Abstract
The metastasis of cancer is a multistage process involving complex biological interactions and difficult to predict outcomes. Accurate assessment of the extent of metastasis is critical for clinical practice; unfortunately, medical imaging methods capable of identifying the early stages of invasion and metastasis are lacking. Photoacoustic imaging is capable of providing noninvasive, real-time imaging of significant anatomical and physiological changes. indicating the progression of cancer invasion and metastasis. Preclinically, photoacoustic methods have been used to image lymphatic anatomy, including the sentinel lymph nodes, to identify circulating tumor cells within vasculature and to detect micrometastases. Progress has begun toward the development of clinically applicable photoacoustic imaging systems to assist with the determination of cancer stage and likelihood of metastatic invasion.
Keywords: circulating tumor cells, lymphatic system, medical imaging, metastasis, photoacoustics, sentinel lymph node
Photoacoustic (PA) imaging and detection are promising technologies with great potential to impact the clinical assessment and treatment of cancer. In this article, the authors focus on PA methods to detect cancer invasion and metastasis. This is a critical area of research that requires the incorporation of new understandings of the physiology of the lymphatic system and its role in the spread of cancer, as well as knowledge of hematologic indicators of cancer status and prognosis. PA signals, which are pressure waves generated by the transient absorption of laser light and subsequent heating of chromophores, can be received by a simple detection system or can be processed via beam-forming ultrasound transducers, integrated scanners or imaging software algorithms, to produce 2D and 3D images or maps of tissue. The utility of PA imaging for the detection and characterization of cancerous tumors has been previously reviewed [1]. PA methods incorporated in this review include those which provide anatomical imaging of the lymphatics and those which enable the detection of the hematologic spread of cancerous cells. This focus reflects the current need in clinical practice to develop noninvasive imaging modalities for the detection of the early indicators of metastasis.
Cancer invasion & metastasis
The extent of invasion and metastasis of a cancerous tumor is directly correlated to patient prognosis [2]. Cancer invasion and metastasis is characterized by the dissemination of cancer cells from the local tissue into the circulatory system, followed by the initiation of tumors in distant organs [3]. Cancer invasion into the vasculature is partially driven by the initiation of a cascade of events leading to the formation of new blood vessels (angiogenesis) that begins once the tumor has reached a size, typically around 2–3 mm3 [4], which prevents the delivery of sufficient nutrients and oxygen by diffusion alone. The newly formed vascular network not only provides nutrients to the growing tumor, but the `leaky' vasculature also provides easy access for cancer cells to enter the tumor-associated capillaries, facilitating the spread of tumor cells into the systemic circulation. Once the cancer cells invade the vascular system, they are arrested in the capillaries surrounding a particular organ where the cancer cells extravasate into the tissue and initiate secondary tumors [5]. The metastatic capacity of the cancer cells is highly dependent on the cancer cell phenotype, but typically only about 0.01% of the cells disseminating from the primary tumor form metastases. Therefore, the detection of disseminated cancer cells in the vasculature could be an early indicator of the potential of a particular primary tumor to become metastatic.
Cancer cells that have invaded the interstitium surrounding a tumor can access the lymphatic circulation. This process is enabled by the absence of tight junctions between the cells forming lymphatic capillaries and the overall high permeability of lymphatic vessels, allowing for relatively easy passage of cancer cells into the lymphatic system [6,7]. Previous studies [8,9] have shown that the presence of the metastatic cells in the sentinel lymph node (SLN), which is the first lymph node to which a primary tumor drains, provides one of the earliest signs of cancer metastasis. Clinical studies have demonstrated that cancer metastasis happens in an orderly manner with cancer cells first proliferating at the local site, then spreading through the lymphatic system before being disseminated by the vascular system [10]. However, 20% of metastases bypass the lymphatic system and disseminate directly via the vascular system as circulating tumor cells (CTCs) [10]. Hence, detection of indicators of cancer invasion and metastasis into both the lymphatic and vascular systems is crucial to image and treat cancer metastasis.
Tumors consist of a heterogeneous mixture of cell phenotypes with different capacities for invading the host system and forming metastases in remote organs [11]. While cancer cells have the ability to change their phenotype during the progression of cancer, this change in phenotype is dependent on the local micro-environment, including the presence of various tumor–stroma interactions [12], angiogenesis and lymphangiogenesis, matrix metalloproteinases [13–15] and growth factors [6,16,17]. Growth factors modulate the tumor–stroma interactions, which in turn can determine the fate of invasion and metastasis. Growth factor receptors [18–20], as well as biomarkers of angiogenesis [21–23] and lymphangiogenesis [24,25], are recognized as therapeutic targets for metastasis.
Clinical detection of cancer invasion & early-stage metastasis
In clinical practice, medical imaging technologies combined with invasive pathology techniques are relied upon to provide critical information about the extent of metastatic invasion to accurately determine cancer stage and to assess therapeutic impact. Clinical assessment of early cancer invasion and metastasis uses computed tomography (CT) and MRI to image the anatomical size and physical features of lymph nodes, with enlargement indicating possible lymphatic invasion [26,27]. However, the anatomical information provided by these imaging methods offers insufficient diagnostic sensitivity. An invasive biopsy, followed by time-consuming immunohistology and analysis, is still required to determine the extent of metastasis within the lymphatic system. Ultrasound, as a nonionizing and real-time imaging alternative, can be combined with ultrasound-guided fine needle aspiration of lymph node tissue, but this method has low sensitivity to detect small metastatic deposits (<5 mm) and micrometastases (<2 mm) [28]. PET using the functional radionuclide fluorine-18 fluorodeoxy-glucose is highly sensitive for the detection of metastasis greater than 80 mm3, but at a higher cost than MRI and CT alone, and with a significant loss in spatial resolution [29]. For clinical assessment of the nodal status of breast cancer and melanoma patients, optical imaging of vital blue dye (e.g., isosulfan blue) and lymphoscintigraphy using a radionuclide contrast agent are combined to provide a 2D map of the lymph nodes intraoperatively [26]. Lymphoscintigraphy is a highly sensitive technique, but its poor spatial resolution necessitates visual coregistration using blue dye. Since lymphoscintigraphy suffers from very poor temporal resolution, the near infrared fluorescent dye indocyanine green (ICG) has been applied in clinical trials and shown to provide increased sensitivity, along with higher temporal and spatial resolution in comparison to lymphoscintigraphy [30,31]. Limitations to this approach include the low quantum efficiency of ICG, and the lack of functional groups on ICG to allow conjugation to targeting moieties [31]. While lymphoscintigraphy and optical methods enable the identification of the SLN, the node must still be biopsied for subsequent laboratory immunohistological processing and analysis [26]. An intraoperative biopsy would enable an immediate decision regarding whether to remove ancillary lymph nodes during the same operation, avoiding a second invasive surgery. Optical imaging methods (intravital microscopy [32], and multiphoton microscopy [33]) have been explored preclinically and could be used for the detection of tumor cells in resected lymph nodes, but optical techniques are limited by insufficient spatial resolution at significant tissue depths.
Clinical detection methods capable of detecting the hematologic spread of cancer are lacking given that the correlation of the early detection of CTCs to overall patient survival is more significant in comparison to the correlation between the presence of lymphatic spread of metastases, detected using traditional radiology techniques, and overall patient survival [34]. Clinically, labor-intensive and time-consuming laboratory separation techniques, including centrifugation, filtration and magnetic enrichment, followed by immunological assays and microscopic analyses of the captured CTCs, are relied upon to provide information for cancer staging [35]. Detection of CTCs is challenging due to the low number of CTCs present in the background of normal blood cells, and the quantity of CTCs is likely to change with time, reflecting the discontinuous process of cell migration from a tumor into the vasculature. Thus, current laboratory methods are providing an incomplete picture of the CTC count of a particular patient and therapeutic scenario. The ability to perform in vivo longitudinal studies of CTC count could greatly improve the accuracy of cancer prognosis and provide significant improvements in therapy [36].
New techniques aimed at improving the detection of cancer invasion and early metastasis should have high specificity and sensitivity in a minimally invasive system with high spatial and temporal resolution. Many preclinical optical methods for in vivo imaging of cancer metastasis have been investigated, including multiphoton microscopy [33] and fluorescent detection [27], but typically these methods require ballistic light transport to achieve high resolution, limiting the effective imaging depth to less than 1 cm. Therefore, most optical techniques to detect cancer metastasis have been applied as intravital microscopy methods [32]. For most light-based imaging techniques, contrast agents will likely be required to achieve the needed sensitivity to be capable of detecting small numbers of cells in either the lymphatics or vasculature. Longer term, there is great potential to combine imaging techniques providing anatomical information with advanced imaging methods providing molecular and functional information, enabling the translation of basic cellular and molecular insights to clinical use [27]. The development of imaging and detection methods aimed at improving the detection of malignant cell invasion and resulting metastases would greatly improve patient diagnosis, treatment and survival.
PAs to assess the lymphatic spread of cancer
PA imaging has the potential to provide noninvasive, real-time detection of anatomical and physiological changes indicating cancer invasion and metastasis. During PA imaging, signals generated from the PA effect, consisting of pressure waves generated by the thermoelastic expansion and relaxation of a material after transient light absorption and heating, are collected by an ultrasound transducer and processed to generate images [37,38]. As with optical imaging, PA imaging can be performed spectroscopically by varying the wavelength of laser light, producing PA signals whose strength correlates to the extinction coefficient of the optical absorber [39,40]. Unlike optical imaging, the resolution of PA imaging is not greatly impacted by optical scattering, enabling the generation of high resolution PA images at several centimeters depth within highly scattering tissue [41]. High-resolution PA imaging of endogeneous chromophores within tissue, including hemoglobin and lipids, has been demonstrated [42,43]. To increase image contrast, a variety of exogenous contrast agents have been utilized, including dyes and metallic plasmonic nanoparticles [44–47]. The use of bioconjugated or chemically responsive contrast agents can provide many unique opportunities for real-time, in vivo functional and molecular imaging [40,48].
PA imaging could provide a minimally invasive alternative to the surgical techniques currently required to locate and biopsy SLNs. Lymphatics do not possess endogenous optically absorbing molecules capable of generating PA signal; therefore, contrast agents are typically introduced. During in vivo studies using rats, a PA imaging system successfully identified SLNs using the optically absorbing contrast agent methylene blue [49]. Carbon nanotubes can also be used as PA contrast agents, demonstrating good accumulation rates within the lymph node (90 min to achieve the maximum signal) and good contrast to noise ratios [50]. However, the wideband absorption of carbon nanotubes prevents their spectroscopic distinction from the surrounding endogenous tissues.
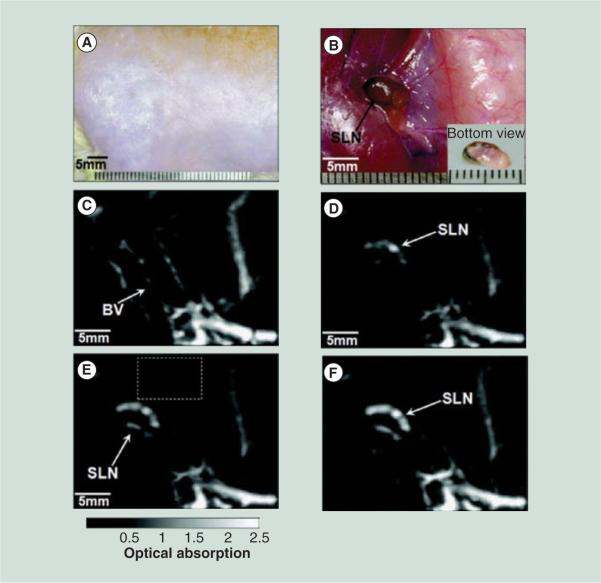
A nano-sized PA contrast agent with a high optical absorption within the near-infrared tissue optical window can be administered to further improve contrast between the lymph vessels and the surrounding tissue. Plasmonic metallic nanoparticles, having optical absorption cross-sections 5–9 orders of magnitude higher than optically absorbing dyes [51], have demonstrated contrast enhancement during PA imaging [39,52–54]. Gold nanoparticles may present toxicity and accumulation hazards [55,56]. Alternative methods of synthesizing plasmonic metallic nanoparticles, including the assembly of biodegradable clusters of primary seed nanoparticles to encourage clearance of the gold nanoparticles from the body by the kidneys, could reduce or even eliminate these hazards [57]. In comparison to molecules or small particles (less than 11 nm), which are quickly transported to lymphatic vessels and cleared from the body, nanoparticles may be retained longer in the lymphatics, allowing for increased contrast and improved imaging capabilities [58]. Particles up to 100 nm in size can be extravasated from the vasculature into the interstitial space and subsequently phagocytosed by macrophages, which travel to the lymph nodes, while particles larger than 100 nm will remain trapped in the interstitium [59]. The size, shape and surface chemistry of the nanoparticles can be tuned to provide optimal delivery and retention kinetics within SLNs, improving detection sensitivity, and reducing the false-positive identification of echelon lymph nodes. Imaging of the accumulation of gold nanorods [60], gold nanocages [61,62], and gold nanobeacons [63] within the lymphatics has been demonstrated (Figure 1). Concerns about the toxicity of metallic nanoparticles has led to the investigation of alternative contrast agents, including enzymatically biodegradable porphysome nanovesicles [64], dye-loaded perfluorocarbon nanoparticles [65] and phospholipid nanoparticles containing copper [66]. Additionally, fluorescent ICG can be used as a contrast agent for multimodal PA and fluorescent imaging [67,68]. For optimal integration of PA and fluorescent imaging, contrast agents must convert light into both heat and fluorescence. Since these are unique energy-relaxation pathways, an increase in PA efficiency corresponds to a decrease in fluorescence efficiency. While a single element transducer-based PA imaging system provides high spatial resolution, the significant length of time required to acquire an image of a small region limits its use in a clinical setting. Recent efforts have been made to address this issue, starting with a 2D array ultrasound probe with laser light delivery to improve image acquisition time to 10 min (2.3 × 2.5 × 5 cm volume) [69–71]. Additionally, a multimodal contrast agent, radiolabeled methylene blue, has been used for combined PA imaging and single-photon emission CT. This approach could streamline clinical studies by colocalizing the contrast agent for single-photon emission CT and PA imaging, to provide corroboration of the PA data [72].
Figure 1. In vivo noninvasive photoacoustic images showing accumulation of gold nanocages within a rat sentinel lymph node.
Photograph of axillary region of rat before (A) and after, with skin removed, (B) the photoacoustic images had been recorded. Photoacoustic images acquired before (C) and at 5 min (D), 59 min (E) and 140 min (F) after the nanocage injection.
BV: Blood vessels; SLN: Sentinel lymph node.
Reproduced with permission from [61] © American Chemical Society (2009).
While the above techniques can identify the locations of SLNs noninvasively, ultimately PA imaging could be developed to detect micrometastases in SLNs, avoiding the biopsy entirely. Toward this goal, ex vivo porcine SLNs were implanted with as few as 500 melanin-containing cells, and it was verified that PA signal was generated when laser light at 532 nm was absorbed by the cells [73]. Limitations of this work include the absence of blood and surface tissue during the imaging, reducing relevance of the results for in vivo situations, and that an exogenous contrast agent, such as those detailed above, would likely need to be introduced to label metastatic cells. Alternatively, this technique could be viable as an intraoperative method, to indicate whether a full lymphadenectomy is required immediately following the biopsy of an identified SLN. PA methods have imaged a resected human lymph node with metastasized melanoma, as shown in Figure 2 [74]; however, fundamental development of the technique is still in progress. To fully integrate SLN mapping with noninvasive methods to assess node status, thereby avoiding a biopsy entirely, recent work has utilized a metastatic orthotopic mouse model of oral cancer [75]. After injection of gold nanospheres targeted to overexpressed surface receptors found on the metastatic cells, PA images positively identified targeted cancer cells that had metastasized to the lymph node. PA imaging studies of gold nanoparticles endocytosed by mesenchymal stem cells have been able to distinguish as few as 200 cells within a tissue phantom [76], indicating PA imaging can provide sufficient sensitivity to detect micrometastases when used in combination with a contrast agent. Additionally, PA imaging of micrometastatic target organs, such as the highly vascularized liver, can be achieved through the use of more sophisticated PA signal generation and processing techniques [77–79].
Figure 2. Images of a resected human lymph node showing photoacoustic signal corresponding to locations within the tumor that contain melanoma cells.
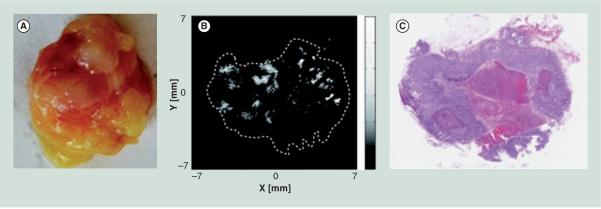
(A) Photograph of the resected lymph node. (B) Photoacoustic signal acquired from the approximate center of the node. (C) Hematoxylin and eosin stained histopathological section of the approximate center of the node, with the dark regions indicating melanoma cells and the light region in the center indicating an area of necrosis.
Reproduced with permission from [74] © Society of Photo Optical Instrumentation Engineers (2011).
PAs for detecting the hematologic spread of cancer
During the development of cancer metastasis, CTCs are typically present in the cardiovascular system before the establishment of detectable metastatic tumors. Therefore, clinical methods to detect CTCs could provide earlier indications of metastasis as well as a metric for patient responsiveness to therapy. For the detection of the hematologic spread of cancer, an indication of the presence or absence of a CTC could be sufficient, making the formation of an image of the tissue area unnecessary, reducing the time to process signals and eliminating the need for expert image analysis. The sensitivity and accuracy of a PA-based CTC detection system will depend heavily upon both the instrumentation and contrast agent development. Flow cytometry-based PA methods have been developed for ex vivo analysis of blood samples containing CTCs with endogenous melanin. Owing to the limited number of endogenous chromophores present in the body, EGFR-labeled black latex microspheres or gold nanoparticles targeted to breast cancer [80], melanoma [81,82] and prostate cancer CTCs [83] were used to improve PA contrast. In the described ex vivo detection method, significant cost and time may still be incurred due to blood sampling and preprocessing steps. Additionally, there are inherent limitations to the sensitivity of an ex vivo method, since there is a limit to how much blood can be sampled.
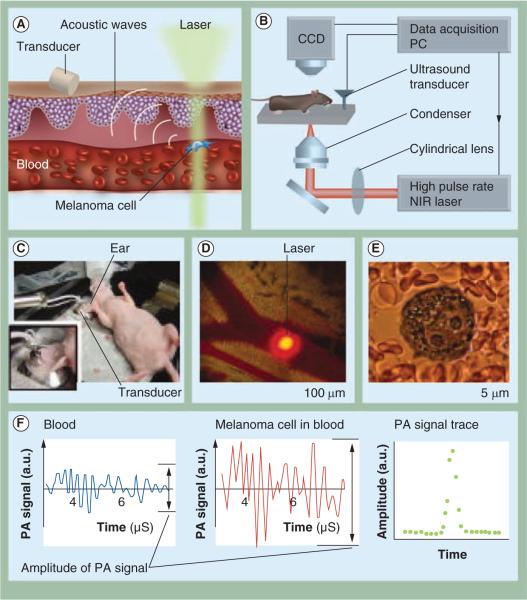
PA signal detection could provide a continuous, longitudinal and highly sensitive method to identify CTCs in vivo. Blood circulates throughout the human vasculature within approximately 1 h (assuming a blood vessel of 3 mm with a flow rate of 100 ml/min) [84]; in theory, the entire blood volume could be screened in a relatively short period of time. Toward this goal, cancer cells that had nonspecifically endocytosed gold nanorods or ICG were injected into the circulation of a mouse and signals from single cells were detected in microscopically visible lymph vessels in the ear and mesentery [85,86]. Owing to the temporal resolution, 1000 cells within the mouse blood circulation were necessary to positively detect the labeled circulating cells, but this sensitivity was improved by using a higher repetition frequency laser to image circulating metastatic melanoma cells, as shown in Figure 3 [87], with a demonstrated capability of detecting 1 CTC/ml of blood volume [88]. Within the larger volume of CTCs, stem CTCs may be present and methods to specifically target and detect these CTCs have also been demonstrated [89]. CTCs may also be present in agglomerations with platelets, termed circulating tumor microemboli, or cell surface markers may change in the circulation, for example, through epithelial to mesenchymal transformation, making labeled detection of CTCs complex [55]. The generation of PA signals from CTCs also demonstrates the prospective use of this system for the noninvasive and real-time identification of CTCs in vivo. It is noted that PA flow cytometry concept has been applied to the detection of CTCs within the lymphatics [90,91]. However, the sensitivity of CTC detection within the lymphatics may be hindered by slow lymphatic flow. While currently published studies have not shown the acquisition of PA signals originating from CTCs at a significant tissue depth, it is very conceivable that appropriately bioconjugated contrast agents could be injected intravenously and used to target specific CTCs, improving the sensitivity of the PA detection method. This requires both an integration of understanding of the biomarkers variably expressed on the surface of the CTCs and improvements to the currently used PA detection systems.
Figure 3. Description of an in vivo photoacoustic flow cytometer.
The principle of operation for the detection of melanoma circulating tumor cells (A), the instrumentation set up (B), the in vivo experimental set up (C) and image of a mouse ear blood vessel and the laser beam (D), an image of a melanoma cell within the vessel (E) and the resulting photoacoustic signals (F) from blood (left) from a single B16F10 melanoma cell within blood (middle), and the photoacoustic signal trace in the presence of a melanoma cell (right) (F) are all shown.
CCD: Charge-coupled device; NIR: Near-Infrared; PA: Photoacoustic; PC: Personal computer.
(A)&(B): Modified with permission from [87]. (C)–(F): Reproduced with permission from [87] © International Society for Advancement of Cytometry (2011).
Expert commentary
The use of PA-based imaging and detection techniques to determine the extent of cancer invasion and metastasis within the lymphatic and vascular systems could provide clear clinical benefit. While it is unlikely that any single imaging modality would be appropriate for all oncological imaging, there are specific characteristics of PA imaging well-suited to solve many clinical imaging challenges. PA detection methods provide good spatial and temporal resolution at significant depth, utilizing a variety of optically absorbing contrast agents, as demonstrated by the PA identification of SLNs and CTCs. In comparison to alternative optical methods, such as two photon microscopy or fluorescent imaging with dyes or quantum dots, it is anticipated that PA imaging of the cancer metastatic processes will be less invasive, since the removal of external tissue layers will not be required to image deep vasculature and lymphatics. Challenges include the difficulty of performing full-body PA imaging and the current absence of clinical PA imaging systems. Optically absorbing chromophores, which are absent in most metastatic cell types, are needed to generate PA contrast; therefore, the development of appropriately targeted contrast agents will be critical for the successful implementation of PA techniques to detect metastasis. Like ultrasound imaging, PA imaging is not ideal for imaging in materials with high acoustic impedance, such as for the detection of metastases in bone. PA imaging presents many opportunities to improve the detection of the spread of cancer, providing high in vivo sensitivity and resolution within highly vascularized tumors and surrounding tissues, and within the vasculature and lymphatics themselves.
Five-year view
While PA methods for the detection of cancer invasion and metastasis has thus far focused on anatomical information, used for the identification of SLNs and CTCs, PA imaging is capable of providing functional and molecular information as well. In combination with appropriate in vivo models, functional and molecular PA imaging and detection could provide new knowledge of the underlying biological processes leading to invasion and metastasis. To enable molecular PA detection, contrast agents can be specifically tailored to molecular targets of relevance to cancer metastasis, including those biomarkers expressed during lymphangiogenesis and those expressed by CTCs. Multiwavelength PA imaging of contrast agents with differing optical absorption spectra, targeted to unique cell types, can enable the generation of multiplex molecular images [39,40]. The identification of biomarkers relevant to lymphangiogenesis [53] or vascular angiogenesis could provide critical molecular information about the status of the cancer invasion and metastasis. Newly identified lymph biomarkers, including Prox-1, podoplanin and lymphatic endothelial specific hyaluronan receptor-1 [7], may prove to be important targets to determine the status of cancer metastasis and metastatic potential. The high temporal and spatial resolution of PA imaging make it particularly useful to visualize the transport of contrast agents within the lymphatics, which could lead to systematic contrast agent improvements, as well as refined methods to study cancer invasion and metastasis in vivo. For example, the ability to perform functional imaging of the lymphatics may result in the discovery of additional criteria, such as lymph flow velocity, which could be used to improve accuracy of cancer staging. Critically, PAs is capable of imaging the vasculature and lymphatic systems simultaneously, enabling the investigation of their interconnected roles in the invasion and metastasis of cancer [92].
The introduction of multimodal imaging and therapy systems could also provide improved detection and treatment of metastatic cancer. The combination of PA and ultrasound imaging will likely create the first multimodal imaging systems to achieve clinical usage within the cancer and metastasis field, due to the similarities in ultrasound and PA signal acquisition and processing. PA imaging could also be combined with optical imaging techniques to provide complementary high-resolution images at a few millimeters depth, or with functional PET imaging to provide higher sensitivity. Since the generation of PA signal originates from optical absorption and heating effects, a natural synergy exists between PA imaging and photothermal therapy. Researchers have incorporated PA imaging and laser therapy within in vivo animal models and demonstrated their ability to locate and ablate cancer cells [53,93]. PA imaging may be best applied clinically as a multimodal system capable of both imaging and eradicating the invading cancer cells.
Ultimately, the success of PA medical imaging depends upon the development of clinical PA imaging systems. Initial steps toward this objective have been taken, but sources and improved integration of the light source with the PA transducer is necessary to produce a system that is accepted in the clinic for the detection of cancer metastasis.
Key issues
Indicators of cancer metastatic potential are directly correlated to patient prognosis and survival, but this area of cancer diagnosis is underserved by the existing clinical imaging modalities.
Photoacoustic imaging provides high contrast, noninvasive, nonionizing and real-time imaging. These features are advantageous for imaging biological markers of the early stages of metastasis, including circulating tumor cells and lymphatic micrometastases.
The development of photoacoustic imaging has progressed from microscopy-based single element transducer systems to clinical arrays to provide rapid image acquisition.
Frontiers in molecular and functional in vivo imaging of cancer invasion remain to be explored, but photoacoustic imaging is uniquely suited to development in this area.
Improved targeting methods and improved understanding of the temporal and spatial variation of biomarker expression will enable sophisticated contrast agent design and enhanced imaging of lymphatics, micrometastases and circulating tumor cells, leading to improvements in the detection and treatment of cancer metastases.
Acknowledgments
The authors would like to acknowledge support from the NIH under grants CA159913 (CL Bayer) and EB008101.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Mallidi S, Luke GP, Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011;29(5):213–221. doi: 10.1016/j.tibtech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review of the current progress toward photoacoustic (PA) imaging of cancer.
- 2.Duffy MJ. Inhibiting tissue invasion and metastasis as targets for cancer therapy. Biotherapy. 1992;4(1):45–52. doi: 10.1007/BF02171709. [DOI] [PubMed] [Google Scholar]
- 3.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80(Suppl. 8):1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]; • Overview of the mechanisms of cancer metastasis.
- 4.Dudek A, Gupta K, Ramakrishnan S, Mukhopadhyay D. Tumor angiogenesis. J. Oncol. 2010;2010:761671. doi: 10.1155/2010/761671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kufe DW, Pollock RE, Weichselbaum RR, et al. Cancer Medicine. 6th Edition Decker Publishing Inc; ON, USA: 2003. Invasion and metastases. [Google Scholar]
- 6.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438(7070):946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 7.Duong T, Koopman P, Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J. Oncol. 2012;2012:204946. doi: 10.1155/2012/204946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCready DR, Yong WS, Ng AK, Miller N, Done S, Youngson B. Influence of the new AJCC breast cancer staging system on sentinel lymph node positivity and false-negative rates. J. Natl. Cancer Inst. 2004;96(11):873–875. doi: 10.1093/jnci/djh142. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y. Emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat. Rev. Cancer. 2005;5(9):735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 10.Leong SP, Cady B, Jablons DM, et al. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25(2):221–232. doi: 10.1007/s10555-006-8502-8. [DOI] [PubMed] [Google Scholar]
- 11.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217(4564):998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 12.Nannuru KC, Singh RK. Tumor-stromal interactions in bone metastasis. Curr. Osteoporos. Rep. 2010;8(2):105–113. doi: 10.1007/s11914-010-0011-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Fu X, Brown PD, Crimmin MJ, Hoffman RM. Matrix metalloproteinase inhibitor BB-94 (batimastat) inhibits human colon tumor growth and spread in a patient-like orthotopic model in nude mice. Cancer Res. 1994;54(17):4726–4728. [PubMed] [Google Scholar]
- 14.Taraboletti G, Garofalo A, Belotti D, et al. Inhibition of angiogenesis and murine hemangioma growth by batimastat, a synthetic inhibitor of matrix metalloproteinases. J. Natl. Cancer Inst. 1995;87(4):293–298. doi: 10.1093/jnci/87.4.293. [DOI] [PubMed] [Google Scholar]
- 15.Gore M, A'Hern R, Stankiewicz M, Slevin M. Tumour marker levels during marimastat therapy. Lancet. 1996;348(9022):263–264. doi: 10.1016/s0140-6736(96)24030-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, He B, Weber GF. Growth factor signaling induces metastasis genes in transformed cells: molecular connection between Akt kinase and osteopontin in breast cancer. Mol. Cell. Biol. 2003;23(18):6507–6519. doi: 10.1128/MCB.23.18.6507-6519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25(3):435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 18.Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat. Rev. Clin. Oncol. 2011;8(8):492–503. doi: 10.1038/nrclinonc.2011.45. [DOI] [PubMed] [Google Scholar]
- 19.Hurvitz SA, Hu Y, O'Brien N, Finn RS. Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat. Rev. 2012 doi: 10.1016/j.ctrv.2012.04.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki M, Yamashita Y, Kubo N, et al. Concurrent biological targeting therapy of squamous cell carcinoma of the esophagus with cetuximab and trastuzumab. Oncol. Rep. 2012;28(1):49–54. doi: 10.3892/or.2012.1803. [DOI] [PubMed] [Google Scholar]
- 21.Bertolini F, Mancuso P, Shaked Y, Kerbel RS. Molecular and cellular biomarkers for angiogenesis in clinical oncology. Drug Discov. Today. 2007;12(19–20):806–812. doi: 10.1016/j.drudis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Brown AP, Citrin DE, Camphausen KA. Clinical biomarkers of angiogenesis inhibition. Cancer Metastasis Rev. 2008;27(3):415–434. doi: 10.1007/s10555-008-9143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br. J. Cancer. 2010;102(1):8–18. doi: 10.1038/sj.bjc.6605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van den Eynden GG, Vandenberghe MK, van Dam PJ, et al. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin. Cancer Res. 2007;13(18 Pt 1):5391–5397. doi: 10.1158/1078-0432.CCR-07-1230. [DOI] [PubMed] [Google Scholar]
- 25.Nagahashi M, Ramachandran S, Rashid OM, Takabe K. Lymphangiogenesis: a new player in cancer progression. World J. Gastroenterol. 2010;16(32):4003–4012. doi: 10.3748/wjg.v16.i32.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett T, Choyke PL, Kobayashi H. Imaging of the lymphatic system: new horizons. Contrast Media Mol. Imaging. 2006;1(6):230–245. doi: 10.1002/cmmi.116. [DOI] [PubMed] [Google Scholar]
- 27.Condeelis J, Weissleder R. In vivo imaging in cancer. Cold Spring Harb. Perspect. Biol. 2010;2(12):a003848. doi: 10.1101/cshperspect.a003848. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review of the contributions of imaging towards the understanding cancer progression and metastasis.
- 28.Thompson JF, Haydu LE, Sanki A, Uren RF. Ultrasound assessment of lymph nodes in the management of early-stage melanoma. J. Surg. Oncol. 2011;104(4):354–360. doi: 10.1002/jso.21963. [DOI] [PubMed] [Google Scholar]
- 29.Yang WT, Le-Petross HT, Macapinlac H, et al. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res. Treat. 2008;109(3):417–426. doi: 10.1007/s10549-007-9671-z. [DOI] [PubMed] [Google Scholar]
- 30.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 2009;16(10):2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen JC, Tan IC, Marshall MV, Fife CE, Sevick-Muraca EM. Lymphatic imaging in humans with near-infrared fluorescence. Curr. Opin. Biotechnol. 2009;20(1):74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timpson P, Serrels A, Canel M, Frame MC, Brunton VG, Anderson KI. Quantitative real-time imaging of molecular dynamics during cancer cell invasion and metastasis in vivo. Cell Adh. Migr. 2009;3(4):351–354. doi: 10.4161/cam.3.4.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provenzano PP, Eliceiri KW, Keely PJ. Shining new light on 3D cell motility and the metastatic process. Trends Cell Biol. 2009;19(11):638–648. doi: 10.1016/j.tcb.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging – predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 2006;12(21):6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 35.Panchapakesan B, Caprara R, Velasco V, et al. Micro- and nanotechnology approaches for capturing circulating tumor cells. Cancer Nanotechnol. 2010;1(1–6):3–11. doi: 10.1007/s12645-010-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riethdorf S, Pantel K. Advancing personalized cancer therapy by detection and characterization of circulating carcinoma cells. Ann. N. Y. Acad. Sci. 2010;1210:66–77. doi: 10.1111/j.1749-6632.2010.05779.x. [DOI] [PubMed] [Google Scholar]
- 37.Kruger RA. Photoacoustic ultrasound. Med. Phys. 1994;21(1):127–131. doi: 10.1118/1.597367. [DOI] [PubMed] [Google Scholar]
- 38.Diebold GJ, Beveridge AC, Hamilton TJ. The photoacoustic effect generated by an incompressible sphere. J. Acoust. Soc. Am. 2002;112(5 Pt 1):1780–1786. doi: 10.1121/1.1508788. [DOI] [PubMed] [Google Scholar]
- 39.Li PC, Wang CR, Shieh DB, et al. In vivo photoacoustic molecular imaging with simultaneous multiple selective targeting using antibody-conjugated gold nanorods. Opt. Express. 2008;16(23):18605–18615. doi: 10.1364/oe.16.018605. [DOI] [PubMed] [Google Scholar]
- 40.Bayer CL, Chen YS, Kim S, Mallidi S, Sokolov K, Emelianov S. Multiplex photoacoustic molecular imaging using targeted silica-coated gold nanorods. Biomed. Opt. Express. 2011;2(7):1828–1835. doi: 10.1364/BOE.2.001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su JL, Wang B, Wilson KE, et al. Advances in clinical and biomedical applications of photoacoustic imaging. Expert Opin. Med. Diagn. 2010;4(6):497–510. doi: 10.1517/17530059.2010.529127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003;21(7):803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 43.Oraevsky AA, Karabutov AA. Optoacoustic tomography. In: Vo-Dinh T, editor. Biomedical Photonics Handbook. CRC Press; FL, USA: 2003. [Google Scholar]
- 44.Oraevsky AA, Karabutov AA, Savateeva EV. Enhancement of optoacoustic tissue contrast with absorbing nanoparticles. Proc. SPIE. 2001;4434:60–69. [Google Scholar]
- 45.Wang XD, Ku G, Xie XY, et al. Laser-induced photoacoustic tomography enhanced with an optical contrast agent. Proc. SPIE. 2004;5320:77–82. [Google Scholar]
- 46.Mallidi S, Larson T, Aaron J, Sokolov K, Emelianov S. Molecular specific optoacoustic imaging with plasmonic nanoparticles. Opt. Express. 2007;15(11):6583–6588. doi: 10.1364/oe.15.006583. [DOI] [PubMed] [Google Scholar]; • First demonstration of molecular PA imaging.
- 47.Luke GP, Yeager D, Emelianov SY. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012;40(2):422–437. doi: 10.1007/s10439-011-0449-4. [DOI] [PubMed] [Google Scholar]
- 48.Kim S, Chen YS, Luke GP, Emelianov SY. In vivo three-dimensional spectroscopic photoacoustic imaging for monitoring nanoparticle delivery. Biomed. Opt. Express. 2011;2(9):2540–2550. doi: 10.1364/BOE.2.002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song KH, Stein EW, Margenthaler JA, Wang LV. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008;13(5):054033. doi: 10.1117/1.2976427. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First publication demonstrating detection of sentinel lymph nodes using a PA imaging system.
- 50.Pramanik M, Song KH, Swierczewska M, Green D, Sitharaman B, Wang LV. In vivo carbon nanotube-enhanced non-invasive photoacoustic mapping of the sentinel lymph node. Phys. Med. Biol. 2009;54(11):3291–3301. doi: 10.1088/0031-9155/54/11/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B. 2006;110(14):7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 52.Agarwal A, Huang SW, O'Donnell M, et al. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys. 2007;102(6):064701. [Google Scholar]
- 53.Kim JW, Galanzha EI, Shashkov EV, Moon HM, Zharov VP. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat. Nanotechnol. 2009;4(10):688–694. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Homan K, Kim S, Chen YS, Wang B, Mallidi S, Emelianov S. Prospects of molecular photoacoustic imaging at 1064 nm wavelength. Opt. Lett. 2010;35(15):2663–2665. doi: 10.1364/OL.35.002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 56.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 2011;40(3):1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 57.Tam JM, Tam JO, Murthy A, et al. Controlled assembly of biodegradable plasmonic nanoclusters for near-infrared imaging and therapeutic applications. ACS Nano. 2010;4(4):2178–2184. doi: 10.1021/nn9015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma R, Wendt JA, Rasmussen JC, Adams KE, Marshall MV, Sevick-Muraca EM. New horizons for imaging lymphatic function. Ann. NY Acad. Sci. 2008;1131:13–36. doi: 10.1196/annals.1413.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moghimi SM, Rajabi-Siahboomi R. Advanced colloid-based systems for efficient delivery of drugs and diagnostic agents to the lymphatic tissues. Prog. Biophys. Mol. Biol. 1996;65(3):221–249. doi: 10.1016/s0079-6107(96)00012-0. [DOI] [PubMed] [Google Scholar]
- 60.Song KH, Kim C, Maslov K, Wang LV. Noninvasive in vivo spectroscopic nanorod-contrast photoacoustic mapping of sentinel lymph nodes. Eur. J. Radiol. 2009;70(2):227–231. doi: 10.1016/j.ejrad.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song KH, Kim C, Cobley CM, Xia Y, Wang LV. Near-infrared gold nanocages as a new class of tracers for photoacoustic sentinel lymph node mapping on a rat model. Nano Lett. 2009;9(1):183–188. doi: 10.1021/nl802746w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai X, Li W, Kim CH, Yuan Y, Wang LV, Xia Y. In vivo quantitative evaluation of the transport kinetics of gold nanocages in a lymphatic system by noninvasive photoacoustic tomography. ACS Nano. 2011;5(12):9658–9667. doi: 10.1021/nn203124x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan D, Pramanik M, Senpan A, et al. Near infrared photoacoustic detection of sentinel lymph nodes with gold nanobeacons. Biomaterials. 2010;31(14):4088–4093. doi: 10.1016/j.biomaterials.2010.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lovell JF, Jin CS, Huynh E, et al. Porphy-some nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011;10(4):324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 65.Akers WJ, Kim C, Berezin M, et al. Noninvasive photoacoustic and fluorescence sentinel lymph node identification using dye-loaded perfluorocarbon nanoparticles. ACS Nano. 2011;5(1):173–182. doi: 10.1021/nn102274q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan D, Cai X, Yalaz C, et al. Photoacoustic sentinel lymph node imaging with self-assembled copper neodecanoate nanoparticles. ACS Nano. 2012;6(2):1260–1267. doi: 10.1021/nn203895n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim C, Song KH, Gao F, Wang LV. Sentinel lymph nodes and lymphatic vessels: noninvasive dual-modality in vivo mapping by using indocyanine green in rats – volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology. 2010;255(2):442–450. doi: 10.1148/radiol.10090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim C, Song KH, Wang LV. In vivo dual-modality imaging of lymphatic systems using indocyanine green in rats: three-dimensional photoacoustic imaging and planar fluorescence imaging. SPIE Photonics West, Photons Plus Ultrasound: Imaging and Sensing; San Francisco, CA, USA. 23 February 2010. [Google Scholar]
- 69.Erpelding TN, Kim C, Pramanik M, et al. In vivo photoacoustic and ultrasonic mapping of rat sentinel lymph nodes with a modified commercial ultrasound imaging system. SPIE Photonics West, Photons Plus Ultrasound: Imaging and Sensing; San Francisco, CA, USA. 24 January 2010. [Google Scholar]
- 70.Erpelding TN, Kim C, Pramanik M, et al. Sentinel lymph nodes in the rat: noninvasive photoacoustic and US imaging with a clinical US system. Radiology. 2010;256(1):102–110. doi: 10.1148/radiol.10091772. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Integration of PA imaging with a clinical US system for the identification of sentinel lymph nodes.
- 71.Kim C, Erpelding TN, Jankovic L, Wang LV. Performance benchmarks of an array-based hand-held photoacoustic probe adapted from a clinical ultrasound system for non-invasive sentinel lymph node imaging. Philos. Transact. A. Math. Phys. Eng. Sci. 2011;369(1955):4644–4650. doi: 10.1098/rsta.2010.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akers WJ, Edwards WB, Kim C, et al. Multimodal sentinel lymph node mapping with single-photon emission computed tomography (SPECT)/computed tomography (CT) and photoacoustic tomography. Transl. Res. 2012;159(3):175–181. doi: 10.1016/j.trsl.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCormack D, Al-Shaer M, Goldschmidt BS, et al. Photoacoustic detection of melanoma micrometastasis in sentinel lymph nodes. J. Biomech. Eng. 2009;131(7):074519. doi: 10.1115/1.3169247. [DOI] [PubMed] [Google Scholar]
- 74.Jose J, Grootendorst DJ, Vijn TW, et al. Initial results of imaging melanoma metastasis in resected human lymph nodes using photoacoustic computed tomography. J. Biomed. Opt. 2011;16(9):096021. doi: 10.1117/1.3631705. [DOI] [PubMed] [Google Scholar]; • Use of PA imaging to identify metastasis in ex vivo human lymph nodes as an intraoperative detection method.
- 75.Luke GP, Papagiannaros A, Tam JO, Sokolov K, Emelianov SY. Noninvasive detection of sentinel lymph node metastasis using molecular photoacoustic imaging. Wold Molecular Imaging Congress; San Diego, CA. September 8, 2011. [Google Scholar]
- 76.Nam SY, Ricles LM, Suggs LJ, Emelianov SY. In vivo ultrasound and photoacoustic monitoring of mesenchymal stem cells labeled with gold nanotracers. PLoS ONE. 2012;7(5):e37267. doi: 10.1371/journal.pone.0037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Razansky D, Vinegoni C, Ntziachristos V. Multispectral photoacoustic imaging of fluorochromes in small animals. Opt. Lett. 2007;32(19):2891–2893. doi: 10.1364/ol.32.002891. [DOI] [PubMed] [Google Scholar]
- 78.Mallidi S, Larson T, Tam J, et al. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009;9(8):2825–2831. doi: 10.1021/nl802929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen YS, Xu D, Frey W, Emelianov SY. Silica-coated gold nanorods optimized for 1064-nm photoacoustic molecular imaging. SPIE Photonics West, Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications; San Francisco, CA, USA. 23–25 January 2012. [Google Scholar]
- 80.Thomas TS, Dale PS, Weight RM, Atasoy U, Magee J, Viator JA. Photoacoustic detection of breast cancer cells in human blood. SPIE Photonics West, Photons Plus Ultrasound: Imaging and Sensing; San Francisco, CA, USA. 20 January 2008. [Google Scholar]
- 81.McCormack DR, Bhattacharyya K, Kannan R, Katti K, Viator JA. Enhanced photoacoustic detection of melanoma cells using gold nanoparticles. Lasers Surg. Med. 2011;43(4):333–338. doi: 10.1002/lsm.21060. [DOI] [PubMed] [Google Scholar]
- 82.McCormack DR, Bhattacharyya K, Kannan R, Katti K, Viator JA. Enhanced detection of circulating melanoma cells using gold nanoparticles as photoacoustic contrasting agents. SPIE Photonics West, Photons Plus Ultrasound: Imaging and Sensing; San Francisco, CA, USA. 24 January 2010. [Google Scholar]
- 83.Viator JA, Gupta S, Goldschmidt BS, et al. Gold nanoparticle mediated detection of prostate cancer cells using photoacoustic flowmetry with optical reflectance. J. Biomed. Nanotechnol. 2010;6(2):187–191. doi: 10.1166/jbn.2010.1105. [DOI] [PubMed] [Google Scholar]
- 84.He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc. Natl Acad. Sci. USA. 2007;104(28):11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zharov VP, Galanzha EI, Shashkov EV, Khlebtsov NG, Tuchin VV. In vivo photoacoustic flow cytometry for monitoring of circulating single cancer cells and contrast agents. Opt. Lett. 2006;31(24):3623–3625. doi: 10.1364/ol.31.003623. [DOI] [PubMed] [Google Scholar]; •• First paper describing the detection of circulating cancer cells using PA flow cytometry.
- 86.Zharov VP, Galanzha EI, Shashkov EV, Kim JW, Khlebtsov NG, Tuchin VV. Photoacoustic flow cytometry: principle and application for real-time detection of circulating single nanoparticles, pathogens, and contrast dyes in vivo. J. Biomed. Opt. 2007;12(5):051503. doi: 10.1117/1.2793746. [DOI] [PubMed] [Google Scholar]
- 87.Nedosekin DA, Sarimollaoglu M, Ye JH, Galanzha EI, Zharov VP. In vivo ultra-fast photoacoustic flow cytometry of circulating human melanoma cells using near-infrared high-pulse rate lasers. Cytometry. A. 2011;79(10):825–833. doi: 10.1002/cyto.a.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galanzha EI, Shashkov EV, Spring PM, Suen JY, Zharov VP. In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009;69(20):7926–7934. doi: 10.1158/0008-5472.CAN-08-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galanzha EI, Kim JW, Zharov VP. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in-vivo detection and killing of circulating cancer stem cells. J. Biophotonics. 2009;2(12):725–735. doi: 10.1002/jbio.200910078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galanzha EI, Shashkov EV, Kokoshka MS, Myhill JA, Zharov VP. In vivo noninvasive detection of metastatic melanoma in vasculature and sentinel lymph nodes by photoacoustic cytometry. American Society for Laser Medicine and Surgery Twenty-Eighth Annual Conference; Kissimmee, FL, USA. 2–6 April, 2008. [Google Scholar]
- 91.Galanzha EI, Shashkov EV, Tuchin VV, Zharov VP. In vivo multispectral, multiparameter, photoacoustic lymph flow cytometry with natural cell focusing, label-free detection and multicolor nanoparticle probes. Cytometry. A. 2008;73(10):884–894. doi: 10.1002/cyto.a.20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Galanzha EI. Blood and lymph circulating cells: well-known systems, well-forgotten interdependence. J. Blood Lymph. 2011;1:e104. [Google Scholar]
- 93.Galanzha EI, Kokoska MS, Shashkov EV, Kim JW, Tuchin VV, Zharov VP. In vivo fiber-based multicolor photoacoustic detection and photothermal purging of metastasis in sentinel lymph nodes targeted by nanoparticles. J. Biophotonics. 2009;2(8–9):528–539. doi: 10.1002/jbio.200910046. [DOI] [PMC free article] [PubMed] [Google Scholar]