Abstract
Background
Currently, a constant shortage in the supply of platelets has become an important medical and society challenge, especially in developing country, and the in vitro production of megakaryocytic progenitor cells (MPs) from cord blood could represent an effective platelet substitute. In the present study, our objective was to determine the safety and feasibility of ex vivo generated MPs in patients.
Methods and Findings
MPs were produced and characterized from cord blood mononuclear cells under a serum free medium with cytokines. We investigated the feasibility of expansion and infusion of cord blood-derived MPs in 24 patients with advanced hematological malignancyes. The primary end point was the safety and tolerability of the infusion of cord blood-derived MPs. No adverse effects were observed in patients who received ex vivo-generated cells at concentrations of up to a median value of 5.45×106cells/kg of body weight. With one year follow-up, acute and chronic GVHD had not been observed among patients who received MPs infusion, even without ABO blood group and HLA typing matching.
Conclusions
These initial results in patients are very encouraging. They suggest that infusion of cord blood-derived MPs appears safe and feasible for treatment of thrombocytopenia.
Trial Registration
www.chictr.org ChiCTR-TCH-09000333.
Introduction
Thrombocytopenia is a common and potentially fatal complication of chemotherapy and hematopoietic stem cell transplantation. Infusion of platelets from unrelated donors is currently the only effective treatment to prevent fatal hemorrhage. However, owing to their short storage time and increased demand, a constant shortage in the supply of platelets has become an important medical and society challenge. [1] Therefore, investigation of alternative sources of platelets would be beneficial.
Hematopoietic stem cells (HSCs) can be used to generate functional megakaryocytic progenitors (MPs), megakaryocytes, and platelets on a large scale. [2], [3], [4] Functional MPs and platelets have successfully been produced in vitro from CD34+ hematopoietic cells from bone marrow, cord blood, and peripheral blood. [5], [6], [7] Several studies have reported that transplantation of in vitro auto-producing MPs can promote platelet recovery after high-dose therapy and HSC transplantation. [8], [9].
Umbilical cord blood is an abundant source of HSCs. [10], [11] Furthermore, cord blood is highly enriched in committed hematopoietic progenitor cells, including those of the megakaryocytic lineage. In vitro large scale production of MPs from cord blood could represent an effective platelet substitute. In vitro cord blood-derived MPs can be used in many non-emergency conditions, allowing platelets to be used for the emergency conditions. However, there are no reports in the literature that document the safety and efficacy of ex vivo–expanded cord blood-derived MPs in clinical trials.
Following high-dose chemotherapy, neutropenia and thrombocytopenia places patients at an increased risk of developing severe infectious and bleeding complications. The transplantation of ex vivo-generated hematopoietic progenitor cells in addition to chemotherapy represents a possible treatment for high-dose chemotherapy-induced cytopenia. Theoretically, the additional transplantation of ex vivo generated progenitor and post-progenitor cells might lead to the production of sufficient numbers of mature functional cells within a few days after transplantation. The feasibility and efficacy of this approach with regard to neutrophil recovery has previously been demonstrated. [12], [13] However, potential clinical benefits of transplanted cord blood derived MPs has not yet been shown.
We successfully generated MPs from cord blood using a combination of cytokines. Based on promising results of our preclinical study, state food and drug administration (SFDA) of China approved our group to conducts a clinical trial of MP injection to patients with hematologic malignancy. We report the results of our phase 1 study of cord blood-derived MPs in 24 patients with hematological malignancy. We believe this to be the first report of MPs from cord blood in a human clinical trial.
Methods
Trial Protocols
The protocols for this trial are available as supporting information; see Protocol S1 and S2.
Ethics Statement
The study was approved by Research Ethics Committee of Peking University Cancer Hospital & Institute and by the SFDA of China. All patients provided written informed consent in accordance with the principles of the Declaration of Helsinki before enrollment. We obtained informed written consent from the next of kin, parents or guardians on the behalf of the minors/children participants involved in our study.
Patient Inclusion and Exclusion Criteria
Twenty-four consenting patients with advanced hematological malignancy were enrolled in this study. The target size of our study is 20–30 patients. Patients with hematological malignancy were eligible to receive MPs if they met the following criteria: 12–60 years of age; post-chemotherapy; 20≤PLT≤60 (×109/L); no serious damage to liver and kidney function; written informed consent. The exclusion criteria: thrombocytopenia without cancer chemotherapy; patients with primary disease in important organs (liver, kidney), patients accompanied by the immune system disease, serious infections, mental disorders or organ transplant recipients; pregnant, breast-feeding women; patients did not sign informed consent form; infusion contraindication about injection of umbilical cord blood MPs.
Study Design
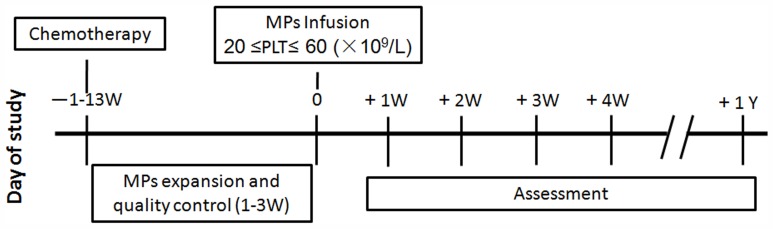
This was a single-center, open-label, phase 1 clinical trial designed to assess the safety profile of infusion of ex vivo-expanded cord blood-derived MPs after chemotherapy. MPs from cord blood were infused when the platelet of patients was 20≤PLT≤60 (109/L) after chemotherapy, and the toxicity was evaluated. The interval time between the cord blood MPs preparation and patient infusion was two hours. Before MP infusion, the patients was given 10 ml 10% calcium gluconate to prevent anaphylaxis. The overall infusion time was within two hours. Platelet counts were assessed at least twice per week. After treatment with cord blood-derived MPs, we conducted one-year follow-up. The study flow chart is depicted in Figure 1. This study was registered with http://www.chictr.org, number ChiCTR-TCH-09000333.
Figure 1. Study flow chart.
In vitro Suspension Culture
After informed consent had been obtained, cord blood from healthy volunteers was collected into sterile heparinized tubes. Mononuclear cells were obtained by centrifugation on Lymphoprep density gradient. Ex vivo cultures were set up with cord blood MNCs at 3×106 cells/mL in StemSpan medium supplemented with 50 ng/mL thrombopoietin (TPO) (Shenyang Sunshine Pharmaceutical Company Limited), 20 ng/mL interleukin-3 (IL-3) (Peprotech), 50 ng/mL stem cell factor (SCF) (Peprotech) and 50 ng/ml IL-6 (Peprotech) using 75 cm2 tissue culture flasks (Corning). At indicated time points, the cultured cells were harvested, washed, and prepared for immediate infusion.
Flow Cytometry Analysis
Ex vivo-generated cells were characterized by flow cytometry on day 0, 7, 10, and 14. All of the conjugated antibodies and the corresponding isotype controls were purchased from BD Pharmingen. The antibodies used were CD3, CD14, CD19, CD41, CD56, and CD61. MPs were collected and washed twice in PBS with 0.1% BSA and stained in accordance with the manufacturer’s suggested concentration of conjugated antibody for 30 min at 4°C. The stained cells were then washed twice in PBS with 0.1% BSA and fixed with the wash buffer supplemented with 1% paraformaldehyde. The samples were then analyzed using a flow cytometer (Becton Dickinson). Cell populations were analyzed with the CellQuest program(Becton Dickinson). Three experiment were performed. We also analyzed the cell size and the co-staining of CD41 on the cultured MPs.
Colony Forming Unit-MK Assay
To demonstrate Colony forming unit-MK colonies, 5×104 in vitro cultured cells were cultured with MegaCult-C media (StemCell Technologies) in the presence of 10 ng/mL recombinant human IL-3 (Peprotech), 20 ng/mL recombinant human IL-6 (Peprotech), and 50 ng/mL recombinant human TPO (Shenyang Sunshine Pharmaceutical Company Limited) according to the instruction manual. After 10–14 days, the cells were fixed, dried, and stained with an anti-CD41 specific antibody or isotype control antibody according to the manufacturer’s instructions. CFU-MK colonies were defined as colonies with at least 3 megakaryocytes. Three experiment were performed.
Megakaryocytes Analysis
For morphological analysis of mature megakaryocytes in the culture, day 14 cultured cells were centrifuged using a cytospin centrifuge. Slides were then stained with Wright-Giemsa. We also analyzed the platelets generation in the day 14 culture by flow cytometry. Culture-derived platelets were enumerated as CD41+ events with the same scatter properties as blood platelets. Ten thousand events were counted per sample. Three experiment were performed.
End Points and Definitions
The primary end point was the safety and tolerability of the infusion of cord blood-derived MPs. Patients were monitored after the infusion of MPs for infusional toxicity and adverse events, which were evaluated according to World Health Organization (WHO) criteria. Secondary end points included platelet recovery after infusion of MPs. The ABO blood group and HLA typing of patients and paired cord blood were carried out at Department of hematology, Peking University People’s Hospital, (Beijing, China).
Results
Patients and MP Characteristics
Twenty-four patients (nine females/fifteen males) were treated between March 2009 and May 2010. The study flow chart is depicted in Figure 1. The median age was 37 years (range, 15–65 years), and the median weight was 68.5 kg (range, 50–92 kg). The patient group included 22 patients with non-Hodgkin’s lymphoma (NHL), one patient with Hodgkin’s lymphoma (HL), and one patient with multiple myeloma (MM). The median number of previous chemotherapy courses were of five (range, 1–17), and the median interval time between the last chemotherapy and MP infusion was 14 days (range, 3–91 days). Baseline characteristics are presented in Table 1.
Table 1. Patient characteristics.
| No | Age | Gender | Diagnosis | Time ofDiagnosis | Chemotherapycourses | LastChemotherapy | Recruitmenttime | Intervaltime | Chemotherapyregimen |
| 1 | 39 | F | NHL IVA | 2008-6-26 | 9 | 2009-4-7 | 2009-4-14 | 8 | GEMOX+ENDOSTAR |
| 2 | 21 | M | NHL IVB | 2008-9-12 | 10 | 2009-5-5 | 2009-5-19 | 14 | DICE+ ENDOSTAR |
| 3 | 29 | M | HL IVB | 1992-6-14 | 10 | 2009-6-1 | 2009-6-26 | 26 | AraC |
| 4 | 56 | M | NHL IVB | 2009-2-16 | 7 | 2009-6-22 | 2009-7-10 | 19 | DICE+ ENDOSTAR |
| 5 | 26 | M | NHL IVB | 2009-5-4 | 3 | 2009-7-8 | 2009-7-21 | 14 | CAT |
| 6 | 62 | F | MM III | 2009-7-9 | 1 | 2009-7-19 | 2009-7-30 | 12 | BTD |
| 7 | 47 | F | NHL IVB | 2009-6-23 | 1 | 2009-7-30 | 2009-8-7 | 9 | R-CHOP |
| 8 | 40 | M | NHL IVB | 2009-8-11 | 3 | 2009-8-11 | 2009-8-21 | 11 | MTX |
| 9 | 30 | M | NHL IVA | 2009-4-17 | 5 | 2009-8-28 | 2009-9-22 | 26 | VALP |
| 10 | 65 | F | NHL IVB | 2004-9-16 | 16 | 2009-9-9 | 2009-10-16 | 37 | FC |
| 11 | 19 | F | NHL IVB | 2009-7-16 | 5 | 2009-10-14 | 2009-10-28 | 15 | DICE+ ENDOSTAR |
| 12 | 35 | M | NHL | 2009-11-4 | 1 | 2009-11-4 | 2009-11-12 | 9 | VDLP |
| 13 | 27 | M | NHL IVB | 2009-10-29 | 1 | 2009-11-6 | 2009-11-20 | 15 | VDLP |
| 14 | 45 | M | NHL IVA | 2008-2-26 | 17 | 2009-11-25 | 2009-12-7 | 14 | AraC+ATO+Tioprinin |
| 15 | 45 | M | NHL IVA | 2008-5-21 | 9 | 2009-12-13 | 2009-12-15 | 3 | GEMOX |
| 16 | 31 | F | NHL IVA | 2007-12-26 | 16 | 2009-12-14 | 2009-12-26 | 13 | VDLP |
| 17 | 57 | F | NHL IVB | 2009-7-1 | 5 | 2009-12-12 | 2010-1-15 | 33 | RFC |
| 18 | 59 | F | NHL IIIB | 1992-4-10 | 17 | 2010-1-14 | 2010-1-26 | 13 | DICE+ ENDOSTAR |
| 19 | 48 | M | NHL IIIB | 2009-11-18 | 3 | 2010-1-25 | 2010-2-9 | 16 | EP |
| 20 | 31 | M | NHL IA | 2009-12-14 | 4 | 2010-3-12 | 2010-3-23 | 12 | MTX |
| 21 | 50 | F | NHL IVA | 2009-8-7 | 6 | 2010-1-15 | 2010-4-16 | 91 | FC |
| 22 | 15 | M | NHL IVA | 2010-1-25 | 1 | 2010-4-10 | 2010-4-21 | 11 | BFM-90 |
| 23 | 32 | M | NHL IVA | 2009-10-12 | 6 | 2010-4-19 | 2010-5-9 | 20 | MTX |
| 24 | 45 | M | NHL IIIB | 2007-2-1 | 9 | 2010-3-31 | 2010-5-24 | 54 | R-COP |
Our preclinical studies had established the method to efficiently inducing and expansing cord blood mononuclear cells (MNCs) to MPs. The in vitro producing MPs were characterized by morphological observation, flow cytometry analysis, and colony formation detection. Cell morphology changed obviously with the extension of culture time (Figure S1A). Flow cytometry analysis showed that the expression of CD41 (a MPs marker, Figure S1E) and CD61 (MPs, Figure S1G) was greater at day 14 than day 0 (7.9 fold and 11.6 fold), while the expression of CD3 (T cells, Figure S1B), CD19 (B cells, Figure S1D), CD56 (NK cells, Figure S1F) was lower at day 14 than day 0 (6.7 fold, 95 fold and 17.5 fold). And the expression of CD14 (monocytes, Figure S1C) was also lower at day 14 than day 0 (1.6 fold). Expansion of CFU-Mk were compared in different culture time. Ex vivo expansion achieved a 64±7 fold increase in CFU-Mk by 14 days culture (Figure S1H). Morphological features of day 14 cultured cells and the presence of polyploid nuclei were confirmed by microscopy (Figure S1I). This results showed that the ex vivo-generated-MPs can mature to megakaryocytes at day 14 culture. Although, the number of mature megakaryocytes were still low. And we also showed that the MKs in culture produced platelets. The platelets in culture were enumerated by flow cytometry as particles having the same scatter properties as blood platelets and expressing CD41 antigen. The number of platelets released remained relatively low with a mean of 5.5±0.9% of the total expanded population on day 14. Moreover, we analyzed the cell size and co-staining of CD41. And the results of a representative experiment was shown in Figure S2.
Twenty-four umbilical cord blood units identified for MP manufacture contained a median of 8.55×107 MNCs (range, 1.9–29 cells) and a median of 76 mL (range 47–106 mL). The results of ex vivo expansion culture are summarized in Table 2. The median expansion was 73.5-fold (range, 3-365-fold). After expansion culture, the median proportion of CD41+_cells was 35% (range, 8%–77%). The dose of infused MPs for individual patients is summarized in Table 2.
Table 2. Ex vivo expansion: Summary of product characteristics before and after expansion.
| NO. | Before expansion | After expansion | Composition of Transplanted Grafts | |||||||||
| Volume of CB (ml) | MNC×107 | % CD41+ | Expansion time (day) | MNC×107 | % CD41+ | AbsoluteCD41+(×106) | Cell Viability | Fold expansion | Body Weight | MNC per kg b.w. (×106) | CD41+ cells per kg b.w. (×106) | |
| 1 | 78 | 4.5 | 6 | 11 | 58.5 | 52 | 304 | 91.4% | 113 | 70 | 8.4 | 4.3 |
| 2 | 54 | 8 | 11 | 5 | 48 | 26 | 125 | 94.0% | 14 | 61 | 7.9 | 2.0 |
| 3 | 69 | 24 | 4 | 7 | 264 | 33 | 871 | 98.7% | 91 | 67 | 39.4 | 13.0 |
| 4 | 47 | 4.6 | 8 | 6 | 611 | 22 | 1344 | 98.0% | 365 | 57 | 107.2 | 23.6 |
| 5 | 93 | 8.1 | 3 | 8 | 72.9 | 27 | 197 | 98.9% | 81 | 92 | 7.9 | 2.1 |
| 6 | 82 | 16 | 8 | 7 | 128 | 22 | 282 | 99.0% | 22 | 54 | 23.7 | 5.2 |
| 7 | 106 | 18 | 6 | 4 | 54 | 13 | 70 | 99.9% | 6 | 60 | 9.0 | 1.2 |
| 8 | 52 | 4.6 | 9 | 17 | 87.4 | 64 | 559 | 97.1% | 135 | 81 | 10.8 | 6.9 |
| 9 | 77 | 1.9 | 13 | 20 | 32.3 | 55 | 178 | 92.2% | 7 | 67 | 4.8 | 2.7 |
| 10 | 62 | 6 | 6 | 4 | 36 | 9 | 32 | 99.0% | 9 | 57 | 6.3 | 0.6 |
| 11 | 70 | 5 | 12 | 12 | 130 | 72 | 936 | 87.8% | 156 | 79 | 16.5 | 11.8 |
| 12 | 77 | 7 | 5 | 14 | 119 | 67 | 797 | 93.0% | 228 | 67 | 17.8 | 11.9 |
| 13 | 72 | 10 | 6 | 4 | 20 | 8 | 16 | 95.0% | 3 | 63 | 3.2 | 0.3 |
| 14 | 78 | 9 | 9 | 12 | 198 | 77 | 1525 | 91.0% | 188 | 70 | 28.3 | 21.8 |
| 15 | 86 | 10 | 8 | 14 | 310 | 54 | 1674 | 84.4% | 209 | 86 | 36.0 | 19.5 |
| 16 | 99 | 15 | 6 | 7 | 120 | 23 | 276 | 93.7% | 31 | 82 | 14.6 | 3.4 |
| 17 | 56 | 11 | 5 | 4 | 44 | 13 | 57 | 98.0% | 10 | 78 | 5.6 | 0.7 |
| 18 | 93 | 9 | 5 | 8 | 135 | 37 | 500 | 84.0% | 111 | 80 | 16.9 | 6.2 |
| 19 | 89 | 12 | 8 | 13 | 192 | 58 | 1114 | 95.1% | 116 | 66 | 29.1 | 16.9 |
| 20 | 70 | 20 | 5 | 13 | 380 | 63 | 2934 | 92.0% | 293 | 80 | 47.5 | 29.9 |
| 21 | 72 | 6 | 9 | 22 | 96 | 32 | 307 | 94.2% | 57 | 50 | 19.2 | 6.1 |
| 22 | 102 | 29 | 6 | 6 | 132 | 18 | 238 | 93.9% | 14 | 88 | 15.0 | 2.7 |
| 23 | 65 | 6.6 | 11 | 16 | 85 | 52 | 442 | 92.0% | 61 | 78 | 10.9 | 5.7 |
| 24 | 75 | 5.1 | 8 | 11 | 56 | 48 | 269 | 90.2% | 66 | 61 | 9.2 | 4.4 |
| Median(range) | 76(47–106) | 8.55(1.9–29) | 7(3–13) | 9.5(4–22) | 107.5(20–611) | 35(8–77) | 305.5(16–2934) | 93.95%(84–99.90%) | 73.5(3–365) | 68.5(50–92) | 14.8(3.2–107.2) | 5.45(0.3–29.9) |
CB, cord blood; MNC: mononuclear cells.
MP Infusion Toxicity Profile
The infusion toxicity profile is summarized in Table 3. Severe adverse events associated with infusion of cultured cord blood-derived MPs were not observed. Two patients experienced fever (<38°C), four patients exhibited increased transaminases, one patient experienced increased jaundice (jaundice was present prior to infusion of MPs), and one patient experienced vomiting. All conditions were resolved with standard clinical management. With regard to four patients with elevated transaminases, we recorded the transaminase changes before and after the MP infusion. The results showed that all four patients experienced increased transaminitis prior to infusion of MPs. (Figure S3) In the present clinical trial, we did not carry out the ABO blood group and HLA matching. However, after one year follow-up acute and chronic GVHD had not been observed among patients who received MPs infusion. The ABO blood group and HLA typing data of patients and paired cord blood were shown in Table S1.
Table 3. Adverse reaction in MP-infusion patients.
| Adverse events | No | Patient Number |
| Fever | 2 | 10, 15 |
| Anaphylaxis | 0 | NA |
| Hemolysis | 0 | NA |
| Shock | 0 | NA |
| Embolism | 0 | NA |
| Hepatolienomegaly | 0 | NA |
| Abnormal electrocardiogram | 0 | NA |
| Transaminitis | 4* | 1, 2, 7, 20 |
| Abnormal renal function | 0 | NA |
| Jaundice | 1† | 7 |
| Vomiting | 1 | 20 |
| Diarrhea | 0 | NA |
| Erythra | 0 | NA |
| GVHD | 0 | NA |
All four patients exhibited increased transaminitis before infusion of MPs.
Jaundice existed before infusion of MPs.
Clinical Reactions
The median dose of infused MPs was 14.8×106 MNCs/kg body weight (range, 3.2×106−107.2×106), and 5.45×106 CD41+ cells/kg body weight (range 0.3×106−29.9×106). Platelet recovery occurred in 17 patients with a median platelet recovery >60×109/L on day 3 (median, 1 to 16 days) and in 13 patients with a median platelet recovery >100×109/L on day 6 (median, 3 to 14 days). However, 5 patients received transfusion of one unit of platelets after infusion of MPs. In the absence of platelet transfusion, platelet recovery was prompt in 12 patients with a median platelet recovery >60×109/L on day 3 (median, 1 to 11 days), and 11 patients with a median platelet recovery >100×109/L on day 6 (median, 3 to 14 days). The summary of platelet recovery after infusion of MPs is shown in Table 4. However, it was difficult to determine whether there is any benefit to the administration of MPs after chemotherapy without appropriate controls. And definitive results will require the completion of a randomized phase 2 clinical trial.
Table 4. Platelet recovery parameters after infusion of ex vivo-generated cells.
| Patient No | PLT Day 0×109/L | Platelets (PLT) | Number of PLT transfusions | ||
| Days with PLT | Days to PLT | ||||
| Less than 20×109/L | More than 60×109/L | More than 100×109/L | |||
| 1 | 52 | 13 | NA | NA | 0 |
| 2 | 57 | NA | 4 | 8 | 0 |
| 3 | 46 | NA | 2 | 5 | 1 |
| 4 | 49 | 3 | 2 | 6 | 0 |
| 5 | 45 | 4 | NA | NA | 0 |
| 6 | 37 | NA | 3 | 5 | 0 |
| 7 | 4 | 0 | 10 | NA | 1 |
| 8 | 49 | NA | 10 | NA | 0 |
| 9 | 53 | NA | 2 | 7 | 0 |
| 10 | 56 | NA | 3 | NA | 0 |
| 11 | 56 | NA | 1 | 3 | 0 |
| 12 | 53 | NA | 2 | 5 | 0 |
| 13 | 51 | NA | 11 | 14 | 0 |
| 14 | 24 | NA | 7 | 14 | 0 |
| 15 | 24 | 2 | NA | NA | 1 |
| 16 | 63 | 4 | NA | NA | 1 |
| 17 | 41 | NA | 16 | NA | 0 |
| 18 | 23 | NA | NA | NA | 1 |
| 19 | 30 | NA | 2 | 6 | 0 |
| 20 | 46 | NA | 3 | 5 | 0 |
| 21 | 25 | NA | NA | NA | 0 |
| 22 | 35 | NA | 10 | 12 | 0 |
| 23 | 44 | NA | 4 | 6 | 0 |
| 24 | 47 | NA | NA | NA | 0 |
NA, not applicable.
Discussion
To our knowledge, this is the first report of the use of ex vivo–expanded cord blood-derived MPs in humans. We investigated the feasibility of in vitro expansion and transplantation of cord blood-derived MPs in 24 patients with advanced hematological malignancy. Administration of ex vivo-generated cell numbers up to a median of 5.45×106/kg body weight was performed, with no adverse effects. Administration of cord blood-derived MPs appears to be safe and feasible for treatment of thrombocytopenia after chemotherapy.
The additional transplantation of ex vivo-generated cell populations containing up to a median of 14.8×106 MNCs/kg (range, 3.2×106−107.2×106), and a median of 5.45×106 CD41+ cells/kg (range, 0.3×106−29.9×106), is comparable to the number of additionally transplanted megakaryocytic in previous studies. [8], [9] Our present study showed that there were no significant detriment to the administration of dose escalated MP. Now, many studies try to improve the efficiency of MP differentiation and expansion from stem cells. [14], [15], [16] The most recent studies also show that platelets can be efficiently produced from pluripotent stem cell in vitro. [17]–[20] All of these studies indicate promise for in vitro-produced platelet substitutes from stem cells.
Our study hadn’t showed definitive efficacy of MP infusion on thrombocytopenia after chemotherapy. Other similar clinical studies have used autologous MPs or megakaryocyte infusion; however, these studies also lacked a control group, and thus cannot demonstrate the exact effect of infusion of ex-vivo-expanded studies. [2], [3], [8], [9] Platelet recovery after chemotherapy can be influenced by patient condition, different chemotherapy regimens, and dosage and opportunity of infusion. Therefore, the efficacy of infusion of cord blood-derived MPs needs to be verified by larger phase 2 clinical trials.
There are limits in our present study. The efficiency of in vitro expansion should be improved, and platelet recovery effects need to be further explored by phase 2 study. In our study, patients had advanced hematological malignancy, and repeated chemotherapy can result in damage to the hematopoietic microenvironment, possibly influencing the effects of MP infusion.
In conclusion, this study demonstrates the safety of ex vivo-expanded MPs from CB infused after chemotherapy in patients with hematological malignancy. Our study also demonstrated the feasibility of manufacturing 3×108 MPs from cord blood units to provide a cell dosage of 3.75×106 cells/kg for an 80-kg recipient. However, definitive efficacy will require the completion of a randomized phase 2 clinical trial.
Supporting Information
Characterization of megakaryocytic progenitors from cord blood mononuclear cells. (A) Cell morphology of different culture time by microscope observation. Scale bars: 100 µm. Surface marker expression of CD3 (B), CD14 (C), CD19 (D), CD41 (E), CD56 (F), and CD61 (G) in expanded CB population during 14 days of culture. (H) CFU-Mk expansions after 14 days of culture. Data are shown as mean±SD from three experiments. (I) Typical morphology of mature megakaryocytes on day 14 culture was shown. Scale bars: 50 µm.
(TIF)
FACS plots showed the cell size and co-staining of CD41+ cells at day 14 culture. (A) and (B) showed the results of isotype IgG. (C) and (D) showed the results of CD41 expression.
(TIF)
Transaminase changes of four patients before and after MPs infusion. (A) Data of patient 1. The chemotherapy time is 2009/4/7, and the MPs infusion time is 2009/4/14. (B) Data of patient 2. The chemotherapy time is 2009/5/5, and the MPs infusion time is 2009/5/19. (C) Data of patient 7. The chemotherapy time is 2009/7/30, and the MPs infusion time is 2009/8/7. (D) Data of patient 20. The chemotherapy time is 2010/3/12, and the MPs infusion time is 2010/3/23. All the four patients had different degrees of elevated transaminases before MP treatment. Dashed lines shows the normal value of ALT and AST. ALT: alanine transarninase, AST: aspartate aminotransferase.
(TIF)
ABO blood group and HLA typing data of patients and paired cord blood.
(DOC)
(DOC)
(DOC)
Funding Statement
This work was supported by the National High Technology Research and Development Program of China (Grant Nos: 2011AA020109 and 2011AA020105), the Major State Basic Research Program of China (Grant No: 2011CB964804). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Reems J-A, Pineault N, Sun S (2010) In Vitro Megakaryocyte Production and Platelet Biogenesis: State of the Art Transfusion Medicine Reviews. 24: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Decaudin D, Vantelon JM, Bourhis JH, Farace F, Bonnet ML, et al. (2004) Ex vivo expansion of megakaryocyte precursor cells in autologous stem cell transplantation for relapsed malignant lymphoma Bone Marrow Transplant. 34: 1089–1093. [DOI] [PubMed] [Google Scholar]
- 3. Chen TW, Hwang SM, Chu IM, Hsu SC, Hsieh TB, et al. (2009) Characterization and transplantation of induced megakaryocytes from hematopoietic stem cells for rapid platelet recovery by a two-step serum-free procedure Exp Hematol. 37: 1330–1339. [DOI] [PubMed] [Google Scholar]
- 4. Matsunaga T, Tanaka I, Kobune M, Kawano Y, Tanaka M, et al. (2006) Ex Vivo Large-Scale Generation of Human Platelets from Cord Blood CD34+ Cells STEM CELLS. 24: 2877–2887. [DOI] [PubMed] [Google Scholar]
- 5. Kishore V, Eliason JF, Matthew HW (2011) Covalently immobilized glycosaminoglycans enhance megakaryocyte progenitor expansion and platelet release J Biomed Mater Res A. 96: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noroozi Aghideh A, Kheirandish M, Abolghasemi H, Gharehbaghian A (2010) Platelet growth factors suppress ex vivo expansion and enhance differentiation of umbilical cord blood CD133+ stem cells to megakaryocyte progenitor cells Growth Factors. 28: 409–416. [DOI] [PubMed] [Google Scholar]
- 7. Bruyn CD, Delforge A, Martiat P, Bron D (2005) Ex Vivo Expansion of Megakaryocyte Progenitor Cells: Cord Blood Versus Mobilized Peripheral Blood Stem Cells and Development. 14: 415–424. [DOI] [PubMed] [Google Scholar]
- 8. Bertolini F, Battaglia M, Pedrazzoli P, Antonio Da Prada G, Lanza A, et al. (1997) Megakaryocytic Progenitors Can Be Generated Ex Vivo and Safely Administered to Autologous Peripheral Blood Progenitor Cell Transplant Recipients Blood. 89: 2679–2688. [PubMed] [Google Scholar]
- 9. Scheding S, Bergmannn M, Rathke G, Vogel W, Brugger W, et al. (2004) Additional transplantation of ex vivo generated megakaryocytic cells after high-dose chemotherapy Haematologica. 89: 630–631. [PubMed] [Google Scholar]
- 10. Barker JN, Wagner JE (2002) Umbilical cord blood transplantation: current state of the art Curr Opin Oncol. 14: 160–164. [DOI] [PubMed] [Google Scholar]
- 11. Barker JN, Wagner JE (2003) Umbilical-cord blood transplantation for the treatment of cancer Nat Rev Cancer. 3: 526–532. [DOI] [PubMed] [Google Scholar]
- 12. Reiffers J, Cailliot C, Dazey B, Attal M, Caraux J, et al. (1999) Abrogation of post-myeloablative chemotherapy neutropenia by ex-vivo expanded autologous CD34-positive cells The Lancet. 354: 1092–1093. [DOI] [PubMed] [Google Scholar]
- 13. McNiece I, Jones R, Bearman SI, Cagnoni P, Nieto Y, et al. (2000) Ex vivo expanded peripheral blood progenitor cells provide rapid neutrophil recovery after high-dose chemotherapy in patients with breast cancer Blood. 96: 3001–3007. [PubMed] [Google Scholar]
- 14. Pineault N, Cortin V, Boyer L, Garnier A, Robert A, et al. (2011) Individual and synergistic cytokine effects controlling the expansion of cord blood CD34+ cells and megakaryocyte progenitors in culture Cytotherapy. 13: 467–480. [DOI] [PubMed] [Google Scholar]
- 15. Mercher T, Cornejo MG, Sears C, Kindler T, Moore SA, et al. (2008) Notch Signaling Specifies Megakaryocyte Development from Hematopoietic Stem Cells Cell Stem Cell. 3: 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poirault-Chassac S, Six E, Catelain C, Lavergne M, Villeval J-L, et al. (2010) Notch/Delta4 signaling inhibits human megakaryocytic terminal differentiation Blood. 116: 5670–5678. [DOI] [PubMed] [Google Scholar]
- 17. Takayama N, Nishimura S, Nakamura S, Shimizu T, Ohnishi R, et al. (2010) Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells The Journal of Experimental Medicine. 207: 2817–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu S-J, Li F, Yin H, Feng Q, Kimbrel EA, et al. (2011) Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice Cell Res. 21: 530–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gekas C, Graf T (2010) Induced pluripotent stem cell-derived human platelets: one step closer to the clinic The Journal of Experimental Medicine. 207: 2781–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takayama N, Nishikii H, Usui J, Tsukui H, Sawaguchi A, et al. (2008) Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors Blood. 111: 5298–5306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of megakaryocytic progenitors from cord blood mononuclear cells. (A) Cell morphology of different culture time by microscope observation. Scale bars: 100 µm. Surface marker expression of CD3 (B), CD14 (C), CD19 (D), CD41 (E), CD56 (F), and CD61 (G) in expanded CB population during 14 days of culture. (H) CFU-Mk expansions after 14 days of culture. Data are shown as mean±SD from three experiments. (I) Typical morphology of mature megakaryocytes on day 14 culture was shown. Scale bars: 50 µm.
(TIF)
FACS plots showed the cell size and co-staining of CD41+ cells at day 14 culture. (A) and (B) showed the results of isotype IgG. (C) and (D) showed the results of CD41 expression.
(TIF)
Transaminase changes of four patients before and after MPs infusion. (A) Data of patient 1. The chemotherapy time is 2009/4/7, and the MPs infusion time is 2009/4/14. (B) Data of patient 2. The chemotherapy time is 2009/5/5, and the MPs infusion time is 2009/5/19. (C) Data of patient 7. The chemotherapy time is 2009/7/30, and the MPs infusion time is 2009/8/7. (D) Data of patient 20. The chemotherapy time is 2010/3/12, and the MPs infusion time is 2010/3/23. All the four patients had different degrees of elevated transaminases before MP treatment. Dashed lines shows the normal value of ALT and AST. ALT: alanine transarninase, AST: aspartate aminotransferase.
(TIF)
ABO blood group and HLA typing data of patients and paired cord blood.
(DOC)
(DOC)
(DOC)