Abstract
Japanese encephalitis virus (JEV), a mosquito-borne zoonotic pathogen, is one of the major causes of viral encephalitis. To reduce the impact of Japanese encephalitis among children in the Republic of Korea (ROK), the government established a mandatory vaccination program in 1967. Through the efforts of this program only 0–7 (mean 2.1) cases of Japanese encephalitis were reported annually in the ROK during the period of 1984–2009. However, in 2010 there was an outbreak of 26 confirmed cases of Japanese encephalitis, including 7 deaths. This represented a >12-fold increase in the number of confirmed cases of Japanese encephalitis in the ROK as compared to the mean number reported over the last 26 years and a 3.7-fold increase over the highest annual number of cases during this same period (7 cases). Surveillance of adult mosquitoes was conducted during the 2010 outbreak of Japanese encephalitis in the ROK. A total of 6,328 culicine mosquitoes belonging to 12 species from 5 genera were collected at 6 survey sites from June through October 2010 and assayed by reverse-transcription polymerase chain reaction (RT-PCR) for the presence of JEV. A total of 34/371 pooled samples tested positive for JEV (29/121 Culex tritaeniorhynchus, 4/64 Cx. pipiens, and 1/26 Cx. bitaeniorhynchus) as confirmed by sequencing of the pre-membrane and envelope protein coding genes. The maximum likelihood estimates of JEV positive individuals per 1,000 culicine vectors for Cx. tritaeniorhynchus, Cx. pipiens, and Cx. bitaeniorhynchus were 11.8, 5.6, and 2.8, respectively. Sequences of the JEV pre-membrane and envelope protein coding genes amplified from the culicine mosquitoes by RT-PCR were compared with those of JEV genotypes I-V. Phylogenetic analyses support the detection of a single genotype (I) among samples collected from the ROK in 2010.
Introduction
Japanese encephalitis virus (JEV), the prototype member of the JEV serocomplex within the genus Flavivirus, family Flaviviridae, is a single stranded positive sense RNA virus. The genome of JEV is approximately 11,000 base pairs (bp) in length and contains of 3 structural proteins (capsid, membrane, and envelope proteins) and 7 nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5) [1]–[3]. JEV is one of the major causes of viral encephalitis worldwide and the most significant arthropod-borne viral encephalitis causing agent in east and southeast Asia [4]. An estimated three billion persons live in JEV-endemic countries [5], and the annual incidence of Japanese encephalitis (JE) is 30,000–50,000 cases [6]. The global economic and human health impacts of JE are impressive with 10,000–15,000 deaths attributed to this disease annually and an estimated 709,000 disability-adjusted life years reported for 2002 [6], [7].
JEV is transmitted principally by rice paddy-breeding Culex mosquitoes in an enzootic cycle involving an avian reservoir and porcine (domestic and feral) amplifying hosts. Humans and other non-avian vertebrates (e.g., horses) are only infected with JEV incidentally and are considered “dead-end hosts” because they usually fail to produce viremia of sufficient titer to infect mosquitoes. The prototype JEV strain was isolated in Japan in 1935 [8], and the virus has since been found throughout east and southeast Asia, with the geographical borders of viral activity extending north to maritime Siberia [9], west to Pakistan [10], southeast to Australia [11], and northeast to Japan and the Korean Peninsula [12]. JEV strains are divided into five genotypes distributed throughout this geographical range. China is a highly epidemic area of JE activity [13] and more than 100 JEV strains belonging to genotypes I, III, and V have been isolated from different hosts in this country since the 1950s [2]. JEV genotype III was predominant in China prior to 2001; however, since the first detection of a genotype I virus in this country (isolated in 1979), the detection of this genotype has become increasingly common [14]. JEV genotype I viruses have recently been isolated from mosquitoes, swine, and humans in China and suggest that this virus type may become the predominant genotype in this country [1]–[3], [14], [15]. JEV genotype III strains were most common in the nearby Republic of Korea (ROK) prior to 1993. Genotypes I and III were both detected in ROK in 1994 and since then, only genotype I has been isolated [12].
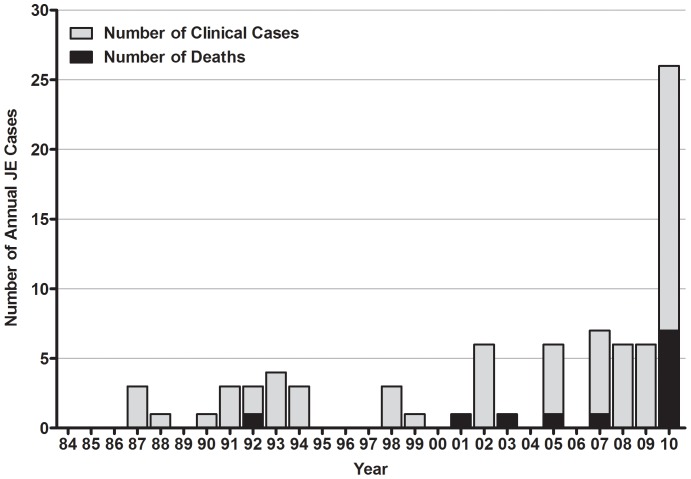
In order to reduce the impact of JE among children in ROK, the government established a mandatory JEV vaccination program in 1967 that was expanded annually until all school-age children were included by 1971. Live attenuated JEV vaccine (SA14-14-2 strain) developed in China, as well as inactivated vaccine produced by formalin treatment of Nakayama strain virus cultivated in specific pathogen-free mouse brain, have been used in this vaccination program [16], [17]. Through the efforts of the vaccination program in ROK, only 0–7 cases (mean 2.1) of JE were reported annually during the period of 1984–2009 [18]. However, during 2010, there was an outbreak of 26 confirmed cases of JE including 7 deaths. This represented a >12-fold increase in the number of confirmed cases of Japanese encephalitis in ROK as compared to the mean reported over the last 26 years (55 cases including 5 deaths during 1984–2009) and a 3.7-fold increase over the highest annual number of cases (7 cases) [19] (Fig. 1).
Figure 1. Clinical cases and deaths of Japanese encephalitis in Republic of Korea, 1984–2010.
Figure is based on data provided by the Korea Center for Disease Control and Prevention, 2011.
Monitoring for the presence of JEV in mosquitoes can be used to estimate levels of potential JEV exposure, intensity of viral activity, and genetic variation of JEV throughout surveyed areas. Although the prevalence of JEV in mosquitoes has been previously reported in ROK, sampling efforts have been focused at or near United States (US) military installations and training sites [20], [21]. Thus, information on the nationwide prevalence of JEV in mosquitoes in ROK is limited [22]. The objectives of this study were to identify JEV genotypes circulating in ROK during the outbreak of JE in 2010, investigate the genetic variation and relative prevalence of virus strains, and identify mosquito species potentially involved in the transmission of JEV.
Materials and Methods
Survey Area and Mosquito Collection
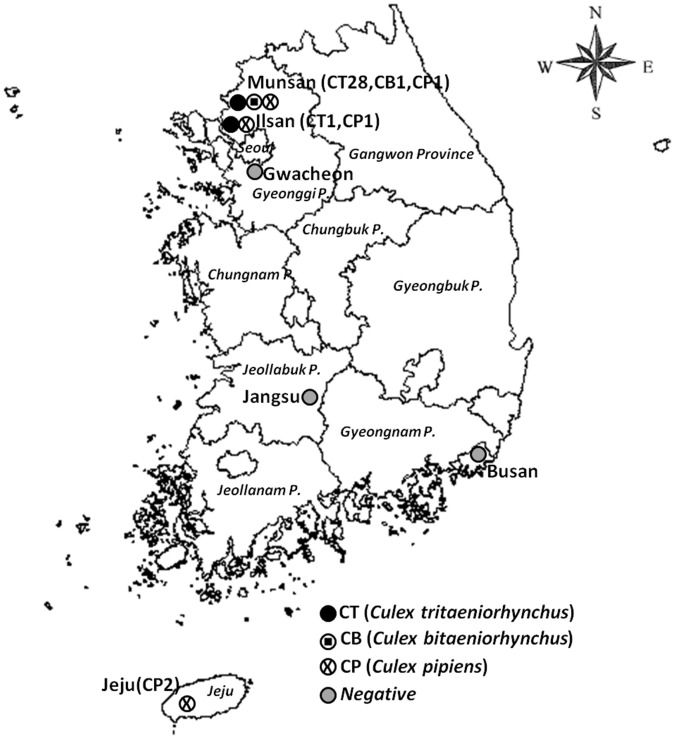
As part of a national vector surveillance program for arboviral infectious diseases, adult mosquitoes were collected using a Mosquito Magnet (Pro-Model, American Biophysics Corp., Greenwich, RI, USA) at selected sites throughout ROK from April through October 2010. The Foreign Animal Disease Division, Animal, Plant, and Fisheries Quarantine and Inspection Agency (QIA, Anyang, ROK), and the 5th Medical Detachment, 168th Multifunctional Medical Battalion, 65th Medical Brigade, collected mosquitoes biweekly at 6 survey sites in ROK: Munsan, Ilsan, Gwacheon, Jangsu, Busan, and Jeju Island (Fig. 2). The Ilsan, Gwacheon, Jangsu, Busan, and Jeju collection sites were at horse farms, whereas the Munsan collection site was at Warrior Base, a US military training site surrounded by rice paddies near the demilitarized zone (DMZ). All necessary permits were obtained for the described field studies from QIA, ROK Racing Authority, ROK Army, and the US Army. Up to 30 specimens of culicine mosquitoes were pooled in a 2 mL cryovials (Nalge Nunc International, NY, USA) according to species and date of collection, packaged with dry ice, and sent to the QIA where they were assayed for JEV.
Figure 2. Collection sites and of Japanese encephalitis virus-positive pools, Republic of Korea, 2010.
Abbreviations in parentheses indicate the number of Japanese encephalitis virus-positive pools by mosquito species.
Reverse-Transcription Polymerase Chain Reaction, Genetic Sequencing, Assessment of Nucleotide Sequence Similarity, and Phylogenetic Analyses
Mosquito samples were homogenized in the laboratory and clarified by centrifugation. Total RNA was extracted from mosquito homogenate using a BioRobot M48 workstation apparatus (Qiagen, GmBH, Hilden, Germany) with a MagAttract Virus Mini M48 kit, (Qiagen). Nucleic acids were eluted in 50 µL of buffer and stored at −70°C.
RNA was assayed by reverse transcriptase polymerase chain reaction (RT-PCR) to detect JEV by targeting pre-membrane protein (prM) and envelope protein (E) coding genes using the Maxime™ RT PreMix (Intron Biotechnology, Seoungnam-si, Gyeonggi-do, ROK). The polymerase chain reaction (PCR) contained 2 µL of prepared cDNA and 10 pmol of each primer (JEE F/JEE R and JEV-prMF/JEV-prMR for reactions targeting the coding regions of the E and prM proteins, respectively) [14], [23] in Maxime PCR PreMix (Intron Biotechnology). Amplified products were visualized by electrophoresis on a 1.2% agarose gel stained with ethidium bromide (0.5 µg/mL) using 1x TAE migration buffer (pH 8.0; 40 mmol/L Tris-acetate, 1 mmol/L EDTA). Target JEV prM and E products were 674 and 1,541 bp, respectively.
The number of JEV positive mosquitoes per 1,000 individuals was estimated from assay results using maximum likelihood estimation. Maximum likelihood estimation takes into account the number of pooled samples, number of positive pooled samples, and variation in pooled sample size thereby relaxing the assumption of the minimum field infection rate that only one infected mosquito exists in a positive pooled sample. Maximum likelihood estimation may therefore be a more accurate measure of infection rate [24]–[25]. Maximum likelihood estimates (MLE) were calculated using PooledInfRate software [26].
Nineteen RT-PCR positive products were selected for cloning and sequencing based on the locality and time of their collection. PCR positive products were purified using a QIA Quick Purification Kit (Qiagen) and cloned into the pGEM-T Easy Vector System I (Promega, Madison, WI, USA). The plasmid clones were purified with a QIAprep Spin Miniprep Kit (Qiagen), and verified by digesting the plasmid DNA with EcoRI (New England Biolabs, UK) and separating it in a 1.2% agarose gel. Products were sequenced by Macrogen (Seoul, ROK). Sequences were deposited in GenBank under the accession numbers JX018147-JX018168 and JX018131-JX018146.
Similarity among genetic sequences for JEV prM and E proteins analyzed as part of this study and those publically available on the GenBank database was assessed using the National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) BLAST network service. Sequences were aligned using the Clustal W method in MegAlign version 7.1 (DNA-STAR, Madison, WI, USA) and compared to published sequences for JEV strains collected from human, porcine, mosquito, and unreported sources collected at locations in Asia and Oceania as available from the GenBank database using phylogenetic analyses. The geographical origin, source, year of detection/isolation, strain name, and GenBank accession numbers for sequences used in phylogenetic analyses are reported in Tables 1 and 2. Phylogenetic trees were generated using neighbor-joining algorithms and the Jukes and Cantor matrix. Support for topology was calculated using 1,000 bootstrap replications.
Table 1. Strains of Japanese encephalitis virus reported on GenBank and used in phylogenetic analysis of pre-membrane protein coding genes.
| Genotype | Strain | Source of Virus | Geographical Origin | Collection Date | Accession No. |
| 1 | JX61 | Pig Serum | China | 2008 | GU556217 |
| 1 | JX66 | Pig | China | 2008 | FJ179364 |
| 1 | SX09S-01 | Swine brain | China | 2009 | HQ893545 |
| 1 | SH17M-07 | NAa | China | 2007 | EU429297 |
| 1 | K01-GN | NAa | Republic of Korea | 2005b | AY965852 |
| 1 | K01-JB | NAa | Republic of Korea | 2005b | AY965850 |
| 1 | K01-JN | NAa | Republic of Korea | 2005b | AY965851 |
| 1 | K94P05 | NAa | Republic of Korea | 1999b | AF045551 |
| 1 | KV1899 | NAa | Republic of Korea | 2003b | AY316157 |
| 1 | 4790-85 | Homo sapiens | Thailand | 2009b | GQ902062 |
| 1 | JEV-eq-Tottori | Horse cerebrum | Japan | 2003 | AB594829 |
| 1 | JEV-sw-Mie-40 | Swine serum | Japan | 2004 | AB241118 |
| 2 | Bennett | Homo sapiens | Republic of Korea | 1951 | HQ223285 |
| 2 | B-1381-85 | Pig | Thailand | 2009b | GQ902061 |
| 2 | FU | Human serum | Australia | 199 9b | AF217620 |
| 2 | JKT654 | Mosquito | Indonesia | 1978 | HQ223287 |
| 3 | 47 | Cerebrospinal fluid | China | 1950 | JF706269 |
| 3 | Beijing-1 | Human brain | China | 1949 | JEVBEICG |
| 3 | CTS | Human brain | China | 2003b | AY243814 |
| 3 | G35 | Mosquito | China | 2003b | AY243815 |
| 3 | GB30 | Murina aurata | China | 1997 | FJ185037 |
| 3 | JEV-NJ1 | Culex | China | 2009 | HM234674 |
| 3 | LYZ | Human brain | China | 2003b | AY243818 |
| 3 | P3 | Human brain | China | 2003b | AY243844 |
| 3 | SA14-12-1-7 | NAa | China | 2001b | AF416457 |
| 3 | SA14-14-2* | SA-14 derivate | China | 1953 | AF315119 |
| 3 | YUNNAN0902 | Sus scrofa | China | 2009 | JQ086763 |
| 3 | CH2195 | Naa | Taiwan | 1994 | AF030550 |
| 3 | CJN-L1 | NAa | Taiwan | 2003b | AY303794 |
| 3 | HVI | Aedes albopictus | Taiwan | 1998b | AF098735 |
| 3 | T1P1-L4 | NAa | Taiwan | 2003b | AY303792 |
| 3 | Indonesia | NAa | Indonesia | 1993b | JEU03692 |
| 3 | Nakayama* | Human brain | Japan | 1395 | JEU03694 |
| 3 | JaOH0566 | NAa | Japan | 1997 | AY508813 |
| 3 | CNU-LP2x | NAa | Republic of Korea | 2009b | GQ199609 |
| 4 | JKT6468 | Mosquito | Indonesia | 1968 | AY184212 |
| 5 | XZ0934 | NAa | China | 2009c | JF915894 |
Not available
Submitted date
Published date.
Vaccine strains that have been used in the Republic of Korea.
Table 2. Strains of Japanese encephalitis virus reported on GenBank and used in phylogenetic analysis of envelope protein coding genes.
| Genotype | Strain | Source of Virus | Geographical Origin | Collection Date | Accession No. |
| 1 | SC04-15 | Culex tritaeniorhynchus | China | 2006b | DQ404091 |
| 1 | LX10P-09 | Cerebrospinal fluid | China | 2009 | HM204528 |
| 1 | LY5P-09 | Cerebrospinal fluid | China | 2009 | HM204530 |
| 1 | XP174M0-08 | Culex tritaeniorhynchus | China | 2008 | HM204527 |
| 1 | B2239 | Pig | Thailand | 1984 | JEU70391 |
| 1 | P19Br | Human | Thailand | 1982 | JEU70416 |
| 1 | M859 | Mosquito | Cambodia | 1967 | JEU70410 |
| 1 | K01-JB | Mosquito | Republic of Korea | 2001 | FJ938221 |
| 1 | K96A07 | Mosquito | Republic of Korea | 1996 | FJ938219 |
| 2 | JKT1749 | Mosquito | Indonesia | 1979 | JEU70405 |
| 2 | WTP-70-22 | Mosquito | Malaysia | 1970 | JEU70421 |
| 3 | Beijing 1 | Human brain | China | 1949 | JEU70389 |
| 3 | CH2195 | NAa | China | 1994 | JEU92644 |
| 3 | FJ03-97 | Homo sapiens | China | 2006b | DQ404127 |
| 3 | GZ04-43 | Culex sp | China | 2006b | DQ404113 |
| 3 | HLJ08-01 | Swine | China | 2008 | GQ495004 |
| 3 | HLJ08-02 | Swine | China | 2008 | GQ495005 |
| 3 | SA14-14-2* | SA-14 derivate | China | 1953 | AF315119 |
| 3 | SH04-10 | Culex tritaeniorhynchus | China | 2006b | DQ404107 |
| 3 | YNDL04-1 | Culex tritaeniorhynchus | China | 2006b | DQ404137 |
| 3 | Chiang Mai | Human | Thailand | 1964 | JEU70393 |
| 3 | B18A | Mosquito | Japan | 1978 | JEU70390 |
| 3 | Mie44-1 | Mosquito | Japan | 1969 | JEU70411 |
| 3 | Nakayama* | Human brain | Japan | 1935 | JEU70413 |
| 3 | Osaka | Mosquito | Japan | 1979 | JEU70414 |
| 3 | Sagiyama | Mosquito | Japan | 1957 | JEU70419 |
| 3 | JaNAr0990 | Mosquito | Japan | 1990 | AY427797 |
| 3 | JaOH0566 | Human brain | Japan | 1966 | JEU70399 |
| 3 | JaOH3767 | Human brain | Japan | 1967 | JEU70400 |
| 3 | K83P34 | Mosquito | Republic of Korea | 1983 | FJ938231 |
| 3 | K84A071 | Mosquito | Republic of Korea | 1984 | FJ938224 |
| 3 | K87A071 | Mosquito | Republic of Korea | 1987 | FJ938226 |
| 3 | K88A071 | Mosquito | Republic of Korea | 1988 | FJ938228 |
| 3 | K94A071 | Mosquito | Republic of Korea | 1994 | FJ938217 |
| 3 | H49778 | Human | Sri Lanka | 1987 | JEU70395 |
| 3 | Indonesia | Mosquito | Indonesia | 1996b | JEU70397 |
| 3 | JKT6468 | Mosquito | Indonesia | 1981 | JEU70407 |
| 3 | 826309 | Human | India | 1982 | JEU70403 |
| 3 | P20778 | Human | India | 1958 | JEU70415 |
| 3 | R53567 | NAa | India | 1996b | JEU70418 |
| 3 | PhAn1242 | Pig serum | Philippines | 1984 | JEU70417 |
| 3 | VN118 | Mosquito | Vietnam | 1979 | JEU70420 |
| 4 | JKT7003 | Mosquito | Indonesia | 1981 | JEU70408 |
| 4 | JKT9092 | Mosquito | Indonesia | 1981 | JEU70409 |
| 4 | 2372 | Human | Thailand | 1979 | JEU70401 |
| 5 | XZ0934 | Mosquito | China | 2009 | JF915894 |
Not available
Submitted date
Vaccine strains that have been used in the Republic of Korea.
Nucleotide sequence information for the E protein was translated for JEV strains identified in mosquitoes as part of this study and vaccine strains currently used in ROK. Deduced amino acid differences were identified among strains and between those detected in mosquitoes and those used in the national vaccination program.
Results
Detection of JEV from Mosquito Samples
A total of 6,328 culicine mosquitoes, representing 12 species from 5 genera, were captured at 6 localities in ROK from June through October 2010. The most frequently collected species was Culex tritaeniorhynchus (45.5%, n = 2,880), followed by Aedes vexans nipponii (33.0%, n = 2,091), Cx. pipiens (11.6%, n = 736), Cx. bitaeniorhynchus (5.4%, n = 344), Ochlerotatus koreicus (2.9%, n = 181), Aedes albopictus (1.0%, n = 66), Armigeres subalbatus (0.4%, n = 23), Cx. orientalis (<0.1%, n = 3), Aedes lineatopennis (<0.1%, n = 1), Cx. inatomii (<0.1%, n = 1), Mansonia uniformis (<0.1%, n = 1), and Ochlerotatus nipponicus (<0.1%, n = 1) (Table 3). A total of 34/371 pools (9.2%) tested positive for JEV (Table 3). JEV was most frequently identified in pools of Cx. tritaeniorhynchus (24.0%, 29/121 pools), followed by Cx. pipiens (6.3%, 4/64 pools) and Cx. bitaeniorhynchus (3.8%, 1/26 pools) (Table 3). All other culicine species tested negative for JEV (Table 3). MLE for the number of JEV RNA-positive mosquitoes per 1,000 individuals were 11.8, 5.6, and 2.8 for Cx. tritaeniorhynchus, Cx. pipiens, and Cx. bitaeniorhynchus (Table 3).
Table 3. Total number of culicine mosquitoes collected at 6 localities in four provinces in Republic of Korea in 2010 and number of Japanese encephalitis virus positive pools (up to 30 mosquitoes) as detected using RT-PCR.
| Species | Total Number Tested(% of Total) | Pools Tested (% of Total) | Positive Pools (MLE)a |
| Aedes albopictus | 66 (1.0) | 15 (4.0) | 0 |
| Aedes lineatopennis | 1 (<0.1) | 1 (0.3) | 0 |
| Aedes vexans nipponii | 2,091 (33.0) | 106 (28.6) | 0 |
| Armigeres subalbatus | 23 (0.4) | 9 (2.4) | 0 |
| Culex bitaeniorhynchus | 344 (5.4) | 26 (7.0) | 1 (2.8) |
| Culex inatomii | 1 (<0.1) | 1 (0.3) | 0 |
| Culex orientalis | 3 (<0.1) | 2 (0.5) | 0 |
| Culex pipiens | 736 (11.6) | 64 (17.3) | 4 (5.6) |
| Culex tritaeniorhynchus | 2,880 (45.5) | 121 (32.6) | 29 (11.8) |
| Mansonia uniformis | 1 (<0.1) | 1 (0.3) | 0 |
| Ochlerotatus koreicus | 181 (2.9) | 24 (6.5) | 0 |
| Ochlerotatus nipponicus | 1 (<0.1) | 1 (0.3) | 0 |
| Total | 6,328 (100.0) | 371 | 34 (5.8) |
Up to 30 specimens of culicine mosquitoes were pooled based on the locality and time of their collection.
Maximum likelihood estimation (MLE) = estimated number of viral RNA-positive mosquitoes per 1,000.
Although JEV-positive mosquitoes were identified at horse farms in Ilsan and Jeju, no JEV-positive mosquitoes were identified at horse farms in Busan, Jangsu, or Gwacheon (Fig. 2). Most of the JEV-positive mosquitoes were collected in Munsan at Warrior Base, a US military complex located 4 km from the DMZ separating North and South Korea. One mosquito species, Cx. pipiens (MLE = 4.4), was positive for JEV at Jeju; two species, Cx. tritaeniorhynchus (MLE = 4.7) and Cx. pipiens (MLE = 16.1), were positive for JEV at Ilsan; while three species, Cx. tritaeniorhynchus (MLE = 13.3), Cx. pipiens (MLE = 5.8), and Cx. bitaeniorhynchus (MLE = 2.8), were positive for JEV at Munsan.
Sequence Similarity and Phylogenetic Analysis of the prM and E Genes
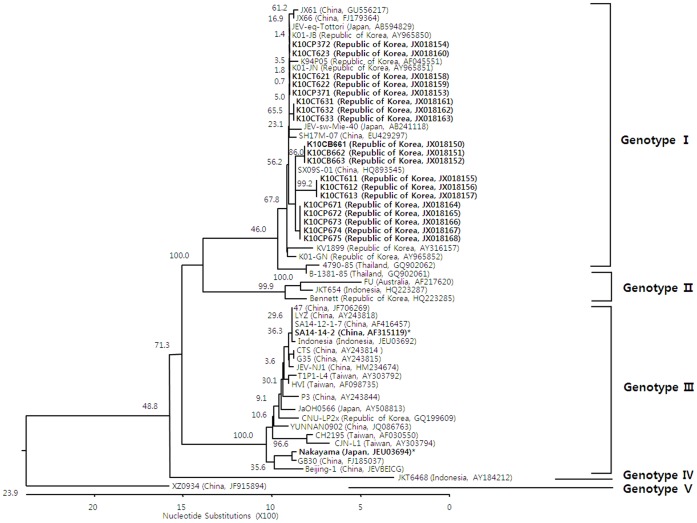
All nucleotide sequences for the prM protein coding region of JEV strains derived from the mosquitoes and sampled in ROK in the present study clustered with viruses previously classified as genotype I (Fig. 3). The prM protein coding nucleotide sequences from ROK analyzed in this study were 79.2–100% similar to other JEV strains isolated in China, Japan, Thailand, Taiwan, Indonesia, and Australia. Sequence similarity for prM protein coding sequences were 88.2–100% between JEV strains previously identified in ROK and those identified in this study.
Figure 3. Phylogenetic tree illustrating the genetic relationship of nucleotide sequences for pre-membrane protein genes of Japanese encephalitis virus (JEV) strains identified in mosquitoes, Republic of Korea, 2010 (indicated in bold font) and reference sequences from other geographic regions as reported on GenBank.
Genotypes of JEV strains are indicated on the right of the phylogenetic tree and were assigned according to Chen et al. [45], [46]. Bootstrap support values are shown. The scale bar indicates the number of mutations. Abbreviations for strains reported in this study are as follows: K10CT = Republic of Korea (ROK), 2010, Culex tritaeniorhynchus; K10CB = ROK, 2010, Culex bitaeniorhynchus; and K10CP = ROK, 2010, Culex pipiens. Vaccine strains that have been used in ROK are indicated in bold font and with an asterisk (*).
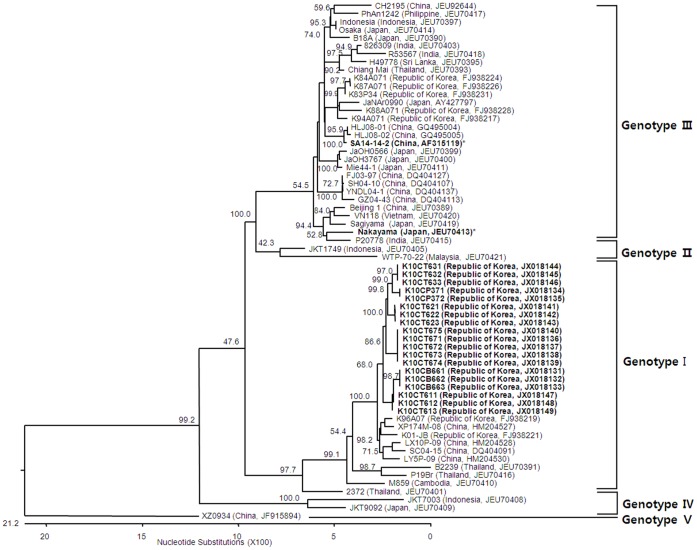
Nucleotide sequences for the E protein coding region of JEV strains from mosquitoes sampled in ROK also formed clustered with genotype I viruses (Fig. 4). Nucleotide sequence similarity between the E protein coding region of viruses identified in this study and previously published sequences was 76.9–99.1%. Similarity of the E protein coding region was 87.5–99.1% when considering only strains of JEV originating from ROK.
Figure 4. Phylogenetic tree illustrating the genetic relationship of nucleotide sequences for envelope protein coding genes of Japanese encephalitis virus (JEV) strains identified in mosquitoes, Republic of Korea, 2010 (indicated in bold font) and reference sequences for JEV strains from other geographic regions as reported on GenBank.
Genotypes of JEV strains are indicated on the right of the phylogenetic tree and were assigned according to Chen et al. [45], [46]. Bootstrap support values are shown. The scale bar indicates the number of mutations. Abbreviations for strains reported in this study are as follows: K10CT = Republic of Korea (ROK), 2010, Culex tritaeniorhynchus; K10CB = ROK, 2010, Culex bitaeniorhynchus; and K10CP = ROK, 2010, Culex pipiens. Vaccine strains that have been used in ROK are indicated in bold font and with an asterisk (*).
Comparison between JEV Strains in Mosquitoes and Vaccine Strains Currently Used in ROK
JEV strains identified in this study had 87.9–88.7% nucleotide sequence similarity and 96.5–97.9% amino acid sequence similarity compared to vaccine strain SA14-14-2 (China, AF315119) currently used in ROK based on analysis of prM protein coding region sequences. Nucleotide and amino acid sequence similarity were 87.5–88.7% and 94.3–95.7% when comparing prM sequences for JEV strains in mosquitoes with another vaccine strain used in ROK, Nakayama (Japan, JEU03694). E protein coding region sequences of JEV strains identified in this study were 87.6–88.0% and 96.4–97.2% similar with regard to nucleotide and amino acid similarity when compared with vaccine strain SA14-14-2. Nucleotide and amino acid sequence similarity was 87.6–88.0% and 96.4–97.2%, respectively, when comparing E protein coding regions of strains from mosquitoes with strain JEV Nakayama. Five amino acid residues of JEV strains identified in mosquitoes were different from vaccine strains used in ROK: E129 (Thr→Met), E176 (Val, Thr→Ile ), E222 (Arg→Ser, Pro), E327 (Ser→Thr), and E366 (Arg→Ser) (Table 4).
Table 4. Comparison of amino acids differences in the envelope protein between the Japanese encephalitis vaccine strains that have been used in Republic of Korea and those identified in mosquitoes for this study.
| Strain | E107 | E123 | E129 | E138 | E176 | E177 | E222 | E264 | E279 | E315 | E327 | E346 | E366 | E439 |
| SA-14-14-2* | F | S | T | K | V | A | A | H | M | V | S | N | A | R |
| Nakayama* | L | S | T | E | T | T | A | Q | K | A | S | N | A | K |
| K10CP371 | L | S | M | E | I | T | P | Q | K | A | T | N | S | K |
| K10CP372 | L | S | M | E | I | T | S | Q | K | A | T | S | S | K |
| K10CT611 | L | S | M | E | I | T | S | Q | K | A | T | S | S | K |
| K10CT612 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CT613 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CT621 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CT622 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CT623 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CT631 | L | S | M | E | I | T | S | Q | K | A | T | S | S | K |
| K10CT632 | L | S | M | E | I | T | S | Q | K | A | T | S | S | K |
| K10CT633 | L | S | M | E | I | T | S | Q | K | A | T | S | S | K |
| K10CB661 | L | S | M | E | I | T | S | Q | E | A | T | N | S | K |
| K10CB662 | L | S | M | E | I | T | S | Q | E | A | T | N | S | K |
| K10CB663 | L | S | M | E | I | T | S | Q | E | A | T | N | S | K |
| K10CP671 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CP672 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CP673 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CP674 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
| K10CP675 | L | S | M | E | I | T | S | Q | K | A | T | N | S | K |
Abbreviations: A, alanine; E, glutamic acid; F, phenylalanine; H, histidine; I, isoleucine; L, leucine; M. methionine; N, asparagines; P, proline; Q, glutamine; R, arginine; S, serine; T, threonine; V, valine. K10CT = Republic of Korea (ROK), 2010, Culex tritaeniorhynchus; K10CB = ROK, 2010, Culex bitaeniorhynchus; and K10CP = ROK, 2010, Culex pipiens.
Vaccine strains that have been used in ROK.
Discussion
JEV vector surveillance provides information regarding the distribution, intensity, and abundance of circulating viruses that can be used for the development and implementation of disease mitigation strategies by public health officials. As part of surveillance activities, it is important that mosquitoes be processed properly so that arboviruses can be identified in the laboratory. Mosquitoes that were trapped over a 24-hr period were transported to the 5th Medical Detachment for identification and maintained at −70°C. Although many of the mosquitoes arrived dead, it has been shown that JEV RNA is stable up to 14 days even under relatively harsh conditions [24]. Thus, methodology for sampling and transport should have ensured accurate results.
The mandatory childhood immunization policy initiated in ROK in 1967 and fully implemented in 1971 greatly decreased the incidence of reported JE cases from epidemic proportions, which often exceeded 1,000 cases prior to 1982. From 1984 through 2009, there were no JEV outbreaks in the Korean population. During this same period, outbreaks/epidemics continued to be reported in India, China, and other countries that did not have comprehensive JEV vaccination programs. In 2010, an outbreak of 26 JE cases, including 7 deaths (26.9%), was reported in ROK [19]. This number likely under-represents the actual number of cases, as only severe cases of encephalitis lead to hospitalization and proper evaluation of JEV infection. Previous JEV vector surveillance programs in ROK have been limited and therefore, although it cannot be empirically evaluated, it is hypothesized that the extended rainy season through mid-September is believed to be responsible for large Cx. tritaeniorhynchus populations, the primary JEV vector in ROK. Additionally, an extended rainy season may have led to increased populations of potential secondary vectors, e.g., Cx. pipiens and Cx. bitaeniorhynchus. Phylogenetic analyses of JEV strains circulating in East Asia indicate that genotype I strains detected in mosquitoes have a relatively distant genetic relationship to genotype III vaccine strains currently used in ROK (Figs. 3 and 4). Additionally, five amino acid residues of JEV strains identified in mosquitoes were different from vaccine strains (Table 4). These data support the circulation of genetically divergent JEV strains in ROK during 2010 as compared to vaccine strains. Howver, there are evidences for partial protection by antibodies that cross-react within the JE serocomplex group of viruses [25]–[27] and therefore vaccine breakthrough may be an insufficient for an alternative explanation for the 2010 outbreak of JE in ROK. A detailed epidemiological analysis of the outbreak may have provided more clues on the underlying factors contributing to the outbreak although this was outside the scope of the current study.
Cx. tritaeniorhynchus is well known to be the primary vector for JEV in ROK and throughout much of Asia. However, in India and other parts of Asia, other Culex spp. are primary (e.g., Cx. vishnui) or secondary vectors (e.g., Cx. pipiens and Cx. bitaeniorhynchus) [28]–[32]. In this study, Cx. tritaeniorhynchus accounted for 85.3% of the JEV-positive pools of culicines while only comprising 32.6% of those tested (Table 3). Maximum likelihood methods estimate 11.8 JEV-positive individuals per 1,000 mosquitos sampled for this species (Table 3). Thus, these data indicate that Cx. tritaeniorhynchus carried JEV at relatively high rates in ROK during the period of the 2010 outbreak and therefore may have contributed to transmission of viruses at this location and time.
Both Cx. tritaeniorhynchus and Cx. bitaeniorhynchus are associated with rice paddies/water impoundments associated with large water birds, while Cx. pipiens is often associated with swine farms. More than one sub-species of Cx. pipiens is found in ROK. Cx. pipiens molestus is autogenous and occurs year-round, whereas Cx. pipiens pallens is not collected during the winter season in ROK. The taxonomy of these species has not been resolved and thus are reported as Cx. pipiens herein. JEV-positive Cx. pipiens were observed in 11.8% of PCR-positive pools while accounting for 17.3% of culicine pools tested (Table 3). Maximum likelihood methods estimated 5.6 JEV-positive individuals per 1,000 mosquitos sampled for this species (Table 3). Laboratory studies have demonstrated low vector competence for Cx. pipiens and therefore this species may be more likely to be a secondary vector when large populations of mosquitoes are present near pig farms [33]–[35]. In the early 1970s, JEV was isolated during the winter on two occasions from Cx. pipiens in ROK [36] which was somewhat surprising as this virus had been identified infrequently in this mosquito species in northern Asia. The role of Cx. pipiens in the transmission of JEV is not fully understood, but this species may contribute to virus spread in urban environments. Thus, Cx. pipiens may also have contributed to the JE outbreak in ROK during 2010.
Cx. tritaeniorhynchus, Cx. bitaeniorhynchus, and Cx. pipiens (a vector of West Nile virus in the United States) readily feed on birds and mammals, including man. Cx. bitaeniorhynchus accounted for 2.9% of JEV PCR-positive culicine pools detected in this study (Table 3). Maximum likelihood methods estimated only 2.8 JEV-positive individuals per 1,000 sampled (Table 3). Although JEV has been identified in Cx. bitaeniorhynchus in India and other countries, this species has only recently been implicated as a potential vector in ROK [37]. Therefore the role of this species in the maintenance and transmission of JEV in ROK is unknown.
Until the latter part of the 20th century, studies of JEV indicated that the predominance of JEV strains detected worldwide could be assigned to genotype III. Since then, there have been reports of JEV genotype I displacing genotype III in many regions [1], [14], [38]–[43], and genotype I is now recognized as the dominant strain in many areas. Although JEV genotype V has recently re-emerged in Asia (China and ROK) after more than a half-century [44], only genotype I viruses have been reported to be circulating in ROK during 1994–2009 [12]. Similarly, all nucleotide sequences for prM and E genes isolated from viruses obtained from mosquitoes and analyzed in this study phylogenetically clustered with JEV genotype I strains. Nucleotide and amino acid similarity results suggest that viruses identified in mosquitoes in the present study were more closely related to JEV strains circulating throughout Asian countries than vaccine strains currently used in ROK (Figs. 3 and 4; Table 4).
Results from this study demonstrate the utility of vector screening for surveillance of JEV in ROK. Additional studies that measure the impact of vectors (e.g., bionomics and vector competence) in the transmission of JEV and that incorporate environmental factors (e.g., weekly rainfall) are needed to define the roles of Culex species in the viral pathogenesis during outbreak and non-outbreak years. Furthermore, long-term longitudinal vector surveillance is necessary to better understand the dynamics of JEV transmission in ROK and to characterize the role of potential secondary vectors, e.g., Cx. pipiens and Cx. bitaeniorhynchus, in the maintenance and human transmission of JEV.
Acknowledgments
The opinions, interpretations, conclusions, and recommendations contained herein are those of the authors and do not reflect official views or policy of the U.S. Army, the U.S. Department of Defense, or the Animal, Plant, and Fisheries Quarantine and Inspection Agency, ROK.
Funding Statement
This work was supported by Korea National Veterinary Research and Quarantine Service (NVRQS) grant N-AD13-2011-13-01. This work was also supported through a joint partnership between the Korean Horse Racing Authority, Seoul, ROK; the Uniformed Services University, Bethesda, MD; the Armed Forces Health Surveillance Center-Global Emerging Infections Surveillance and Response System (AFHSC-GEIS), Silver Spring, MD; and the National Center for Medical Intelligence. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang L, Fu S, Zhang H, Ye X, Yu D, et al. (2010) Identification and isolation of Genotype-I Japanese encephalitis virus from encephalitis patients. Virology journal 7: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y, Li M, Wang H, Liang G (2012) Japanese encephalitis and Japanese encephalitis virus in mainland China. Reviews in Medical Virology doi: 10.1002/rmv.1710. [DOI] [PubMed]
- 3. Cao QS, Li XM, Zhu QY, Wang DD, Chen HC, et al. (2011) Isolation and molecular characterization of genotype 1 Japanese encephalitis virus, SX09S-01, from pigs in China. Virology journal 8: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsai TF (2000) New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine 18 Suppl 21–25. [DOI] [PubMed] [Google Scholar]
- 5.United Nations (2005) The United Nations urbanization prospects: the 2005 revision. POP/DB/WUP/Rev.2005/1/F1 ed. New York: United Nations.
- 6. Solomon T (2006) Control of Japanese encephalitis–within our grasp? The New England journal of medicine 355: 869–871. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (2008) World health report (for years 2000–2004). World Health Organization.
- 8. Lewis L, Taylor HG, Milton B, Sorem MC, John W, et al. (1947) Japanese B encephalitis; clinical observations in an outbreak on Okinawa Shima. Archives of neurology and psychiatry 57: 430–463. [PubMed] [Google Scholar]
- 9. Grascenkov NI (1964) Japanese encephalitis in the Ussr. Bulletin of the World Health Organization 30: 161–172. [PMC free article] [PubMed] [Google Scholar]
- 10. Igarashi A, Tanaka M, Morita K, Takasu T, Ahmed A, et al. (1994) Detection of West Nile and Japanese encephalitis viral genome sequences in cerebrospinal fluid from acute encephalitis cases in Karachi, Pakistan. Microbiology and immunology 38: 827–830. [DOI] [PubMed] [Google Scholar]
- 11. Hanna JN, Ritchie SA, Phillips DA, Shield J, Bailey MC, et al. (1996) An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. The Medical journal of Australia 165: 256–260. [DOI] [PubMed] [Google Scholar]
- 12. Yun SM, Cho JE, Ju YR, Kim SY, Ryou J, et al. (2010) Molecular epidemiology of Japanese encephalitis virus circulating in South Korea, 1983–2005. Virology journal 7: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang JS, Zhao QM, Guo XF, Zuo SQ, Cheng JX, et al. (2011) Isolation and genetic characteristics of human genotype 1 Japanese encephalitis virus, China, 2009. PLoS One 6: e16418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang HY, Takasaki T, Fu SH, Sun XH, Zhang HL, et al. (2007) Molecular epidemiological analysis of Japanese encephalitis virus in China. The Journal of general virology 88: 885–894. [DOI] [PubMed] [Google Scholar]
- 15. Wang H, Fu S, Liu G, Liu H, Gao X, et al. (2012) Isolation and identification of a distinct strain of CulexFlavivirus from mosquitoes collected in Mainland China. Virology journal 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JO, Han EL, Kim YH, Kim SH, Kim HJ, et al.. (2009) Establishment of in vitro potency assay for Japanese encephalitis (inactivated) vaccine - Construction of recombinant Japanese encephalitis virus (JEV) E antigen and neutralizing JEV E monoclonal antibodies. Korea Food and Drug Administration.
- 17. Cho YJ, Kim YJ, Kim DS, Kim YB, Joo YR, et al. (2007) Neutralizing antibody induction and cytotoxic T lymphocyte response to Nakayama-NIH and Beijing-1 as Japanese encephlitis virus vaccine strains. Journal of bacteriology and virology 37: 161–167. [Google Scholar]
- 18. Sohn YM (2000) Japanese encephalitis immunization in South Korea: past, present, and future. Emerging infectious diseases 6: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.K-CDC (2010) Infectious diseases surveillance yearbook. Reported cases of group II notifiable diseases (total/incidence rate): Korea Centers for Disease Control and Prevention. 51.
- 20. Kim HC, Turell MJ, O’Guinn ML, Lee JS, Chong S, et al. (2007) Historical review and surveillance of Japanese encephalitis, Republic of Korea, 2002–2004. Entomological Research 37: 267–274. [Google Scholar]
- 21. Turell MJ, O’Guinn ML, Wasieloski LP Jr, Dohm DJ, Lee WJ, et al. (2003) Isolation of Japanese encephalitis and Getah viruses from mosquitoes (Diptera: Culicidae) collected near Camp Greaves, Gyonggi Province, Republic of Korea, 2000. Journal of medical entomology 40: 580–584. [DOI] [PubMed] [Google Scholar]
- 22.Jeong YE (2011) Activity of Japanese encephalitis virus in the Republic of Korea. Korea Center for Disease Control. 333–337 p.
- 23. Yang DK, Kim BH, Kweon CH, Kwon JH, Lim SI, et al. (2004) Molecular characterization of full-length genome of Japanese encephalitis virus (KV1899) isolated from pigs in Korea. Journal of veterinary science 5: 197–205. [PubMed] [Google Scholar]
- 24. Johansen CA, Hall RA, van den Hurk AF, Ritchie SA, Mackenzie JS (2002) Detection and stability of Japanese encephalitis virus RNA and virus viability in dead infected mosquitoes under different storage conditions. The American journal of tropical medicine and hygiene 67: 656–661. [DOI] [PubMed] [Google Scholar]
- 25. Takasaki T, Yabe S, Nerome R, Ito M, Yamada K, et al. (2003) Partial protective effect of inactivated Japanese encephalitis vaccine on lethal West Nile virus infection in mice. Vaccine 21: 4514–4518. [DOI] [PubMed] [Google Scholar]
- 26. Yamshchikov G, Borisevich V, Kwok CW, Nistler R, Kohlmeier J, et al. (2005) The suitability of yellow fever and Japanese encephalitis vaccines for immunization against West Nile virus. Vaccine 23: 4785–4792. [DOI] [PubMed] [Google Scholar]
- 27. Williams DT, Daniels PW, Lunt RA, Wang LF, Newberry KM, et al. (2001) Experimental infections of pigs with Japanese encephalitis virus and closely related Australian flaviviruses. The American journal of tropical medicine and hygiene 65: 379–387. [DOI] [PubMed] [Google Scholar]
- 28. Vythilingam I, Oda K, Chew TK, Mahadevan S, Vijayamalar B, et al. (1995) Isolation of Japanese encephalitis virus from mosquitoes collected in Sabak Bernam, Selangor, Malaysia in 1992. Journal of the American Mosquito Control Association 11: 94–98. [PubMed] [Google Scholar]
- 29. Sucharit S, Surathin K, Shrestha SR (1989) Vectors of Japanese encephalitis virus (JEV): species complexes of the vectors. The Southeast Asian journal of tropical medicine and public health 20: 611–621. [PubMed] [Google Scholar]
- 30. Rosen L (1986) The natural history of Japanese encephalitis virus. Annual review of microbiology 40: 395–414. [DOI] [PubMed] [Google Scholar]
- 31. Bhattacharya S, Chakraborty SK, Chakraborty S, Ghosh KK, Palit A, et al. (1986) Density of Culex vishnui and appearance of JE antibody in sentinel chicks and wild birds in relation to Japanese encephalitis cases. Tropical and geographical medicine 38: 46–50. [PubMed] [Google Scholar]
- 32. Banerjee K, Deshmukh PK, Ilkal MA, Dhanda V (1978) Transmission of Japanese encephalitis virus by Culex bitaeniorhynchus Giles. The Indian journal of medical research 67: 889–893. [PubMed] [Google Scholar]
- 33. Weng MH, Lien JC, Lin CC, Yao CW (2000) Vector competence of Culex pipiens molestus (Diptera: Culicidae) from Taiwan for a sympatric strain of Japanese encephalitis virus. Journal of medical entomology 37: 780–783. [DOI] [PubMed] [Google Scholar]
- 34. Turell MJ, Mores CN, Dohm DJ, Lee WJ, Kim HC, et al. (2006) Laboratory transmission of Japanese encephalitis, West Nile, and Getah viruses by mosquitoes (Diptera: Culicidae) collected near Camp Greaves, Gyeonggi Province, Republic of Korea 2003. Journal of medical entomology 43: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 35. Turell MJ, Mores CN, Dohm DJ, Komilov N, Paragas J, et al. (2006) Laboratory transmission of Japanese encephalitis and West Nile viruses by molestus form of Culex pipiens (Diptera: Culicidae) collected in Uzbekistan in 2004. Journal of medical entomology 43: 296–300. [DOI] [PubMed] [Google Scholar]
- 36. Lee HW (1971) Study on overwintering mechanisms of Japanese encephalitis virus in Korea. Journal of the Korean Medical Association 14: 871–878. [Google Scholar]
- 37. Kim HC, Klein TA, Takhampunya R, Evans BP, Mingmongkolchai S, et al. (2011) Japanese encephalitis virus in culicine mosquitoes (Diptera: Culicidae) collected at Daeseongdong, a village in the demilitarized zone of the Republic of Korea. Journal of medical entomology 48: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 38. Li YX, Li MH, Fu SH, Chen WX, Liu QY, et al. (2011) Japanese encephalitis, Tibet, China. Emerging infectious diseases 17: 934–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nabeshima T, Loan HT, Inoue S, Sumiyoshi M, Haruta Y, et al. (2009) Evidence of frequent introductions of Japanese encephalitis virus from south-east Asia and continental east Asia to Japan. The Journal of general virology 90: 827–832. [DOI] [PubMed] [Google Scholar]
- 40. Nga PT, del Carmen Parquet M, Cuong VD, Ma SP, Hasebe F, et al. (2004) Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. The Journal of general virology 85: 1625–1631. [DOI] [PubMed] [Google Scholar]
- 41. Nitatpattana N, Dubot-Peres A, Gouilh MA, Souris M, Barbazan P, et al. (2008) Change in Japanese encephalitis virus distribution, Thailand. Emerging infectious diseases 14: 1762–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saito M, Taira K, Itokazu K, Mori N (2007) Recent change of the antigenicity and genotype of Japanese encephalitis viruses distributed on Okinawa Island, Japan. The American journal of tropical medicine and hygiene 77: 737–746. [PubMed] [Google Scholar]
- 43. Wang LH, Fu SH, Wang HY, Liang XF, Cheng JX, et al. (2007) Japanese encephalitis outbreak, Yuncheng, China, 2006. Emerging infectious diseases 13: 1123–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takhampunya R, Kim HC, Tippayachai B, Kengluecha A, Klein TA, et al. (2011) Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virology journal 8: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen WR, Rico-Hesse R, Tesh RB (1992) A new genotype of Japanese encephalitis virus from Indonesia. American journal of tropical medicine and hygiene 47: 61–69. [DOI] [PubMed] [Google Scholar]
- 46. Chen WR, Tesh RB, Rico-Hesse R (1990) Genetic variation of Japanese encephalitis virus in nature. Journal of general virology 71 (Pt 12): 2915–2922. [DOI] [PubMed] [Google Scholar]