Abstract
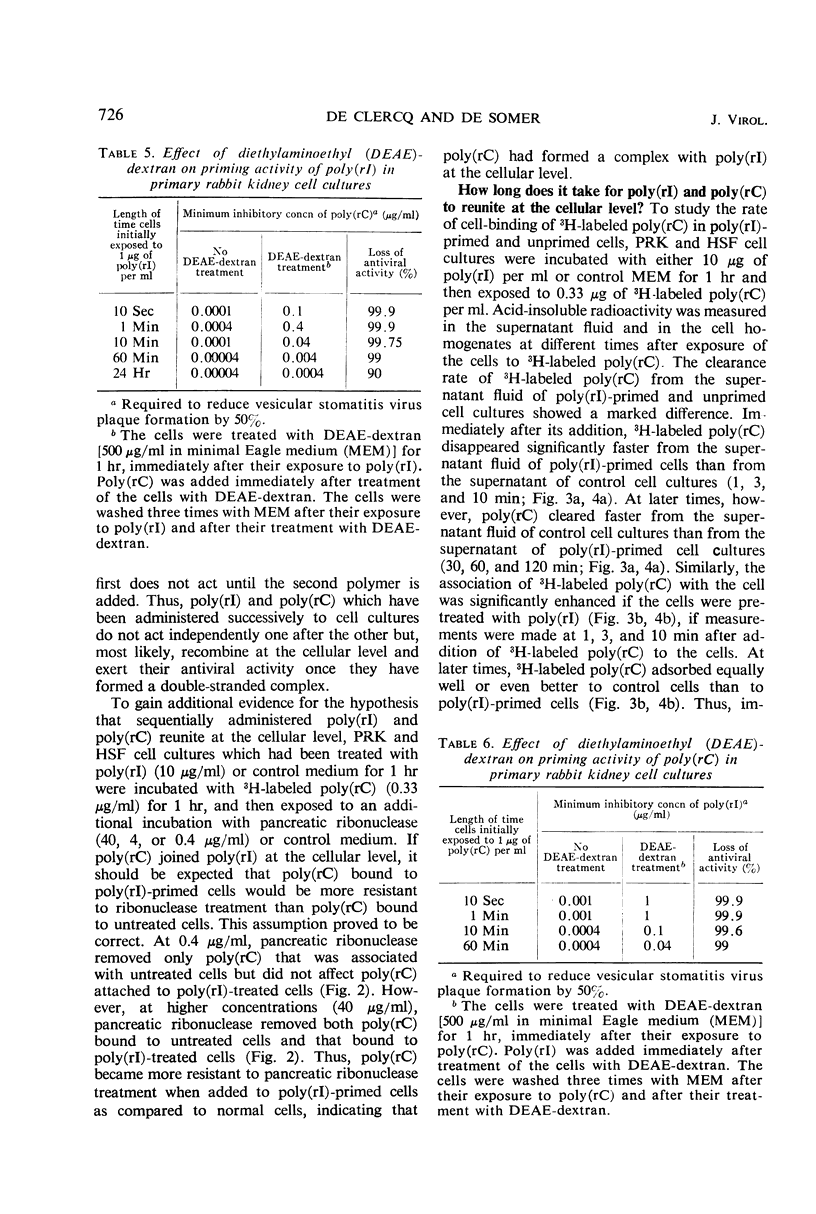
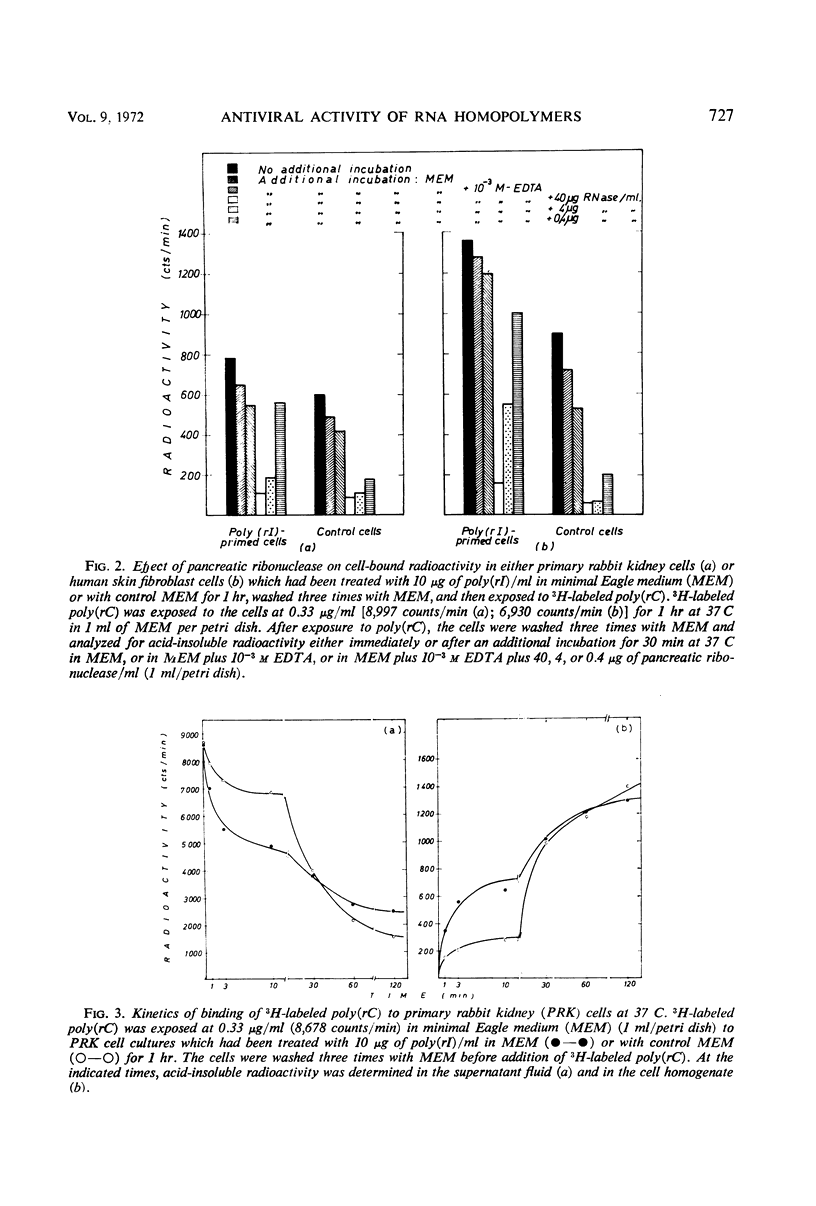
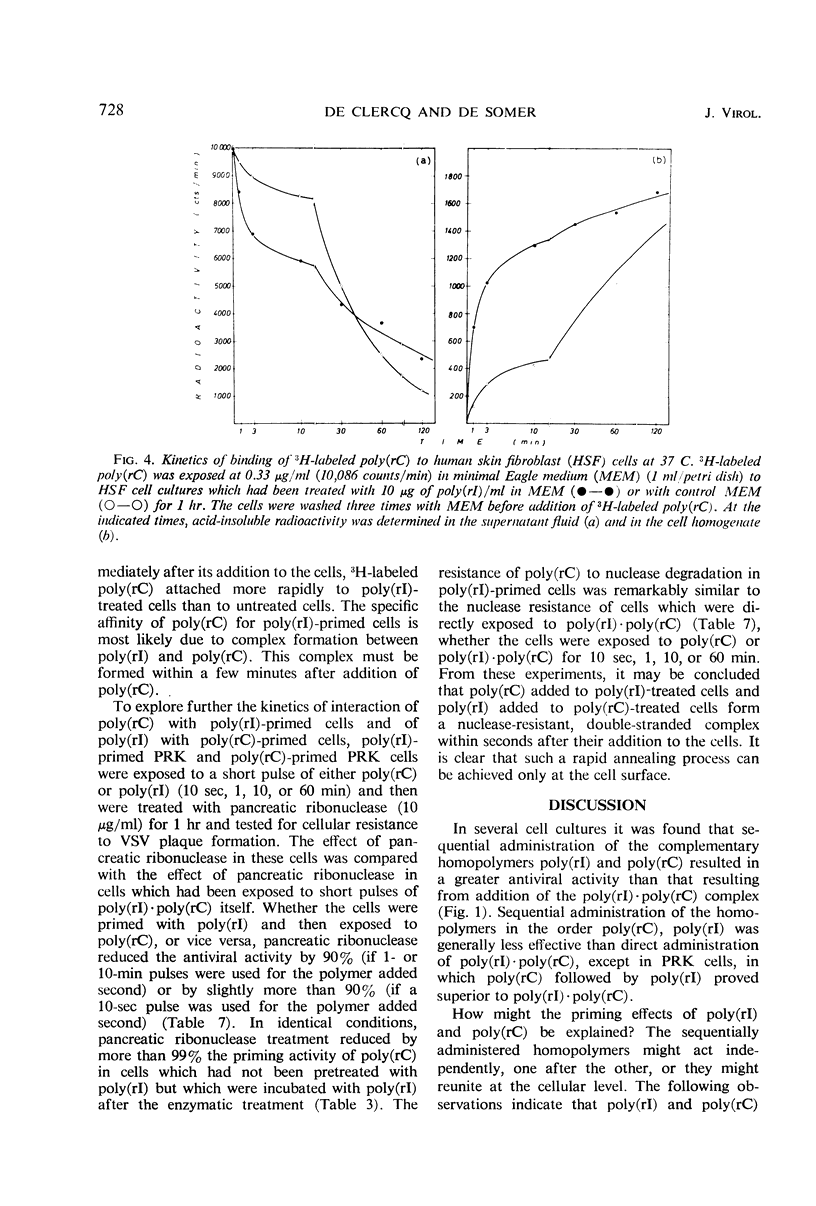
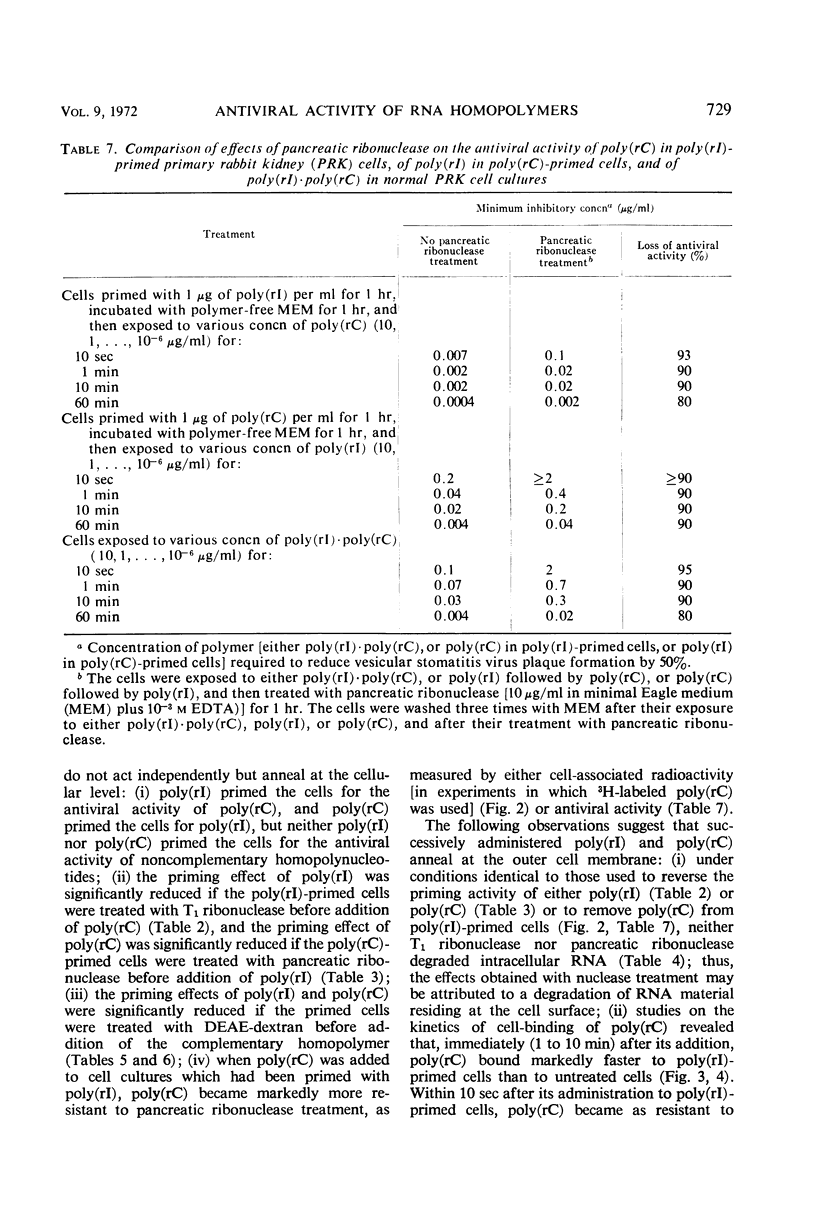
The antiviral activity of the double-stranded complex poly(rI) · poly(rC) in cell culture was restored or even surpassed if the constituent homopolymers were administered separately. Poly(rI) primed the cells for the antiviral activity of poly(rC) and poly(rC) primed for poly(rI), but neither poly(rI) nor poly(rC) primed the cells for the antiviral activity of noncomplementary homopolynucleotides. The priming effect of poly(rI) was significantly reduced if the poly(rI)-primed cells were treated with either T1 ribonuclease or diethylaminoethyl (DEAE)-dextran before addition of poly(rC), and the priming effect of poly(rC) was significantly reduced if the poly(rC)-primed cells were treated with either pancreatic ribonuclease or DEAE-dextran before addition of poly(rI). 3H-labeled poly(rC) bound more rapidly to poly(rI)-treated cells than to control cells. Cell-associated poly(rC) was markedly more resistant to pancreatic ribonuclease treatment if the cells had been incubated with poly(rI) before exposure to poly(rC). Our results clearly indicate that poly(rI) and poly(rC) added successively to cell cultures do not act independently but reunite at the cellular level, most likely at the outer cell membrane.
Full text
PDF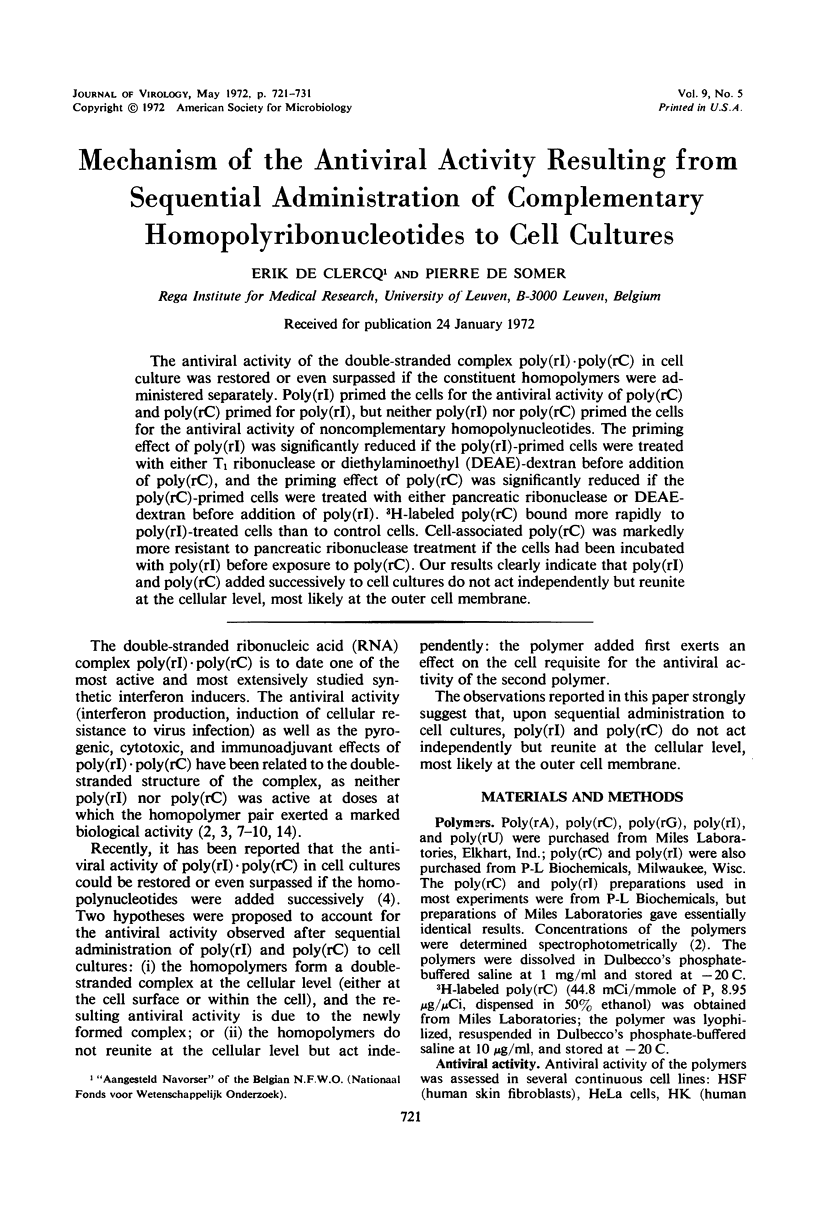



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bausek G. H., Merigan T. C. Cell interaction with a synthetic polynucleotide and interferon production in vitro. Virology. 1969 Nov;39(3):491–498. doi: 10.1016/0042-6822(69)90097-x. [DOI] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Antiviral activity of polyribocytidylic acid in cells primed with polyriboinosinic acid. Science. 1971 Jul 16;173(3993):260–262. doi: 10.1126/science.173.3993.260. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Merigan T. C. Requirement of a stable secondary structure for the antiviral activity of polynucleotides. Nature. 1969 Jun 21;222(5199):1148–1152. doi: 10.1038/2221148a0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Nuwer M. R., Merigan T. C. The role of interferon in the protective effect of a synthetic double-stranded polyribonucleotide against intranasal vesicular stomatitis virus challenge in mice. J Clin Invest. 1970 Aug;49(8):1565–1577. doi: 10.1172/JCI106374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Wells R. D., Grant R. C., Merigan T. C. Thermal activation of the antiviral activity of synthetic double-stranded polyribonucleotides. J Mol Biol. 1971 Feb 28;56(1):83–100. doi: 10.1016/0022-2836(71)90086-6. [DOI] [PubMed] [Google Scholar]
- Egami F., Takahashi K., Uchida T. Ribonucleases in taka-diastase: properties, chemical nature, and applications. Prog Nucleic Acid Res Mol Biol. 1964;3:59–101. doi: 10.1016/s0079-6603(08)60739-4. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance, V. In vitro studies. Proc Natl Acad Sci U S A. 1968 Sep;61(1):340–346. doi: 10.1073/pnas.61.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien P., de Maeyer-Guignard J. Toxic effect of polyriboinosinic-polyribocytidylic acid on mouse hemopoietic stem cells. Int J Cancer. 1971 May 15;7(3):468–475. doi: 10.1002/ijc.2910070312. [DOI] [PubMed] [Google Scholar]
- Lindsay H. L., Trown P. W., Brandt J., Forbes M. Pyrogenicity of poly I. poly C in rabbits. Nature. 1969 Aug 16;223(5207):717–718. doi: 10.1038/223717a0. [DOI] [PubMed] [Google Scholar]
- Niblack J. F., McCreary M. B. Relationship of biological activities of poly I-poly C to homopolymer molecular weights. Nat New Biol. 1971 Sep 8;233(36):52–53. doi: 10.1038/newbio233052a0. [DOI] [PubMed] [Google Scholar]
- Schell P. L. Uptake of polynucleotides by intact mammalian cells (IV): synthetic homoribopolynucleotides. Z Naturforsch B. 1968 Aug;23(8):1117–1119. doi: 10.1515/znb-1968-0825. [DOI] [PubMed] [Google Scholar]
- Schell P. L. Uptake of polynucleotides by intact mammalian cells. 8. Synthetic homoribopolynucleotides. Biochim Biophys Acta. 1971 Jul 29;240(4):472–484. doi: 10.1016/0005-2787(71)90704-0. [DOI] [PubMed] [Google Scholar]
- Schmidtke J. R., Johnson A. G. Regulation of the immune system by synthetic polynucleotides. I. Characteristics of adjuvant action on antibody synthesis. J Immunol. 1971 May;106(5):1191–1200. [PubMed] [Google Scholar]
- Tytell A. A., Lampson G. P., Field A. K., Nemes M. M., Hilleman M. R. Influence of size of individual homopolynucleotides on the physical and biological properties of complexed rIn:rCn (poly I:C). Proc Soc Exp Biol Med. 1970 Dec;135(3):917–921. doi: 10.3181/00379727-135-35170. [DOI] [PubMed] [Google Scholar]



