbic cortex except for hippocampus | ↓ VT with longer cannabis exposure (yr.) |
| Stokes et al. (2011) [160] | [11C]-raclopride-PET | 6/4 | 9/1 | 32.6 (7.7)/ 36.5 (4.5) | ROI | Resting state | 542 | ○ | ||
| Urban et al. (2012) [162] | [11C]-raclopride-PET | 15/1 | 14/2 | 27.3 (6.1)/ 28.1 (6.9) | ROI | Resting state | 30 | ○ | ||
| Albrecht et al. (2012) [161] | [11C]-raclopride-PET | 10/0 | 8/0 | 25.1 (4.6)/ 26.4 (5.6) | ROI | Resting state | ≤ 1 | ○ | ↓ BPND with increase in urine levels of THC-COOH and self-reported recent intake per day | |
| Functional (cognitive task) | ||||||||||
| Block et al. (2002) [164] | H2 15O-PET | 18/0 | 13/0 | 22.3 (0.5)/ 22.6 (0.5) | Whole brainROI | Verbal memory | ≤ 1 | • ○ • ○ ○ ○ • | ↓ rCBF L/R PFC↑ rCBF L > R hippocampus in HC↑ rCBF posterior cerebellum | |
| Eldreth et al. (2004) [166] | H2 15O-PET | 11/0 | 11/0 | 25/29 | Whole brainROI† | Stroop | 25 | • ○ • • ○ ○ ○ | ↑ CBF R paracentral lobule and L occipital lobe↓ CBF R VMPFC and R DLPFCHippocampus: ↑ rCBF L/RACC:↓ rCBF LDLPF: ↑ rCBF L/R | |
| Bolla et al. (2005) [165] | H2 15O-PET | 11/0 | 11/0 | 26 (21–35)/ 31 | Whole brainROI† | Iowa Gambling | 25 | • ○ • • ○ ○ • | ↓ CBF OFC and R middle frontal gyrus↑ CBF L cerebellum, R inferior frontal gyrus and R occipital lobe in moderate CU↑ CBF L cerebellum in heavy CUCerebellum: ↑ rCBF LOFC: ↓ rCBF RDLPFC: ↓ rCBF R | ↓ rCBF R cerebellum, R orbital gyrus and ↑rCBF R parahippocampus with cannabis exposure |
| Gruber et al. (2005) [151] | fMRI 3T | 8/1 | 8/1 | 26 (3.6)/ 26.2 (3.1) | ROI† | Stroop | NE | • | ACC: ↓ BOLD L/RDLPFC: ↑ BOLD R | |
| Chang et al. (2006) [169] | fMRI 4T | 15/9 | 11/8 | 27.9 (10.8)/ 30.6 (8.0) | Whole brainROI† | Visual attention task | ≤ 1 and 1157 | • • ○ • ○ ○ • | ↓ BOLD L/R PFC, R medial and dorsal parietal cortex and cerebellum↑ BOLD frontal, parietal and occipital lobules↑ BOLD cerebellum in current CU | ↑ BOLD R PFC and dorsal parietal cortex and ↓ BOLD cerebellum with age of cannabis onset; ↓ BOLD cerebellum with cannabis exposure; ↑ BOLD R PFC and cerebellum (normalization) with duration of cannabis abstinence |
| Jager et al. (2006) [173] | fMRI 1.5T | 7/3 | 7/3 | 22.7 (4.2)/ 22.8 (2.9) | Whole brainROI† | Working memory | 7 | ○ • ○ ○ ○ ○ ○ | ↑ BOLD L superior parietal cortex | |
| Jager et al. (2007) [152] | fMRI 1.5T | 13/7 | 13/7 | 24.5 (5.2)/ 23.6 (3.9) | Whole brainROI† | Associative memory | 7 | • ○ • ○ ○ ○ ○ | ↓ BOLD R DLPFC and L/R parahippocampusParahippocampus: ↓ BOLD L/R | |
| Hester el al. (2009) [172] | fMRI 3T | 15/1 | 15/1 | 24.6 (1.5)/ 25.2 (1.3) | Whole brainROI† | Error Awareness Task | ≤ 1 | • • ○ ○ • ○ ○ | ↓ BOLD ACC, R insula, L/R inferior parietal and middle frontal regions | |
| Gruber et al. (2009) [170] | fMRI 3T | 14/1 | 14/1 | 25 (8.8)/ 26 (9.0) | ROI | Masked affective stimuli | ≤ 1 | • • | ACC: ↑ BOLD (masked happy faces) and ↓ BOLD (masked angry stimuli)Amygdala: ↓ BOLD (masked angry stimuli) | ↑ BOLD ACC with cannabis exposure (per week) in masked angry faces and with cannabinoid level in masked happy faces; ↑ BOLD amygdala with cannabis exposure (per week) in masked happy faces |
| van Hell et al. (2010) [176] | fMRI 1.5T | 14/1 | 11/2 | 24 (4.4)/ 24 (2.7) | Whole brainROI† | Monetary incentive delay task | 7 | • ○ • • ○ • ○ | Compared to non-smoking HC: ↓ BOLD NAcc, caudate nucleus, L putamen, R inferior and medial frontal gyrus, L/R superior frontal gyrus, L cingulate gyrus; ↑ BOLD L/R temporal gyrus, R cuneus and R parahippocampus gyrusCompared to smoking HC: ↓ BOLD caudate nucleus, L/R superior frontal; ↑ BOLD L middle temporal gyrus | |
| Nestor et al. (2010) [175] | fMRI 3T | 11/3 | 12/2 | 22.1 (1.2)/ 23.1 (1.2) | Whole brain | Monetary incentive delay task | 4.5 | ○ ○ ○ ○ • • ○ | ↑ BOLD R VS (win cue periods)↓ BOLD L insula (after loss and loss avoidance) | ↑ BOLD R VS (win cue periods) with cannabis exposure (yr.) |
| Abdullaev et al. (2010) [168] | fMRI 3T | 10/4 | 10/4 | 19.5 (0.8)/ 19.7 (1.4) | Whole brain† | Attention task | 2 | • • ○ ○ ○ ○ ○ | ↑ BOLD R PFC and L/R parietal cortex | |
| Wesley et al. (2011) [177] | fMRI 1.5T | 9/7 | 6/10 | 26.4 (3.6)/ 26.6 (6.1) | Whole brain | Iowa Gambling | ≤ 1 | • • ○ • ○ ○ • | ↓ BOLD ACC, VMPFC and medial frontal cortex, precuneus, superior parietal, occipital and cerebellum | BOLD activity in ACC, VMPFC and rostral PFC predicted improvement over the task course only in HC |
| King et al. (2011) [174] | fMRI 3T | 16/14 | 16/14 | 23.7/21.7 | ROI | Finger sequencing | ≤ 1 | • • | Lingual gyrus: ↓ BOLDSuperior frontal gyrus: ↑ BOLD | ↑ BOLD superior frontal gyrus with cortisol levels in F CU |
| Vaidya et al. (2012) [167] | H2 15O-PET | 28/18 | 18/16 | 24.3 (3.9)/ 24.7 (5.3) | Whole brain | Iowa Gambling | ≤ 1 | • ○ ○ ○ • ○ • | ↑ rCBF VMPFC and cerebellumVariant IGT: ↑ rCBF VMPFC, cerebellum and anterior insula | ↑ rCBF VMPFC with duration of cannabis use |
| Harding et al. (2012) [171] | fMRI 3T | 11/10 | 10/11 | 36.5 (8.8)/ 31 (11.7) | ROI | MSIT | ≤ 1 | • ○ • • • ○ ○ | ↑ connectivity between ACC, L PFC, L/R anterior insula and the L occipitoparietal cortex | ↑ connectivity with age of onset and lifetime exposure to cannabis |
| ADOLESCENTS | ||||||||||
| Functional (cognitive task) | ||||||||||
| Tapert et al. (2007) [183] | fMRI 1.5T | 12/4 | 12/5 | 18.1 (0.7)/ 17.9 (1.0) | Whole brain | Response inhibition task | 28 | • • ○ • • ○ ○ | ↑ BOLD R DLPFC, L/R medial frontal and inferior and superior parietal and R occipital gyrus↑ BOLD R PFC, insular and parietal cortex during go trials | |
| Padula et al. (2007)** [179] | fMRI 1.5T | 14/3 | 12/5 | 18.1 (0.8)/ 17.9 (1.1) | Whole brain | Spatial working memory | 28 | • • • ○ ○ • ○ | ↑ BOLD R basal ganglia, R precuneus, postcentral gyrus and L/R superior parietal cortex | ↑ BOLD L temporal gyrus and L ACC (↓ in HC) and ↓ BOLD R temporal gyrus, R thalamus and pulvinar, L parahippocampus (↑ in HC) with higher score on SWM task |
| Schweinsburg et al. (2008)*** [180] | fMRI 1.5T | 11/4 | 12/5 | 18.1 (0.7)/ 17.9 (1.0) | Whole brain | Spatial working memory | 28 | • • ○ • ○ ○ ○ | ↓ BOLD R DLPFC and occipital cortex↑ BOLD R posterior parietal cortex | |
| Schweinsburg et al. (2010)† [181] | fMRI 1.5T | 9/4 – 9/4 | 11/7 | 17.1 (0.5)-17.6 (0.9)/17.3 (0.8) | Whole brain† | Spatial working memory | 3 and 38 | • ○ ○ ○ • ○ ○ | ↑ BOLD L superior PFC and L/R anterior insula in recent CU↑ BOLD R precentral gyrus in abstinent CU | |
| Schweinsburg et al. (2011) [182] | fMRI 3T | 4/4 | 16/6 | 18.1 (0.9)/ 17.6 (0.8) | Whole brainROI | Verbal paired associates task | 25 | ○ ○ ○ ○ ○ ○ ○ | ||
Note: Yr. = years; CU = Cannabis users; HC = Healthy controls; M = Male; F = Female; SD = Standard deviation; FL = Frontal lobe; PL = Parietal lobe; TL = Temporal lobe; OL = Occipital lobe; I = Insula; BG = Basal ganglia; Cb = Cerebellum; fMRI = Functional magnetic resonance imaging; SPECT = Single photon emission tomography; PET = Positron emission tomopgraphy; DSC = Dynamic susceptibility contrast; VT = Distribution volume; BPND = Non-displaceable binding potential; FDG = Fludeoxyglucose; L = Left hemisphere; R = Right hemisphere; ROI = Region of interest; MSIT = Multi-Source Interference Task; CBF = Global cerebral blood flow; rCBF = Regional cerebral blood flow; BOLD = blood oxygenation-level dependent; NE = Not specified; PFC = Prefrontal cortex; DLPFC = Dorsolateral prefrontal cortex; VMPFC = Ventromedial prefrontal cortex; OFC = Orbitofrontal cortex; ACC = Anterior cingulated cortex; NAcc = Nucleus accumbens; VS = Ventral striatum; STG = Superior temporal gyrus; SWM = Spatial working task; IGT = Iowa Gambling Task.
If not otherwise specified, results are presented in terms of chronic cannabis users.
○ = Significant differences; ○ = Non-significant differences; = Not examined.
Two subjects in the marijuana group met criteria for alcohol abuse.
Four teens in the chronic cannabis group met criteria for alcohol use disorder, two cases of abuse and two cases of dependence.
One control, three recent users and two abstinent users met criteria for alcohol use disorders.
Multiple comparison correction.
3.2. Cognitive paradigms
We identified 16 studies in adult chronic cannabis users that compared regional activation during the performance of a cognitive task with healthy controls (Table 2), four with PET [164]–[167] and twelve with fMRI [151], [152], [168]–[177].
Attention
Chang et al. (2006) [169] used fMRI to compare a visual-attention task in current and abstinent cannabis users with healthy controls. Despite all groups showing normal task performance, both active and abstinent chronic cannabis users demonstrated decreased activation in the right prefrontal, medial and dorsal parietal cortices and medial cerebellar regions but greater activation in several smaller regions throughout the frontal, posterior parietal, occipital and cerebellum. An apparent normalization of BOLD signal was described in the right prefrontal and medial cerebellar regions in those with a longer duration of abstinence. In addition, early age of onset and estimated cumulative cannabis lifetime exposure were both associated with reduced activation in the right prefrontal cortex and medial cerebellum. More recently, Abdullaev et al. (2010) [168] used two attention tasks [the use generation task and the attention network task (ANT)] to contrast differences between cannabis users and healthy controls. Chronic cannabis users showed poorer performance in the ANT (more errors and longer reaction time), as well as stronger activation within the right prefrontal cortex in both tasks and within the parietal cortices in the ANT, which may indicate a less efficient system for the executive control of attention during conflict resolution tasks. Finally, Harding et al. (2012) [171] demonstrated for the first time that long-term heavy cannabis use is associated with increased functional connectivity between several frontal cortex regions and the occipitoparietal cortex using the Multi-Source Interference Task (MSIT). No differences in behavioural performance were evident between groups. The authors suggest that their findings may suggest a compensatory role for these regions in mitigating the effects of abnormal attentional and visual processing following chronic cannabis exposure [171].
Memory
In a H215O-PET study, Block et al. (2002) [164] found that cannabis users performed verbal memory tasks more poorly than controls. This was associated with reduced activation in the prefrontal cortex and greater activation in the posterior cerebellum, as well as with an absence of lateralization of hippocampal activity. Consistent with this, Jager et al. (2007) [152] described attenuated activity in the right dorsolateral prefrontal cortex and bilateral (para) hippocampal gyri in cannabis users despite normal performance in an associative memory task. Finally, in a verbal working memory task, Jager et al. (2006) [173] found significantly greater activity in the left superior parietal cortex in the cannabis using group despite there being no differences in task performance, which may be consistent with the idea of a compensatory recruitment effect.
Inhibition and impulsivity
Eldreth et al. (2004) [166] and Gruber et al. (2005) [151] studied the degree of inhibitory control during a Stroop task in current (positive THC urine analysis) and abstinent chronic cannabis users, respectively. Gruber et al. (2005) [151] found lower anterior cingulate activity and higher mid-cingulate and bilateral dorsolateral prefrontal cortex activity in current cannabis users relative to healthy controls, who demonstrated focal increased activity within the right dorsolateral prefrontal cortex. Consistently, Eldreth et al. (2004) [166] found in abstinent cannabis users a reduced anterior cingulate activation using H215O-PET during the performance of a modified Stroop test. However, they also reported a reduced dorsolateral prefrontal cortex activation and a greater activation in the hippocampus bilaterally [166]. Lastly, Hester et al. (2009) [172] administered a go/no-go response inhibition task to active cannabis users to determine inhibitory control and error awareness compared with healthy controls. Although control performance was equivalent between the two groups, cannabis users displayed a significant deficit in awareness of commission errors, which was associated with decreased a activity in the anterior cingulate cortex and right insula, as well as in the bilateral inferior parietal and middle frontal regions [172].
Decision-making
Bolla et al. (2005) [165] and Vaidya et al. (2011) [167] using H215O-PET, and Wesley et al. (2011) [177] using fMRI, studied the brain activation pattern in chronic cannabis users compared to healthy controls during the Iowa Gambling Task (IGT). Bolla et al. (2005) [165] reported dysfunction during the performance of the task in abstinent cannabis users, demonstrating a lower activation in the right orbitofrontal cortex and dorsolateral prefrontal cortex and greater activation in the left parietal and cerebellar cortices. The number of joints used per week was positively correlated with activation in the right parahippocampal gyrus but inversely correlated with activation in the right cerebellum and orbital gyrus. Wesley et al. (2011) [177] also reported a poorer performance on the IGT in active cannabis users. However, there were no differences during the initial strategy development phase, in which cannabis users showed reduced activity in response to losses in anterior cingulate cortex, ventromedial prefrontal cortex, precuneus, superior parietal lobe, occipital lobe and cerebellum compared to controls [177]. Additionally, the functional response to losses in anterior cingulate, ventromedial and rostral prefrontal cortices was positively correlated with improvement over the task course only in the control group, indicating that cannabis users may be less sensitive to negative feedback during the strategy development phase [177]. In contrast, Vaidya et al. (2011) [167] did not find differences on the standard IGT performance between active cannabis users and healthy controls. Nevertheless, cannabis users performed significantly worse than controls on a variant version of the same task [178]. Both groups showed increased activity in ventromedial prefrontal cortex on both versions of the IGT compared to the control task but in contrast to Wesley et al. (2011) [177], cannabis users demonstrated greater activity than controls in the ventromedial prefrontal cortex on the standard IGT, as well as in the cerebellum and the anterior insula on both versions of the IGT [167]. Furthermore, duration of cannabis use was associated with greater activity in ventromedial prefrontal cortex [167]. Nestor et al. (2010) [175] and van Hell et al. (2010) [176] used fMRI to measure brain activity during reward and anticipation of loss with different versions of a monetary reward task. There were no significant behavioural differences between the groups in both studies. Nestor et al. (2010) [175] reported a greater right ventral striatum activity in cannabis users during reward anticipation, which was significantly correlated with years of lifetime cannabis use. In addition, response to loss and loss avoidance outcome notification was related with hypoactivity in left insula, and in the post hoc analysis comparing loss and win cues with no-outcome cues, right ventral putamen showed greater BOLD response [175]. Conversely, comparing cannabis users to non tobacco-smoking controls, van Hell et al. (2010) [176] demonstrated attenuated activity in the nucleus accumbens and caudate nucleus bilaterally during reward anticipation, as well as left putamen and right inferior and medial frontal gyrus, superior frontal gyrus bilaterally and left cingulate gyrus. Cannabis users showed enhanced reward anticipation activity in the middle temporal gyrus bilaterally, right cuneus and right parahippocampal gyrus. When compared to tobacco-smoking controls, cannabis users also showed reduced anticipation activity in the same areas, with the exception of the nucleus accumbens bilaterally, the right medial frontal gyrus and the left cingulated gyrus, indicating that anticipation activity in these regions may be attenuated by both cannabis and nicotine [176]. In accordance with Nestor et al. (2010) [175], response to contrasted outcome notification was associated with greater activity in the putamen bilaterally and the right caudate nucleus compared with non-smoking controls [176]. The putamen was more activated in cannabis users than in non-smokers and tobacco-smoking controls, indicating that changes in this area were mainly due to cannabis use [176].
Motor performance
King et al. (2011) [174] reported that chronic cannabis use was associated with slower and less efficient psychomotor function, especially in male users. Cannabis users showed lesser activation in the lingual gyrus and greater activation of the superior frontal gyrus compared to controls while performing a visually paced finger sequencing task, suggesting that the former group shifted from more automated visually-guided responses to more executive or attention control regions of the brain [174].
Affective processing
Gruber et al. (2009) [170] examined the BOLD signal changes for two target affective conditions (happy and anger). Region of interest analyses revealed that cannabis users demonstrated relatively lower anterior cingulate and amygdalar activity during the presentation of masked angry stimuli sets relative to the control group, who showed relatively higher activation within these regions. In contrast, cannabis users demonstrated a larger pattern of activation during the presentation of masked happy faces within the cingulate as compared to controls, with no increase in amygdalar activation [170]. Furthermore, the total number of smoking episodes per week was positively associated with cingulate activity during the viewing of masked angry faces and positively associated with amygdalar activity during the viewing of masked happy faces [170]. Finally, overall cannabinoid level was positively related to cingulate activity during the viewing of masked happy faces [170]. The disparate activation patterns showed between groups suggest a different way of processing emotional information between groups [170].
4. Functional neuroimaging studies in adolescent chronic cannabis
We included five case-control fMRI studies in adolescent cannabis users comparing brain activity with healthy controls during a cognitive task performance. As an exception, two of them [180], [181] were included despite involving a minor proportion of participants with a co-morbid alcohol dependence given the relatively modest number of studies in this population (for details see Table 2). No resting state studies were identified in the adolescent population.
Memory
Padula et al. (2007) [179] and Schweinsburg et al. (2008, 2010) [180], [181] examined fMRI response during a spatial working memory (SWM) task. In a group of abstinent adolescent cannabis users, Padula et al. (2007) [179] described increased activity in the left temporal gyrus and anterior cingulate cortex but lower activity in right temporal gyrus, thalamus, pulvinar and left parahippocampal gyrus related to higher scores on the task, while the reverse pattern was found in the controls. This may suggest that cannabis users employed more of a verbal strategy to achieve the same level of task performance as the controls [179]. Additionally, cannabis users demonstrated greater performance-related activation in the right basal ganglia, precuneus, postcentral gyrus and bilateral superior parietal lobe [179], again suggesting a compensatory neural effort. Consistent with this, Schweinsburg et al. (2008) [180] also found a different pattern of activation in abstinent adolescent cannabis users who performed the SWM task similarly to the control group. Thus, cannabis users demonstrated higher activation in the right parietal cortex but also lower activity in the right dorsolateral prefrontal and occipital cortices [180]. Finally, in a cross-sectional study, Schweinsburg et al. (2010) [181] compared fMRI responses using the same task among adolescent cannabis users with brief and sustained cannabis abstinence and healthy controls. Although both groups performed at a similar level on the task, recent users showed greater activity in the medial and left superior prefrontal cortices and bilateral insula while abstinent users demonstrated an increased response in the right precentral gyrus [181]. More recently, Schweinsburg et al. (2011) [182] compared fMRI response during a verbal paired associates encoding task in 3 groups of participants that included an abstinent cannabis user group, a binge drinker group and a cannabis user group with co-morbid binge-drinking to healthy controls with very limited alcohol or cannabis experience. In general, each group displayed deviations in BOLD response relative to non-using controls, and binge drinking and cannabis use demonstrated independent as well as interactive effects on brain functioning [182].
Inhibition and impulsivity
In a group of abstinent cannabis users, Tapert et al. (2007) [183] compared the activation pattern on a go/no-go task during fMRI with seventeen healthy subjects. Despite similar level of task performance, cannabis users showed greater activation during inhibitory trials in the right dorsolateral prefrontal, bilateral medial frontal, bilateral inferior and superior parietal lobules and right occipital gyrus compared to the healthy subjects. During the non-inhibitory trials, differences were located in right prefrontal, insular and parietal cortices, with cannabis users showing greater activation in these areas compared to the controls. As observed in adults, these results suggest a greater neurocognitive effort during the task in cannabis users, even after the abstinence period.
Discussion
In this systematic review, we identified 43 studies suitable for inclusion regarding the impact of chronic cannabis use on brain structure and functioning, of which eight (19%) were in the adolescent population. Despite the high degree of heterogeneity among the studies reviewed herein, several relatively consistent findings emerged from this review. These findings, discussed in detail below, include: (1) Structural brain abnormalities, mainly in CB1-rich areas implicated in several cognitive functions, which may be related to the amount of cannabis use; (2) Altered neural activity during resting state and under several different types of cognitive paradigms, that may reflect a different recruitment of brain areas during the tasks, particularly within the prefrontal cortex; and (3) The few studies conducted in adolescents suggest that both structural and functional alterations may appear soon after starting the drug use and may be related to gender.
In terms of structural findings, specific regional brain analyses demonstrated evidence of structural abnormalities when adult chronic cannabis users were compared with healthy controls. The most consistently reported brain alteration was reduced hippocampal volume [145], [146], [148], [149], which was shown to persist even after several months of abstinence in one study [145] and also to be related to the amount of cannabis use [145], [146], [149]. Other frequently reported morphological brain alterations related to chronic cannabis use were reported in the amygdala [146], [149], [156], the cerebellum [146], [155] and the frontal cortex [148], [154]. Lastly, two DTI studies found differences in the mean diffusivity or fractional anisotropy in the corpus callosum and the frontal white matter fibre tract [144], [147], suggesting that chronic cannabis exposure may also alter white matter structural integrity, by either affecting demyelination or causing axonal damage or indirectly through delaying normal brain development. With regard to the few structural MRI studies focusing on the effects of cannabis use on brain morphology in adolescents, some discrepancies were reported related to adult population. These inconsistencies may be explained in terms of the disruption of normal pruning during developmental maturation due to early chronic cannabis use, ultimately resulting in larger regional volumes [156]. Notwithstanding, structural results from adolescent population suggest that the effects of chronic cannabis use may appear soon after starting the drug use, persist after a month of abstinence or even be moderated by gender [145], [154]–[156]. In this context, it has been reported that adolescent female cannabis users may be at increased risk for cannabis-induced morphological effects [154], [156].
Functional neuroimaging studies that have evaluated the resting state in active and abstinent adult chronic cannabis users suggest that resting global [158], prefrontal cortical [157]–[159], cerebellar [157] and striatal [159] blood flow may be lower compared with controls. These brain regions correspond to areas with relatively high concentration of CB1 receptors [19]. Hence, it has been hypothesised that the decreased resting state function may represent a down-regulation of CB1 receptors as a result of regular exposure to cannabis [41]. However, it is important to note that not all studies have consistently demonstrated effects in these regions. Furthermore, it has been recently found that, similar to animal studies, down-regulation of CB1 receptors in humans is region-specific and reversible, occurring in the neocortex and limbic cortex but neither in subcortical brain regions nor in the cerebellum [163]. It is also noteworthy that these brain regions correspond to areas that are engaged in the processing of reward [184]. This is also consistent with the evidence of neuropsychological impairments in chronic cannabis users, such as in attention and working memory [185], decision making [186], and psychomotor speed [187]. Also, consistent with experimental animal studies, no differences in striatal D2/D3 receptor availability were found in four studies of chronic cannabis users compared with healthy controls [159]–[162]. However, in the only study where the chronic cannabis users were not abstinent [161], an inverse correlation between recent cannabis consumption and D2/D3 receptor availability was found, leading the authors to suggest that this effect could be related to a direct effect of cannabis smoking on the expression of striatal DA receptors in heavy cannabis users [161]. Additional studies are needed to better understand the neurochemical basis of this finding.
Functional imaging studies comparing activation in both adult and adolescent chronic cannabis users with healthy controls during the performance of different cognitive tasks indicated that chronic cannabis users would use similar brain areas that engage these cognitive processes but often demonstrating an altered pattern of brain activity [151], [152], [157], [165]–[177], [179], [181]–[183]. However, the level of performance of the cannabis users on the cognitive tasks employed was generally similar to that of controls [164], [165], [168], [171], [174], [177], or at least within what may be considered a normal range of test performance. Therefore, these findings may be interpreted as reflecting neuroadaptation, perhaps indicating the recruitment of additional regions as a compensatory mechanism to maintain normal cognitive performance in response to chronic cannabis exposure [151], [152], [164], [166], [171], [172], [175], [179]–[181], [183], particularly within the prefrontal cortex area [151], [166], [168], [169], [171], [181], [183]. In this regard, the brain seems able to achieve some degree of reorganization, activating brain regions not usually needed to perform the cognitive task in response to an impaired ability of the normally engaged task network. Thus, it is feasible that drug-related compensatory mechanism may work for a period of time until it turns out to be insufficient and differences between groups become apparent. However, the impact of these subtle brain alterations on social, familiar and occupational life as well as its potential relationship with psychiatric disorders remains speculative.
A further important issue emerging out of this review is that few studies have investigated the effects of chronic cannabis use on the brain in adolescence subjects. In light of the popularity of cannabis among teenagers [1], [2] and recent data showing the potential neurotoxic effects of chronic cannabis use on the maturational brain [188], investigation of the possible long-term effects on brain structure and function in the adolescent population should be a priority both from the scientific and population health perspective [34], [188]. Future studies should consider the need for convergent methodology, replication of known facts with greater methodological rigor, and prospective large samples involving subjects of both genders across the life-span from adolescence to adulthood to delineate the evolution and reversibility of previously reported alterations.
Limitations of the review
Results presented here have pointed out some important methodological differences that limit the generalisation of results and comparison between studies and have doubtless contributed to the slightly disparate array of findings. Despite the use of a strict definition of chronic cannabis user and robust application of inclusion and exclusion criteria in an attempt to avoid excessive heterogeneity between samples, studies often diverged on certain socio-demographic characteristics and cannabis use parameters, such as gender-bias, age of onset, lifetime use and abstinence period before the acquisition of imaging data. Moreover, it is well known that the THC content of smoked cannabis varies markedly between sources and preparations, with potency reported to have increased substantially over the past ten years [2]. Thus, comparability of earlier to later studies may not always be appropriate [44]. Furthermore, the exclusion of studies involving recreational and naïve cannabis users implies that the question of whether the brains of these subjects are adversely affected by cannabis is not addressed within the framework of the present review. Another important confounding factor is the inclusion of subjects with concurrent use of tobacco, which may affect neural activity as well as potentially interact with the effects of cannabis use [176]. In addition, it is known that co-morbid misuse of alcohol and other illicit drugs, such as cocaine and methamphetamine, may also be associated with significant neurobiological, neurocognitive and psychiatric abnormalities [189]. In the present review, although we excluded studies involving subjects with alcohol dependence, some included subjects with alcohol misuse (abuse [145], [179] or excessive consumption [150]), or reported differences in alcohol intake parameters despite alcohol consumption was within safe limits [143], [144], [147], [156], [157], [163], [164], [169], [170]. Moreover, given the relatively modest number of studies in the adolescent population, we included four studies which may involve some participants with co-morbid alcohol dependence [154], [155], [180], [181]. In all these studies, the interaction of alcohol with cannabis use, as well as its contribution to the brain effects cannot be ruled out. On the other hand, the exclusion of those with alcohol dependence, often highly co-morbid with cannabis use, may restrict the generalization of the results to the majority of chronic cannabis users [190].
With regard to other methodological limitations, some studies have reported modest sample sizes, sometimes below the threshold that would be currently regarded as acceptable (for instance, for PET or SPECT studies 10 subjects and for fMRI studies 15 subjects) [29]. In this regard, strategies for expanding data-sharing would be a welcome development in future research (i.e. The Function Biomedical Informatics Research Network [191] or the 1000 Functional Connectomes project [192], [193]). However, further obstacles must be addressed to make collaborative analysis efficient, such as between-site differences in scanners and data acquisition parameters, as well as pre- and post-processing schemes. The cross-sectional designs of most of the studies reviewed here complicated the interpretation of results as pre-existing morphological or functional alterations cannot be ruled out. Furthermore, studies that merely compare those subjects exposed to an environmental factor from those that are not, are likely to promote interpretation biases whereby study findings, irrespective of their direction, tend to be interpreted as detrimental. Longitudinal evaluations in larger samples may thus prove particularly useful. With regard to technical limitations, it is remarkable to note that the resting state studies did not control for spontaneous neutral activity and modulation of the BOLD signal, and the functional studies often reported different imaging methods and explored different brain functions using diverse cognitive paradigms, hampering the comparison between the studies. Hence, replication of previous results is critically important. Convergent methodology to sort out the current inconsistencies and controversies among studies would be important for future research in the field.
Supporting Information
Funding Statement
This study has been done in part with Spanish grants: Plan Nacional sobre Drogas, Ministerio de Sanidad y Consumo PNSD/2011/050 and PNSD2006/101 (R. MartÃn-Santos); and the support of DIUE of Generalitat de Catalunya SGR2009/1435 (R. MartÃn-Santos, J.A. Crippa); and the Technology Institute for Translational Medicine (INCT-TM, Conselho Nacional de Desenvolvimiento CientÃfico e TecnolÃ2gico (CNPq), Brazil (J.A. Crippa, R. MartÃn-Santos); S. Bhattacharyya is supported by a Clinician Scientist award from the National Institute of Health Research, UK; and J.A. Crippa receives a CNPq (Brazil) productivity award (IC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.European Monitoring Centre for Drugs and Drug Addiction (2011) The state of the drugs problem in Europe. EMCDDA. Available: http://www.emcdda.europa.eu/publications/annual-report/2011. Accessed 2 February 2012.
- 2.United Nations Office on Drugs and Crime (2011) World drug report 2011. UNODC, Vienna 2011. Available: http://www.unodc.org/unodc/en/data-and-analysis/WDR-2011.html. Accessed 2 February 2012.
- 3. Chen CY, O'Brien MS, Anthony JC (2005) Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000–2001. Drug Alcohol Depend 79: 11–22. [DOI] [PubMed] [Google Scholar]
- 4. Fernandez-Artamendi S, Fernandez-Hermida JR, Secades-Villa R, Garcia-Portilla P (2011) Cannabis and mental health. Actas Esp Psiquiatr 39: 180–190. [PubMed] [Google Scholar]
- 5. Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, et al. (2009) Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry 66: 442–451. [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, et al. (2012) Induction of psychosis by {delta}9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry 69: 27–36. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, et al.. (2012) Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of delta-9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry Jan 31.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Hall W, Degenhardt L (2009) Adverse health effects of non-medical cannabis use. Lancet 374: 1383–1391. [DOI] [PubMed] [Google Scholar]
- 9. Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, et al. (2007) Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370: 319–328. [DOI] [PubMed] [Google Scholar]
- 10. Morrison PD, Nottage J, Stone JM, Bhattacharyya S, Tunstall N, et al. (2011) Disruption of frontal theta coherence by Delta9-tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology 36: 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solowij N, Battisti R (2008) The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev 1: 81–98. [DOI] [PubMed] [Google Scholar]
- 12. Stone JM, Bhattacharyya S, Barker GJ, McGuire PK (2012) Substance use and regional gray matter volume in individuals at high risk of psychosis. Eur Neuropsychopharmacol 22: 114–122. [DOI] [PubMed] [Google Scholar]
- 13. Stone JM, Morrison PD, Brugger S, Nottage J, Bhattacharyya S, et al. (2012) Communication breakdown: delta-9 tetrahydrocannabinol effects on pre-speech neural coherence. Mol Psychiatry 17: 568–569. [DOI] [PubMed] [Google Scholar]
- 14. Sundram S (2006) Cannabis and neurodevelopment: implications for psychiatric disorders. Hum Psychopharmacol 21: 245–254. [DOI] [PubMed] [Google Scholar]
- 15. van Winkel R (2011) Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry 68: 148–157. [DOI] [PubMed] [Google Scholar]
- 16. Chevaleyre V, Takahashi KA, Castillo PE (2006) Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 29: 37–76. [DOI] [PubMed] [Google Scholar]
- 17. Morrison PD, Murray RM (2009) From real-world events to psychosis: the emerging neuropharmacology of delusions. Schizophr Bull 35: 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belue RC, Howlett AC, Westlake TM, Hutchings DE (1995) The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol 17: 25–30. [DOI] [PubMed] [Google Scholar]
- 19. Burns HD, Van LK, Sanabria-Bohorquez S, Hamill TG, Bormans G, et al. (2007) [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad SciUSA 104: 9800–9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaoni Y, Mechoulam R (1971) The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. JAm Chem Soc 93: 217–224. [DOI] [PubMed] [Google Scholar]
- 21. Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR (2007) Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem 14: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landfield PW, Cadwallader LB, Vinsant S (1988) Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Res 443: 47–62. [DOI] [PubMed] [Google Scholar]
- 23. Scallet AC, Uemura E, Andrews A, Ali SF, McMillan DE, et al. (1987) Morphometric studies of the rat hippocampus following chronic delta-9-tetrahydrocannabinol (THC). Brain Res 436: 193–198. [DOI] [PubMed] [Google Scholar]
- 24. Bossong MG, Niesink RJ (2010) Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol 92: 370–385. [DOI] [PubMed] [Google Scholar]
- 25. Adriani W, Laviola G (2004) Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol 15: 341–352. [DOI] [PubMed] [Google Scholar]
- 26. Schneider M, Koch M (2003) Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 27. Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, et al. (2008) Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 33: 1113–1126. [DOI] [PubMed] [Google Scholar]
- 28. Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yucel M (2010) Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse 45: 1787–1808. [DOI] [PubMed] [Google Scholar]
- 29. Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, et al. (2010) Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med 40: 383–398. [DOI] [PubMed] [Google Scholar]
- 30. Bhattacharyya S, Crippa JA, Martin-Santos R, Winton-Brown T, Fusar-Poli P (2009) Imaging the neural effects of cannabinoids: current status and future opportunities for psychopharmacology. Curr Pharm Des 15: 2603–2614. [DOI] [PubMed] [Google Scholar]
- 31. Solowij N, Pesa N (2010) [Cognitive abnormalities and cannabis use]. Rev Bras Psiquiatr 32 Suppl 1S31–S40. [PubMed] [Google Scholar]
- 32. Pope HG Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, et al. (2003) Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend 69: 303–310. [DOI] [PubMed] [Google Scholar]
- 33. Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, et al. (2000) Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis 19: 1–22. [DOI] [PubMed] [Google Scholar]
- 34. Jager G, Ramsey NF (2008) Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: an overview of animal and human research. Curr Drug Abuse Rev 1: 114–123. [DOI] [PubMed] [Google Scholar]
- 35. Crews F, He J, Hodge C (2007) Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav 86: 189–199. [DOI] [PubMed] [Google Scholar]
- 36. D'Souza DC, Sewell RA, Ranganathan M (2009) Cannabis and psychosis/schizophrenia: human studies. Eur Arch Psychiatry Clin Neurosci 259: 413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, et al. (2001) Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry 40: 1012–1020. [DOI] [PubMed] [Google Scholar]
- 38. Jernigan TL, Gamst AC (2005) Changes in volume with age--consistency and interpretation of observed effects. Neurobiol Aging 26: 1271–1274. [DOI] [PubMed] [Google Scholar]
- 39. Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387: 167–178. [DOI] [PubMed] [Google Scholar]
- 40. Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, et al. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang L, Chronicle EP (2007) Functional imaging studies in cannabis users. Neuroscientist 13: 422–432. [DOI] [PubMed] [Google Scholar]
- 42. Crean RD, Crane NA, Mason BJ (2011) An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonzalez R (2007) Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev 17: 347–361. [DOI] [PubMed] [Google Scholar]
- 44. Quickfall J, Crockford D (2006) Brain neuroimaging in cannabis use: a review. J Neuropsychiatry Clin Neurosci 18: 318–332. [DOI] [PubMed] [Google Scholar]
- 45. Bhattacharyya S, Sendt KV (2012) Neuroimaging evidence for cannabinoid modulation of cognition and affect in man. Front Behav Neurosci 6: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, McGuire PK (2012) Neural mechanisms for the cannabinoid modulation of cognition and affect in man: a critical review of neuroimaging studies. Curr Pharm Des 18: 5045–5054. [DOI] [PubMed] [Google Scholar]
- 47. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, et al. (2004) Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 29: 417–426. [DOI] [PubMed] [Google Scholar]
- 49. Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA (2010) Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol 1: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, et al.. (2012) Neural responses associated with cue-reactivity in frequent cannabis users. Addict Biol Jan 20.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51. Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA (2004) Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 176: 239–247. [DOI] [PubMed] [Google Scholar]
- 52. Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, et al. (2011) Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res 220: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murphy K, Dixon V, LaGrave K, Kaufman J, Risinger R, et al. (2006) A validation of event-related FMRI comparisons between users of cocaine, nicotine, or cannabis and control subjects. Am J Psychiatry 163: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 54. Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA (2011) Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage 57: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schneider M (2008) Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol 13: 253–263. [DOI] [PubMed] [Google Scholar]
- 56. Silveri MM, Jensen JE, Rosso IM, Sneider JT, Yurgelun-Todd DA (2011) Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Res 191: 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aasly J, Storsaeter O, Nilsen G, Smevik O, Rinck P (1993) Minor structural brain changes in young drug abusers. . A magnetic resonance study. Acta Neurol Scand 87: 210–214. [DOI] [PubMed] [Google Scholar]
- 58. Amen DG, Waugh M (1998) High resolution brain SPECT imaging of marijuana smokers with AD/HD. J Psychoactive Drugs 30: 209–214. [DOI] [PubMed] [Google Scholar]
- 59. Campbell AM, Evans M, Thomson JL, Williams MJ (1971) Cerebral atrophy in young cannabis smokers. Lancet 2: 1219–1224. [DOI] [PubMed] [Google Scholar]
- 60. Co BT, Goodwin DW, Gado M, Mikhael M, Hill SY (1977) Absence of cerebral atrophy in chronic cannabis users. Evaluation by computerized transaxial tomography. JAMA; 237: 1229–1230. [PubMed] [Google Scholar]
- 61. Hannerz J, Hindmarsh T (1983) Neurological and neuroradiological examination of chronic cannabis smokers. Ann Neurol 13: 207–210. [DOI] [PubMed] [Google Scholar]
- 62. Jacobsen LK, Mencl WE, Westerveld M, Pugh KR (2004) Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci 1021: 384–390. [DOI] [PubMed] [Google Scholar]
- 63. Kuehnle J, Mendelson JH, Davis KR, New PF (1977) Computed tomographic examination of heavy marijuana smokers. JAMA 237: 1231–1232. [PubMed] [Google Scholar]
- 64. O'Leary DS, Block RI, Flaum M, Schultz SK, Boles Ponto LL, et al. (2000) Acute marijuana effects on rCBF and cognition: a PET study. Neuroreport 11: 3835–3841. [DOI] [PubMed] [Google Scholar]
- 65. Sneider JT, Pope HG Jr, Silveri MM, Simpson NS, Gruber SA, et al. (2006) Altered regional blood volume in chronic cannabis smokers. Exp Clin Psychopharmacol 14: 422–428. [DOI] [PubMed] [Google Scholar]
- 66.Ward PB, Solowij N, Peters R, Otton J, Chesher G, et al.. (2002) An fMRI study of regional brain volumes in long-term cannabis users. J Psychopharmacol 16(Suppl. 3).. [Google Scholar]
- 67.Yurgelun-Todd D, Gruber SA, Hanson RA, Baird AA, Renshaw PF, et al.. (1998) Residual effects of marijuana use: an fMRI study. In: Problems of drug dependence 1998: Proceedings of the 60th Annual Scientific Meeting, The College on Problems of Drug Dependence. NIDA Research Monograph. pp.78. [Google Scholar]
- 68. Zalesky A, Solowij N, Yucel M, Lubman DI, Takagi M, et al. (2012) Effect of long-term cannabis use on axonal fibre connectivity. Brain 135: 2245–2255. [DOI] [PubMed] [Google Scholar]
- 69. Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J (2010) The impact of early-onset cannabis use on functional brain correlates of working memory. Prog Neuropsychopharmacol Biol Psychiatry 34: 837–845. [DOI] [PubMed] [Google Scholar]
- 70. Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, et al. (2011) Diminished gray matter in the hippocampus of cannabis users: Possible protective effects of cannabidiol. Drug Alcohol Depend 114: 242–245. [DOI] [PubMed] [Google Scholar]
- 71. Leroy C, Karila L, Martinot JL, Lukasiewicz M, Duchesnay E, et al. (2012) Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study. Addict Biol 17: 981–990. [DOI] [PubMed] [Google Scholar]
- 72. Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Pazos A, et al. (2010) Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res 1317: 297–304. [DOI] [PubMed] [Google Scholar]
- 73. Parkar SR, Ramanathan S, Nair N, Batra SA, Adarkar SA, et al. (2010) Cannabis dependence: Effects of cannabis consumption on inter-regional cerebral metabolic relationships in an Indian population. Indian J Psychiatry 52: 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Smith AM, Longo CA, Fried PA, Hogan MJ, Cameron I (2010) Effects of marijuana on visuospatial working memory: an fMRI study in young adults. Psychopharmacology (Berl) 210: 429–438. [DOI] [PubMed] [Google Scholar]
- 75. Voruganti LN, Slomka P, Zabel P, Mattar A, Awad AG (2001) Cannabis induced dopamine release: an in-vivo SPECT study. Psychiatry Res 107: 173–177. [DOI] [PubMed] [Google Scholar]
- 76. Wiesbeck GA, Taeschner KL (1991) A cerebral computed tomography study of patients with drug-induced psychoses. Eur Arch Psychiatry Clin Neurosci 241: 88–90. [DOI] [PubMed] [Google Scholar]
- 77. Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, et al. (2007) Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry 61: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 78. Nestor L, Roberts G, Garavan H, Hester R (2008) Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage 40: 1328–1339. [DOI] [PubMed] [Google Scholar]
- 79. Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, et al. (2008) Brain imaging study of the acute effects of Delta9-tetrahydrocannabinol (THC) on attention and motor coordination in regular users of marijuana. Psychopharmacology (Berl) 196: 119–131. [DOI] [PubMed] [Google Scholar]
- 80. Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J (2010) Altered parahippocampal functioning in cannabis users is related to the frequency of use. Psychopharmacology (Berl) 209: 361–374. [DOI] [PubMed] [Google Scholar]
- 81. Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE (2009) Marijuana craving in the brain. Proc Natl Acad Sci USA 106: 13016–13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE (2010) Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology 35: 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE (1992) Changes in middle cerebral artery velocity after marijuana. Biol Psychiatry 32: 164–169. [DOI] [PubMed] [Google Scholar]
- 84. Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, et al. (1991) Cerebellar metabolic activation by delta-9-tetrahydro-cannabinol in human brain: a study with positron emission tomography and 18F-2-fluoro-2-deoxyglucose. Psychiatry Res 40: 69–78. [DOI] [PubMed] [Google Scholar]
- 85. Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, et al. (2009) Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res 43: 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, et al. (2009) Altered white matter microstructure in adolescent substance users. Psychiatry Res 173: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF (2010) Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn 72: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cheetham A, Allen NB, Whittle S, Simmons J, Yucel M, et al. (2012) Orbitofrontal Volumes in Early Adolescence Predict Initiation of Cannabis Use: A 4-Year Longitudinal and Prospective Study. Biol Psychiatry 71: 684–692. [DOI] [PubMed] [Google Scholar]
- 89. Chung T, Geier C, Luna B, Pajtek S, Terwilliger R, et al. (2011) Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug Alcohol Depend 115: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Clark DB, Chung T, Thatcher DL, Pajtek S, Long EC (2012) Psychological dysregulation, white matter disorganization and substance use disorders in adolescence. Addiction 107: 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen M, Rasser PE, Peck G, Carr VJ, Ward PB, et al.. (2011) Cerebellar grey-matter deficits, cannabis use and first-episode schizophrenia in adolescents and young adults. Int J Neuropsychopharmacol 1–11. [DOI] [PubMed] [Google Scholar]
- 92. Cornelius JR, Aizenstein HJ, Hariri AR (2010) Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav 35: 644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cowan RL, Joers JM, Dietrich MS (2009) N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: a 3 T magnetic resonance spectroscopy study. Pharmacol Biochem Behav 92: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dekker N, Schmitz N, Peters BD, van Amelsvoort TA, Linszen DH, et al. (2010) Cannabis use and callosal white matter structure and integrity in recent-onset schizophrenia. Psychiatry Res 181: 51–56. [DOI] [PubMed] [Google Scholar]
- 95. Habets P, Marcelis M, Gronenschild E, Drukker M, van OJ (2011) Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry 69: 487–494. [DOI] [PubMed] [Google Scholar]
- 96. Ho BC, Wassink TH, Ziebell S, Andreasen NC (2011) Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr Res 128: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, et al. (2009) White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol 31: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jager G, Block RI, Luijten M, Ramsey NF (2010) Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 49: : 561–72, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. James A, Hough M, James S, Winmill L, Burge L, et al. (2011) Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS). Schizophr Res 128: 91–97. [DOI] [PubMed] [Google Scholar]
- 100. Kumra S, Robinson P, Tambyraja R, Jensen D, Schimunek C, et al. (2012) Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J Am Acad Child Adolesc Psychiatry 51: 171–180. [DOI] [PubMed] [Google Scholar]
- 101. Li CS, Milivojevic V, Constable RT, Sinha R (2005) Recent cannabis abuse decreased stress-induced BOLD signals in the frontal and cingulate cortices of cocaine dependent individuals. Psychiatry Res 140: 271–280. [DOI] [PubMed] [Google Scholar]
- 102. Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF (2007) Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry 48: 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Parkar SR, Ramanathan S, Nair N, Batra SA, Adarkar SA, et al. (2011) Are the effects of cannabis dependence on glucose metabolism similar to schizophrenia? An FDG PET understanding. Indian J Psychiatry 53: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Peters BD, de HL, Vlieger EJ, Majoie CB, den Heeten GJ, et al. (2009) Recent-onset schizophrenia and adolescent cannabis use: MRI evidence for structural hyperconnectivity? Psychopharmacol Bull 42: 75–88. [PubMed] [Google Scholar]
- 105. Rais M, van Haren NE, Cahn W, Schnack HG, Lepage C, et al. (2010) Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur Neuropsychopharmacol 20: 855–865. [DOI] [PubMed] [Google Scholar]
- 106. Roberts GM, Garavan H (2010) Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage 52: 429–435. [DOI] [PubMed] [Google Scholar]
- 107. Safont G, Corripio I, Escarti MJ, Portella MJ, Perez V, et al. (2011) Cannabis use and striatal D2 receptor density in untreated first-episode psychosis: an in vivo SPECT study. Schizophr Res 129: 169–171. [DOI] [PubMed] [Google Scholar]
- 108. Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, et al. (2005) fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend 79: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Solowij N, Yucel M, Respondek C, Whittle S, Lindsay E, et al. (2011) Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychol Med 41: 2349–2359. [DOI] [PubMed] [Google Scholar]
- 110.Takagi M, Lubman DI, Walterfang M, Barton S, Reutens D, et al.. (2011) Corpus callosum size and shape alterations in adolescent inhalant users. Addict Biol Sep 29.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 111. Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, et al. (2007) Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Hum Brain Mapp 28: 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Voytek B, Berman SM, Hassid BD, Simon SL, Mandelkern MA, et al. (2005) Differences in regional brain metabolism associated with marijuana abuse in methamphetamine abusers. Synapse 57: 113–115. [DOI] [PubMed] [Google Scholar]
- 113. Welch KA, McIntosh AM, Job DE, Whalley HC, Moorhead TW, et al. (2011) The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull 37: 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Welch KA, Stanfield AC, McIntosh AM, Whalley HC, Job DE, et al. (2011) Impact of cannabis use on thalamic volume in people at familial high risk of schizophrenia. Br J Psychiatry 199: 386–390. [DOI] [PubMed] [Google Scholar]
- 115. Wobrock T, Sittinger H, Behrendt B, D'Amelio R, Falkai P (2009) Comorbid substance abuse and brain morphology in recent-onset psychosis. Eur Arch Psychiatry Clin Neurosci 259: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yucel M, Zalesky A, Takagi MJ, Bora E, Fornito A, et al. (2010) White-matter abnormalities in adolescents with long-term inhalant and cannabis use: a diffusion magnetic resonance imaging study. J Psychiatry Neurosci 35: 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Barkus E, Morrison PD, Vuletic D, Dickson JC, Ell PJ, et al. (2011) Does intravenous Delta9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol 25: 1462–1468. [DOI] [PubMed] [Google Scholar]
- 118. Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, et al. (2008) Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry 64: 966–973. [DOI] [PubMed] [Google Scholar]
- 119. Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, et al. (2009) Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 34: 759–766. [DOI] [PubMed] [Google Scholar]
- 120. Bossong MG, Jager G, van Hell HH, Zuurman L, Jansma JM, et al. (2012) Effects of Delta9-Tetrahydrocannabinol Administration on Human Encoding and Recall Memory Function: A Pharmacological fMRI Study. J Cogn Neurosci 24: 588–599. [DOI] [PubMed] [Google Scholar]
- 121. Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, et al. (2009) Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry 66: 95–105. [DOI] [PubMed] [Google Scholar]
- 122. Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, et al. (2010) Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol 13: 421–432. [DOI] [PubMed] [Google Scholar]
- 123. Mathew RJ, Wilson WH, Tant SR (1989) Acute changes in cerebral blood flow associated with marijuana smoking. Acta Psychiatr Scand 79: 118–128. [DOI] [PubMed] [Google Scholar]
- 124. Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE (1992) Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab 12: 750–758. [DOI] [PubMed] [Google Scholar]
- 125. Mathew RJ, Wilson WH (1993) Acute changes in cerebral blood flow after smoking marijuana. Life Sci 52: 757–767. [DOI] [PubMed] [Google Scholar]
- 126. Mathew RJ, Wilson WH, Coleman RE, Turkington TG, Degrado TR (1997) Marijuana intoxication and brain activation in marijuana smokers. Life Sci 60: 2075–2089. [DOI] [PubMed] [Google Scholar]
- 127. Mathew RJ, Wilson WH, Turkington TG, Coleman RE (1998) Cerebellar activity and disturbed time sense after THC. Brain Res 797: 183–189. [DOI] [PubMed] [Google Scholar]
- 128. Mathew RJ, Wilson WH, Chiu NY, Turkington TG, Degrado TR, et al. (1999) Regional cerebral blood flow and depersonalization after tetrahydrocannabinol administration. Acta Psychiatr Scand 100: 67–75. [DOI] [PubMed] [Google Scholar]
- 129. Mathew RJ, Wilson WH, Turkington TG, Hawk TC, Coleman RE, et al. (2002) Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Res 116: 173–185. [DOI] [PubMed] [Google Scholar]
- 130. O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, et al. (2002) Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology 26: 802–816. [DOI] [PubMed] [Google Scholar]
- 131. O'Leary DS, Block RI, Turner BM, Koeppel J, Magnotta VA, et al. (2003) Marijuana alters the human cerebellar clock. Neuroreport 14: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 132. O'Leary DS, Block RI, Koeppel JA, Schultz SK, Magnotta VA, et al. (2007) Effects of smoking marijuana on focal attention and brain blood flow. Hum Psychopharmacol 22: 135–148. [DOI] [PubMed] [Google Scholar]
- 133. Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, et al. (2008) Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci 28: 2313–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pillay SS, Rogowska J, Kanayama G, Jon DI, Gruber S, et al. (2004) Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: an fMRI study. Drug Alcohol Depend 76: 261–271. [DOI] [PubMed] [Google Scholar]
- 135. Rabinak CA, Sripada CS, Angstadt M, de WH, Phan KL (2012) Cannabinoid modulation of subgenual anterior cingulate cortex activation during experience of negative affect. J Neural Transm 119: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Stokes PR, Mehta MA, Curran HV, Breen G, Grasby PM (2009) Can recreational doses of THC produce significant dopamine release in the human striatum? Neuroimage 48: 186–190. [DOI] [PubMed] [Google Scholar]
- 137. Stokes PR, Egerton A, Watson B, Reid A, Breen G, et al. (2010) Significant decreases in frontal and temporal [11C]-raclopride binding after THC challenge. Neuroimage 52: 1521–1527. [DOI] [PubMed] [Google Scholar]
- 138. van Hell HH, Bossong MG, Jager G, Kristo G, van Osch MJ, et al. (2011) Evidence for involvement of the insula in the psychotropic effects of THC in humans: a double-blind, randomized pharmacological MRI study. Int J Neuropsychopharmacol 14: 1377–1388. [DOI] [PubMed] [Google Scholar]
- 139. van Hell HH, Jager G, Bossong MG, Brouwer A, Jansma JM, et al. (2012) Involvement of the endocannabinoid system in reward processing in the human brain. Psychopharmacology (Berl) 219: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, et al. (1996) Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res 67: 29–38. [DOI] [PubMed] [Google Scholar]
- 141. Winton-Brown TT, Allen P, Bhattacharyya S, Borgwardt SJ, Fusar-Poli P, et al. (2011) Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: an FMRI study. Neuropsychopharmacology 36: 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wolff V, Lauer V, Rouyer O, Sellal F, Meyer N, et al. (2011) Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke 42: 1778–1780. [DOI] [PubMed] [Google Scholar]
- 143. Block RI, O'Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, et al. (2000) Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport 11: 491–496. [DOI] [PubMed] [Google Scholar]
- 144. Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, et al. (2008) Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage 41: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 145. Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, et al. (2011) Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res 45: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, et al. (2012) Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. Neuroimage 59: 3845–3851. [DOI] [PubMed] [Google Scholar]
- 147. Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D (2011) Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp Clin Psychopharmacol 19: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Matochik JA, Eldreth DA, Cadet JL, Bolla KI (2005) Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend 77: 23–30. [DOI] [PubMed] [Google Scholar]
- 149. Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, et al. (2008) Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry 65: 694–701. [DOI] [PubMed] [Google Scholar]
- 150. DeLisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, et al. (2006) A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gruber SA, Yurgelun-Todd DA (2005) Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res 23: 107–118. [DOI] [PubMed] [Google Scholar]
- 152. Jager G, van Hell HH, De Win MM, Kahn RS, van den Brink W, et al. (2007) Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol 17: 289–297. [DOI] [PubMed] [Google Scholar]
- 153. Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, et al. (2005) Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict 14: 64–72. [DOI] [PubMed] [Google Scholar]
- 154. Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, et al. (2009) Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol 14: 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Medina KL, Nagel BJ, Tapert SF (2010) Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res 182: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. McQueeny T, Padula CB, Price J, Medina KL, Logan P, et al. (2011) Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res 224: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Ponto LL, et al. (2000) Cerebellar hypoactivity in frequent marijuana users. Neuroreport 11: 749–753. [DOI] [PubMed] [Google Scholar]
- 158. Lundqvist T, Jonsson S, Warkentin S (2001) Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol 23: 437–443. [DOI] [PubMed] [Google Scholar]
- 159. Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, et al. (2008) Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 197: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Stokes PR, Egerton A, Watson B, Reid A, Lappin J, et al. (2012) History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol 26: 144–149. [DOI] [PubMed] [Google Scholar]
- 161.Albrecht DS, Skosnik PD, Vollmer JM, Brumbaugh MS, Perry KM, et al.. (2012) Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol Depend Aug 18.[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, et al. (2012) Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry 71: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, et al. (2012) Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry 17: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, et al. (2002) Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav 72: 237–250. [DOI] [PubMed] [Google Scholar]
- 165. Bolla KI, Eldreth DA, Matochik JA, Cadet JL (2005) Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage 26: 480–492. [DOI] [PubMed] [Google Scholar]
- 166. Eldreth DA, Matochik JA, Cadet JL, Bolla KI (2004) Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage 23: 914–920. [DOI] [PubMed] [Google Scholar]
- 167. Vaidya JG, Block RI, O'Leary DS, Ponto LB, Ghoneim MM, et al. (2012) Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology 37: 618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Abdullaev Y, Posner MI, Nunnally R, Dishion TJ (2010) Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav Brain Res 215: 45–57. [DOI] [PubMed] [Google Scholar]
- 169. Chang L, Yakupov R, Cloak C, Ernst T (2006) Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 129: 1096–1112. [DOI] [PubMed] [Google Scholar]
- 170. Gruber SA, Rogowska J, Yurgelun-Todd DA (2009) Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend 105: 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, et al. (2012) Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology 37: 1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hester R, Nestor L, Garavan H (2009) Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology 34: 2450–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Jager G, Kahn RS, van den Brink W, van Ree JM, Ramsey NF (2006) Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) 185: 358–368. [DOI] [PubMed] [Google Scholar]
- 174. King GR, Ernst T, Deng W, Stenger A, Gonzales RM, et al. (2011) Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. J Neurosci 31: 17923–17931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Nestor L, Hester R, Garavan H (2010) Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. Neuroimage 49: 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, et al. (2010) Chronic effects of cannabis use on the human reward system: an fMRI study. Eur Neuropsychopharmacol 20: 153–163. [DOI] [PubMed] [Google Scholar]
- 177. Wesley MJ, Hanlon CA, Porrino LJ (2011) Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res 191: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Bechara A, Tranel D, Damasio H (2000) Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123 ( Pt 11): 2189–2202. [DOI] [PubMed] [Google Scholar]
- 179. Padula CB, Schweinsburg AD, Tapert SF (2007) Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol Addict Behav 21: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, et al. (2008) Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res 163: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, et al. (2010) The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J Psychoactive Drugs 42: 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF (2011) Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction 106: 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, et al. (2007) Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 194: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, et al. (2002) Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 287: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 186. Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, et al. (2001) Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39: 376–389. [DOI] [PubMed] [Google Scholar]
- 187. Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL (2002) Dose-related neurocognitive effects of marijuana use. Neurology 59: 1337–1343. [DOI] [PubMed] [Google Scholar]
- 188.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, et al.. (2012) Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A Aug 27. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Licata SC, Renshaw PF (2010) Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci 1187: 148–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Okuda M, Hasin DS, Olfson M, Khan SS, Nunes EV, et al. (2010) Generalizability of clinical trials for cannabis dependence to community samples. Drug Alcohol Depend 111: 177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Glover GH, Mueller BA, Turner JA, van Erp TG, Liu TT, et al. (2012) Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging 36: 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, et al. (2010) Toward discovery science of human brain function. Proc Natl Acad Sci USA 107: 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Milham MP (2012) Open neuroscience solutions for the connectome-wide association era. Neuron 73: 214–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.