Abstract
Objective
Evaluate nicotinic acetycholine receptor (nAChR) single nucleotide polymorphism (SNP) association with seven day point prevalence abstinence (abstinence) in randomized clinical trials of smoking cessation therapies (RCTs) in individuals grouped by pharmacotherapy randomization to inform the development of personalized smoking cessation therapy.
Methods
We quantified association of four SNPs at three nAChRs with abstinence in eight RCTs. Participants were 2,633 outpatient treatment-seeking, self-identified European ancestry individuals smoking ≥10 cigarettes per day, recruited via advertisement, prescribed pharmacotherapy, and provided with behavioral therapy. Interventions included nicotine replacement therapy (NRT), bupropion, varenicline, placebo or combined NRT and bupropion, and five modes of group and individual behavioral therapy. Outcome measures tested in multivariate logistic regression were end of treatment (EOT) and six month (6MO) abstinence, with demographic, behavioral and genetic covariates.
Results
“Risk” alleles previously associated with smoking heaviness were significantly (P<0.05) associated with reduced abstinence in the placebo pharmacotherapy group (PG) at 6MO [for rs588765 OR (95%CI) 0.41 (0.17–0.99)], and at EOT and at 6MO [for rs1051730, 0.42 (0.19–0.93) and 0.31 (0.12–0.80)], and with increased abstinence in the NRT PG at 6MO [for rs588765 2.07 (1.11–3.87) and for rs1051730 2.54 (1.29–4.99)]. We observed significant heterogeneity in rs1051730 effects (F=2.48, P=0.021) between PGs.
Conclusions
chr15q25.1 nAChR SNP risk alleles for smoking heaviness significantly increase relapse with placebo treatment and significantly increase abstinence with NRT. These SNP-PG associations require replication in independent samples for validation, and testing in larger sample sizes to evaluate whether similar effects occur in other PGs.
Keywords: logistic regression, mediation analysis, nAChR variation, nicotine dependence, pharmacotherapy, randomized clinical trials
Introduction
Tobacco use is the largest preventable cause of death in the United States [1] and worldwide [2]. Most smokers wish to stop, and both behavioral counseling and pharmacotherapies increase abstinence rates two-to-three fold compared to placebo (PLA) abstinence rates in RCTs, though there are differences in therapy effectiveness [3]. Yet, the majority of smokers are not able to quit long-term with either behavioral therapy and/or pharmacotherapy. Thus, there is a critical need to enhance the effectiveness of smoking cessation treatments. One approach to improve cessation rates would be to identify factors that indicate which individuals will benefit most from which treatment and to develop algorithms to incorporate these factors into clinical practice. These factors could include gender, nicotine dependence, comorbidity, the rate of nicotine metabolism, pharmacogenetic variation, or combinations of factors [4,5,6,7,8,9,10,11].
Evidence that reveals interactions between smoker characteristics, medications and cessation success suggests that effective algorithms to assign medication may be possible. For example, there is evidence that the rate of nicotine metabolism predicts which smokers will be more successful at quitting with bupropion (BUP) [12] and with transdermal NRT [8,13], and that more highly dependent smokers benefit more from combination pharmacotherapies than do less dependent smokers [14]. Despite such findings, at present, no algorithm for the assignment of smoking cessation medication has been demonstrated to be useful in clinical practice and none is widely used. More research is needed on this topic. Nicotinic acetylcholine receptor (nAChR) locus single nucleotide polymorphisms (SNPs) have been related to measures of nicotine dependence [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], response to tobacco [43,44,45] and smoking cessation [23,46,47,48,49,50,51,52] and, therefore, may prove useful in optimizing assignment of smoking cessation pharmacotherapies. We choose four nAChR SNPs among many possible nAChR SNPs with a priori evidence for an association with nicotine dependence, with response to nicotine or with smoking cessation. We choose these four based on substantial and repeated a priori evidence of association with nicotine dependence and with abstinence (below). The a priori associations represented by these four nAChR SNPs are the only association signals investigated across the eight RCTs to date.
rs2072661, in the 3′ untranslated region of CHRNB2 at chr1q21.3, has been associated with: abstinence in a RCT randomizing participants to BUP or PLA; initial response to tobacco in adolescent samples; short-term abstinence in a cross-over smoking cessation trial of NRT and PLA; baseline Fagerström Test for Nicotine Dependence score among treatment-seeking smokers; and nausea among treatment-seeking smokers randomized to behavioral therapies and prescribed varenicline (VAR) [35,43,46,53,54]. Candidate gene, genome wide association studies, and meta-analytic studies with a nicotine dependence phenotype have identified three different loci represented by SNPs rs1051730, rs578776 and rs588765 at chr15q25.1 in CHRNA5 and CHRNA3 [30]. rs1051730 and correlated SNPs have been associated with nicotine dependence and lung cancer [18,19,20,22,55], abstinence [23,50], and smoking likelihood during pregnancy [48]. rs578776 and correlated SNPs have been associated with nicotine dependence [18,22,27,30] and abstinence [49]. rs588765 and correlated SNPs have been associated with nicotine dependence [27,30] and with abstinence [51]. Recent research using a single RCT has demonstrated that individuals with chr15q25.1 risk haplotypes [22,23] exhibit statistically significantly reduced abstinence when randomized to PLA versus no effect on abstinence when randomized to active pharmacotherapy [52], encouraging further exploration of chr15q25.1 associations with response to multiple pharmacotherapies and cessation outcomes in treatment-seeking smokers.
The Pharmacogenetics of Nicotine Addiction Treatment (PNAT) Consortium was formed in 2005 to identify the role of pharmacokinetic and pharmacodynamic gene variation on nicotine dependence and metabolism phenotypes, with a focus on smoking cessation and medication response, and to generate the evidence base to optimize the use of pharmacotherapies for smoking cessation. In this analysis, we conduct analyses of the association of nAChR candidate gene variation with abstinence at EOT and at 6MO after the quit date in 2,633 treatment-seeking smokers enrolled in eight RCTs of smoking cessation. We performed analyses by PG, including predictor SNP regression, sensitivity, mediation, and receiver operator curve analyses. We performed these analyses to address the questions: a) are any of the four nAChR SNPs of a priori interest significantly associated with abstinence in smokers grouped by pharmacotherapy, and b) how do the results help our understanding of the pharmacogenetic mechanisms that operate in smoking cessation?
This research employs the largest combined sample and the most comprehensive group of smoking cessation pharmacotherapies to be submitted to pharmacogenetic analyses. In our analyses, we have adjusted for trial randomization arm, participant demographics, nicotine dependence measures, and genetic covariates. This study refines previous pharmacogenetic smoking cessation associations at four nAChR SNPs of current interest, identifies novel associations of two nAChR loci on smoking cessation outcomes in individuals randomized to NRT, and identifies at least two mechanisms by which a nAChR SNP may influence abstinence. The significant SNP PG association results require testing in independent RCT arms to validate the specific PG associated effects. Additional testing in larger numbers of RCTs arms, and using multiple treatment meta-analysis techniques, may establish whether there are specific SNP associations with PGs not identified in this analysis.
Methods
Human Subjects
Informed written consent was obtained by the investigators of each RCT, and approval was obtained from the appropriate institutional review boards [56,57,58,59,60,61,62].
Data Sources, Study Selection and Phenotype Data Extraction
We utilized data from eight RCTs with participant clinical, outcome and genetic data [56,57,58,59,60,61,62] (Table 1 and Supplemental Digital Contents 1–4: Randomized clinical trial design characteristics; Behavioral and demographic variables selected for analysis; Inclusion and exclusion criteria for eight RCTs; Pharmacotherapy and behavioral therapy to EOT and 6MO of eight RCTs by randomization arm). The individuals included in the analysis represented 44% of individuals randomized to treatment in the eight RCTs, and 81% of individuals for whom we had received RCT data and biospecimens or DNA samples. Reasons for exclusion include: 1) a biospecimen was not collected [1595 (27.0%)]; 2) did not self-identify as White [1168 (19.7%)]; 3) were randomized to pharmacotherapy arms not selected for this analysis [490 (8.3%)]; 4) did not enter treatment after randomization [188 (3.2%)]; 5) DNA sample genotype completion rate was below a predetermined threshold [70 (1.2%)]; and/or chromosomal sex did not match clinical gender [22 (0.4%)].
Table 1.
RCT participant characteristics, pharmacotherapy and end of treatment and six month abstinence.
| RCT | 3A | 3B | 5 | 6A | 6B | 9A | 9B | 9C |
|---|---|---|---|---|---|---|---|---|
| Investigator | Lerman | Lerman | Swan | Hall | Hall | Baker | Baker | Baker |
| Nb | 378 | 416 | 487 | 150 | 174 | 173 | 171 | 684 |
| Age yearsb Mean (SD) | 46.7 (11.4) | 44.4 (11.5) | 49.1 (11.5) | 41.8 (9.6) | 57.3 (5.9) | 37.7 (11.2) | 41.3 (10.8) | 44.4 (11.8) |
| BMIb Mean (SD) | 27.5 (5.5) | 26.5 (4.7) | 27.8 (5.8) | 26.5 (4.7) | 26.5 (5.9) | 26.6 (5.7) | 26.7 (5.5) | 28.8 (6.7) |
| College (%) | 51.9 | 46.1 | 25.5 | 51.7 | 58.6 | 19.7 | 18.2 | 22.8 |
| Female (%) | 46.8 | 54.6 | 68.8 | 38.0 | 41.4 | 53.8 | 58.5 | 60.0 |
| Married (%) | 49.2 | 47.3 | 69.0 | 24.7 | 28.7 | 44.8 | 48.0 | 47.2 |
| FTNDb Mean (SD) | 5.55 (2.2) | 5.17 (2.1) | 5.15 (2.1) | 4.82 (2.1) | 4.87 (2.1) | 5.13 (2.4) | 5.76 (2.1) | 5.21 (2.2) |
| CPDb Mean (SD) | 23.7 (9.2) | 21.8 (9.4) | 20.2 (8.3) | 19.1 (7.4) | 20.8 (8.8) | 21.5 (8.3) | 24.1 (9.7) | 21.5 (8.8) |
| Pharmacotherapyc | NRT | BUP, PLA | VAR | NRT+BUP | NRT+BUP | BUP, PLA | BUP, PLA | NRT, BUP, PLA |
| Randomization Arms | 2 | 2 | 3 | 5 | 4 | 4 | 2 | 4 |
| EOT ABSd | 0.325 | 0.272 | 0.554 | 0.642 | 0.672 | 0.272 | 0.216 | 0.418 |
| 6MO ABSe | 0.198 | 0.219 | 0.431 | 0.460 | 0.626 | 0.145 | 0.205 | 0.317 |
N of self-identified White participants with DNA.
Mean (SD).
Nicotine Replacement Therapy (NRT), Bupropion (BUP), Placebo (PLA), Varenicline (VAR), combined NRT and BUP (NRT+BUP).
End of treatment abstinence.
6 month abstinence.
Genotyping and Genotype Data Extraction
Genomic DNA was extracted from saliva [63], whole blood or buffy coat, quantified and normalized to 50ng/ul, and genotyped at the University of Southern California Epigenome Center, and at the University of California San Francisco Institute for Human Genetics Genomics Core Facility. We extracted SNP genotype data from custom 1536 SNP Illumina GoldenGate panels interrogating candidate genes of interest to PNAT [46,64] and imputed genotype data where necessary. All genotyping included HapMap and replicate DNA samples. We reviewed and filtered GoldenGate genotyping data as described [46] for RCTs 3A and 3B and in a similar fashion for the remaining RCTs by manual review of genotype cluster metrics, review of HapMap sample concordance, by successively filtering samples and SNPs with call rates below a defined threshold, and comparison of X chromosome heterozygosity and clinical gender. We estimated principal components of population genetic variation [65] among self-identified White participants using 45 ancestry informative markers genotyped across all individuals. Genotypes were imputed with IMPUTE v2.1.2 [66] using 1000 Genomes CEU (Utah residents with ancestry from northern and western Europe) August 2010 haplotype data at CHRNB2 and chr15q25.1 (chr1:154476304-154616304 and chr15:78747906-79045112 [NCBI build 37], respectively). Imputed dosage was converted to genotypes with a 0.90 dosage probability cutoff using GTOOL v0.6.5. (http://www.well.ox.ac.uk/~cfreeman/software/gwas/gtool.html). rs2072661 and rs1051730 genotype data was extracted from GoldenGate genotyping data, and rs588765 genotype data was imputed for all RCTs. rs578776 genotype data was extracted from GoldenGate genotyping data for RCTs 3A and 3B, and imputed for the remaining RCTs. Among the expected 10,532 genotypes from four SNPs at 2,633 individuals tested for association, the overall missing genotype rate was 1.3%, while 57.0% and 41.6% were extracted from GoldenGate genotyping data or imputed, respectively. 97.7% of rs588765 and 98.8% of rs578776 imputed genotype dosage probabilities were within 10% of modal values. nAChR SNP minor allele frequencies did not differ significantly across the 26 Arms. We evaluated rs2072661 and rs1051730 genotype distributions by randomization arm and observed two arm-by-SNP strata with Hardy-Weinberg equilibrium p-values<0.05, versus 2.5 expected by chance (See Table, Supplemental Digital Content 5: nAChR SNPs counts and Hardy-Weinberg equilibrium P value, by arm).
Logistic Modeling of the Effect of SNPs on EOT and 6MO abstinence
Multiple imputation by chained equations [67] was used to impute missing values 20 times for age (two individuals), education (ten), marital status (seven), cigarettes per day (CPD) (seven), and Fagerström Test for Nicotine Dependence [68] (FTND) score (forty-two). Regression analyses were performed on each imputed data set and the results were combined with adjustment to the variance of regression parameters to reflect the additional variance attributable to the imputations [69]. Regression analyses were run for all SNPs using an additive model (and for rs2072661, with the dominant model [35,46,53,54]), and with adjustment for the other chr15q25.1 SNPs [30], when appropriate. Regression analysis was conducted with data from all 26 arms (except for rs2072661, where we excluded the two arms from the RCT that discovered the SNP association) and included variables for the SNPs, demographics [age (age and age squared), education (presence or absence of college degree), gender, marital status (married or other)], dependence measures [FTND and CPD (coded as in the FTND)], interactions with demographic variables (CPD x age, CPD x gender and FTND x gender), the first ten principal components of population genetic variation, and indicator variables for the 26 RCT arms and the PGs. These analyses were performed as regression analyses including all 2,633 individuals simultaneously, thus the number of variables is a small fraction (~2%) of the number of individuals. Regression analyses assessed the homogeneity of SNP effects between PGs, and quantified SNP effects across all PGs.
Post-hoc analyses performed and general considerations
Regression analysis of chr15q25.1 SNPs evaluated SNP effects excluding dependence covariates. Multiple mediation analyses tested whether nicotine dependence measures mediated the association of rs1051730 with 6MO abstinence, controlling for other chr15q25.1 SNPs, demographics, population genetic variation and relevant RCT arms [70]. Receiver operating characteristic (ROC) analyses of abstinence compared the contribution of nicotine dependence and genetic variables to a base model with demographic variables. Statistical analyses were performed in STATA 12.0 (StataCorp, College Station, TX, USA). Power analyses were performed using Quanto [71]. Alpha for all tests was 0.05.
Results and Discussion
Variation between RCTs
The eight RCTs exhibit similar design features and ascertainment criteria, but differ in prevalence of baseline variables and EOT and 6MO abstinence (Table 1 and see Tables, Supplemental Digital Contents 1–4). RCT 5 was conducted in a health care setting [57], and the other RCTs were conducted at Universities. All RCTs were conducted in United States metropolitan regions. Two RCTs were designed as pharmacogenetic efficacy trials [56], one RCT was designed as a comparative effectiveness trial [57], and the remaining RCTs were designed as comparative treatment efficacy trials [58,59,60,61,62]. All RCTs required ≥10 CPD and age >18 years, although one RCT was focused on older smokers [59]. All RCTs had similar exclusion criteria that included reproductive/lactation criteria for females, severe current cardiovascular, neurological, or psychiatric disorders, medical contraindications for pharmacotherapy treatment, and current use of psychiatric drugs. All RCTs provided multiple sessions of group or individual counseling, where one RCT randomized participants to web-based counseling, proactive telephone-based counseling, or both modalities [57]. Therapy randomization from baseline to EOT was to five different pharmacotherapies [NRT, BUP, PLA, VAR or combined NRT and BUP (NRT+BUP)], which could be combined with different behavioral therapies [group counseling (five or seven sessions), individual counseling (six, seven or eight sessions), and web-based counseling, proactive telephone-based counseling, or both]. Combined PG sizes at EOT were 748, 595, 479, 487, and 324, respectively. Most RCT arms received no further therapy from EOT to 6MO; individuals in the two arms that received NRT+BUP from baseline to EOT were randomized to several pharmacologic and behavioral treatments from EOT to 6MO (See Table, Supplemental Digital Content 4), resulting in a total of seven different PGs at 6MO, the five original PGs, chronic NRT and BUP (CNRT+BUP), and chronic BUP and NRT (CBUP+NRT). Combined PG sizes at 6MO were the same for the first four PGs and 161, 98, and 65, respectively, for the three NRT+BUP PGs. Seven RCTs performed biochemical verification of abstinence [56,58,59,60,61,62]. All RCTs evaluated seven day point prevalence abstinence at EOT (eight to 12 weeks post-quit), and at 6MO.
Association of nAChR SNPs with abstinence by pharmacotherapy randomization
rs2072661 is not significantly associated with reduced abstinence in any PG with either transmission model (See Table, Supplemental Digital Content 6: Effects of rs2072661 on EOT and 6MO abstinence, 24 arms). There are two PG groups that exhibit p-values<0.10, but these differ in transmission model, abstinence time point, and PG.
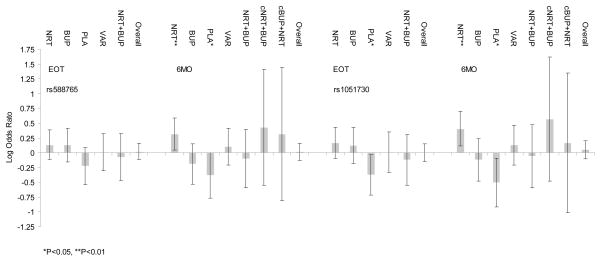
rs588765 and rs1051730 are significantly associated with abstinence (Fig. 1 and See Table, Supplemental Digital Content 7: Effects of chr15q25.1 nAChR SNPs on abstinence, 26 arms). The minor allele of rs588765 is significantly associated with reduced abstinence in the PLA PG at 6MO [0.414 (0.171–0.999) 0.049], and with increased abstinence in the NRT PG at 6MO [2.074 (1.111–3.871) 0.022]. The minor allele of rs1051730 is significantly associated with reduced abstinence in the PLA PG at EOT [0.422 (0.191–0.934) 0.033] and at 6MO [0.312 (0.122–0.802) 0.016], and with increased abstinence in the NRT PG at 6MO [2.540 (1.293–4.987) 0.007]. The effect of rs1051730 on abstinence differs significantly between PGs at 6MO (F6, 28652=2.48, P=0.021). The degrees of freedom of the F statistic reflect imputation of multiple datasets. The significant test of homogeneity is most likely due to the significant and opposite effects of rs1051730 on abstinence in individuals randomized to PLA versus NRT. In sensitivity analyses not adjusting for nicotine dependence measures (See Table, Supplemental Digital Content 8: Effects of chr15q25.1 nAChR SNPs on abstinence, excluding dependence measures, 26 arms), rs1051730 associations with abstinence remain statistically significant with modestly reduced effect sizes.
Figure 1.
Effects of rs588765 and rs1051730 on abstinence at end of treatment (EOT) and six months (6MO) by pharmacotherapy group (PG) [nicotine replacement therapy (NRT), bupropion (BUP), placebo (PLA), varenicline (VAR), NRT and BUP (NRT+BUP), chronic NRT and BUP (cNRT+BUP) and chronic BUP and NRT (cBUP+NRT)].
Mediation analysis
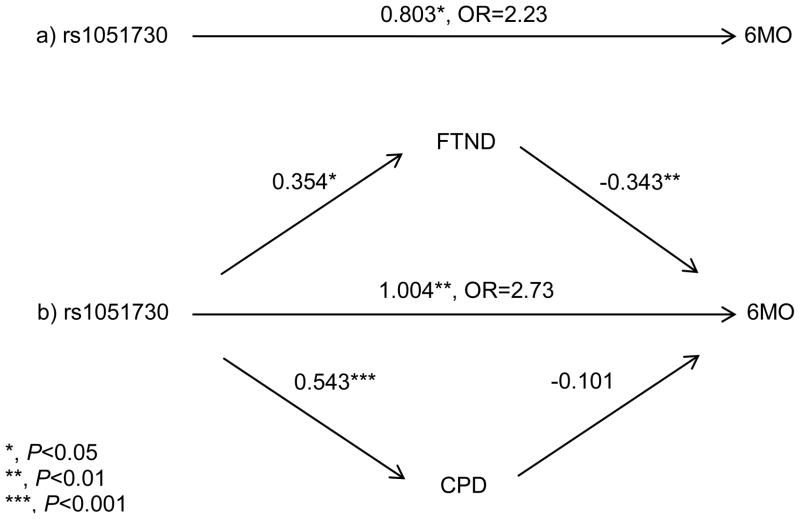
We performed post-hoc multivariate mediation analyses to evaluate the association of rs1051730 with nicotine dependence measures, nicotine dependence measure associations with 6MO abstinence, and rs1051730 direct effects on 6MO abstinence in the PLA and in the NRT PGs. We restricted these analyses to rs1051730 due to the significant effect sizes observed with this locus on 6MO abstinence with and without adjustment for multiple nicotine dependence measures. We observe a significant mediational path through the FTND score in the association of rs1051730 with 6MO abstinence in the NRT PG, but not in the PLA PG, perhaps due to sample size limitations (Fig. 2 and see Table, Supplemental Digital Content 9: Mediation of rs1051730 association with 6MO abstinence by nicotine dependence measures in individuals randomized to NRT and to PLA). The direct effect [OR (95%CI) P] of rs1051730 on abstinence with both FTND and CPD included in the mediation model is 2.73 (1.34–5.53) 0.005, and the pseudo-r2 is 0.083. rs1051730 is significantly and positively associated with CPD and with FTND score (p<0.001 and p=0.016, respectively). FTND score is significantly negatively associated with 6MO abstinence (OR=0.71, 0.57–0.89, p=0.003), while CPD is non-significantly negatively associated with 6MO abstinence. The effect of rs1051730 on 6MO abstinence excluding both nicotine dependence measures from the model is 2.23 (1.12–4.44) 0.022, with pseudo-r2 of 0.058. Thus, rs1051730 has a stronger relation with 6MO abstinence when the dependence measures are included in the model than when they are not, i.e., that the dependence measures are acting as suppressors in this mediation model [72].
Figure 2.
Mediation of rs1051730 association with 6MO abstinence by nicotine dependence measures Fagerstrom Test for Nicotine Dependence (FTND) and cigarettes per day (CPD). a) Association of rs1051730 with 6MO abstinence without adjustment for nicotine dependence measures. The total path from rs1051730 to 6MO abstinence (not including the nicotine dependence measures FTND and CPD) is statistically significant at P<0.05. b) Mediation analyses of rs1051730 with 6MO abstinence with nicotine dependence measures. The direct path has a larger effect size and is more significant (P<0.01), than the total path in (a) above, due to the negative effects of FNTD and CPD on the total path. The path from rs1051730 through FTND to 6MO abstinence is statistically significant at P<0.05. The path from rs1051730 through CPD to 6MO abstinence is not statistically significant, though the association of rs1051730 with CPD is statistically significant at P<0.001.
Receiver Operating Characteristic (ROC) analysis
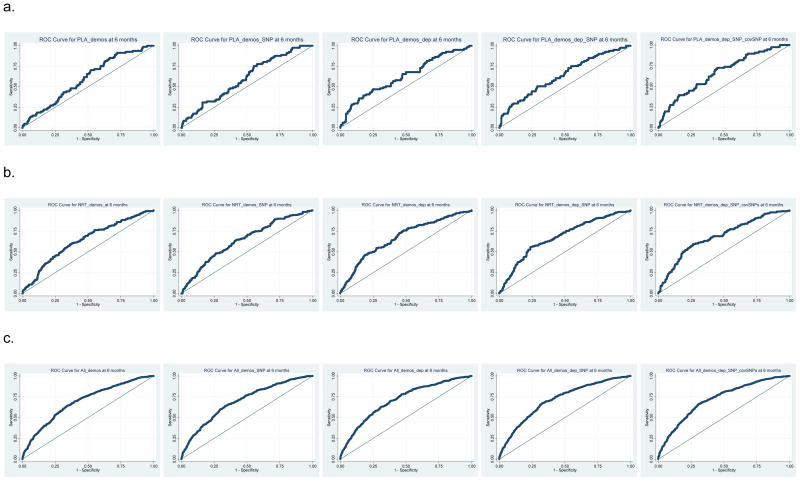
We performed post-hoc ROC analyses to evaluate the contributions of demographic, dependence, and genetic variables to predict abstinence at 6MO. We evaluated ROC models for the association of rs1051730 with abstinence in the PLA PG at 6MO (N~467), in the NRT PG at 6MO (~740), and in all PGs at 6MO (sample size ~2592) (Figure 3 and see Table, Supplemental Digital Content 10: Area Under the Curve (AUC) mean and 95%CI estimates from PLA, NRT and All PG models). The ROC AUC values increase when pharmacotherapy is added, e.g., with the addition of NRT or all PGs, compared to PLA, and, within each set of ROC models, the ROC AUC increases when including additional variables in the model. For the PLA models, the AUC of the full model is significantly greater that PLA models with demographic variables or demographic variables and rs1051730. For NRT or all PG models, the inclusion of dependence variables, dependence variables and rs1051730, or dependence variables, rs1051730 and covariate SNPs (rs588765 and rs578776), results in ROC curves with significantly greater AUC than the models with only demographic variables, or with demographic variables and rs1051730. This suggests that with or without pharmacotherapy, information imparted by dependence measures and covariate SNPs increases the ability to predict abstinence outcomes. E.g., for a specificity of 0.50, the sensitivity of the full model in the PLA, NRT and All PGs setting is 0.73, 0.72 and 0.81, versus 0.68, 0.70, and 0.76, for the model with only demographic variables, respectively.
Figure 3.
Receiver Operating Characteristic (ROC) curves for a. placebo (PLA), b. nicotine replacement therapy (NRT) and c. all pharmacotherapy groups (All PG) models at 6MOs. ROC curves are shown for models including demographic variables (demos), demographic variables and rs1051730 (demos_SNP), demographic and dependence variables (demos_dep), demographics and dependence variables and rs1051730 (demos_dep_SNP), and all variables with other chr15q25.1 SNPs, rs588765 and rs578776 (demos_dep_SNP_covSNPs).
Chr15q25.1 SNPs
Two chr15q25.1 SNPs (rs588765 and rs1051730) exhibit statistically significant associations with quitting success in individuals randomized to placebo and to NRT, but not in individuals randomized to other pharmacotherapies. These results were obtained by analysis of a total of 2,633 self-identified White participants from eight RCTs containing 26 therapy randomization arms, adjusted for PG, RCT arm, demographics, dependence measures, and population genetic variation (and chr15q25.1 SNPs, where appropriate). rs578776, another chr15q25.1 SNP, is not statistically significantly associated with abstinence at either timepoint or in any PG. This may be due to its more modest effect size or its inverse association with smoking heaviness [30]. rs588765 associations with abstinence appear to be somewhat smaller in magnitude than those observed with rs1051730, concordant with previously observed effects on smoking heaviness [30]. Focusing on the results of analysis of rs1051730, we observed that the minor allele is associated with reduced abstinence in the PLA PG at EOT and at 6MO, and with increased abstinence in the NRT PG at 6MO. The directionality of the effect on abstinence in individuals prescribed PLA is expected, as previously shown for one trial included in this analysis [52], but the directionality with the NRT PG is unexpected, given the prior associations of rs1051730 with nicotine dependence [23], with reduced abstinence at four weeks in multiple RCTs that randomize participants to NRT [50], and considering the inverse associations of nicotine dependence and abstinence [5,6]. We adjusted for the nicotine dependence measures CPD and FTND in our models because we previously observed significant inverse associations of these measures with abstinence in the eight RCTs (data not shown), concordant with a published meta-analysis [6]. Association analyses of chr15q25.1 SNPs and abstinence relations that exclude nicotine dependence measures modestly reduced rs1051730 SNP effects, suggesting that the influences of rs1051730 and nicotine dependence on abstinence are related. Mediation analysis examined the relations of rs1051730 with abstinence in the PLA and NRT PGs at 6MO, and the extent to which this relation was mediated by nicotine dependence measures; we observed significant mediation effects only in the NRT PG at 6MO. The mediation analysis suggests that rs1051730 significantly increases measures of nicotine dependence [18,19,25], that nicotine dependence significantly decreases abstinence likelihood [6], and that there is a mechanism other than nicotine dependence through which rs1051730, in the presence of NRT and at 6MO, increases abstinence.
Mechanisms
Mechanisms that underlie the two distinct association results involving rs1051730 can be postulated based on recent studies in neurogenetics, neuroscience, and pharmacology. α5 knockout mice self-administer nicotine more vigorously than wild-type mice [73], show reduced seizure and hyperlocomotive sensitivity to nicotine [74], and exhibit conditioned place preference for nicotine at doses that are aversive in wildtype mice [75]. These properties are thought to be due to the α5 subunit regulation of the medial habenular-interpeduncular nuclear tract [73]. Functional magnetic resonance imaging in healthy human smokers has characterized functional connectivity (circuits) [76], including a dorsal anterior cingulate cortex to ventral striatal circuit inversely associated with FTND and multiple distinct intra-cingulate cortex and cingulate cortex to frontal region circuits strengthened by nicotine patch administration. In additional studies in smokers and non-smokers, and in individuals who do and who do not meet criteria for Axis I disorders [26], rs16969968 (highly correlated with rs1051730 in European ancestry populations, and coding for CHRNA5 p.Asp398Asn, where Asn398 is associated with reduced nAChR function [77]) was observed to be associated with functional connectivity within the same FTND-associated cingulate circuit. In population samples, rs1051730 has been associated with reduced working memory performance [78]. In a laboratory study of abstinent smokers, transdermal nicotine has been associated with improvements in working memory [79]. Finally, reduced working memory performance in abstinent smokers has been associated with relapse over seven days in individuals receiving placebo and exposed to a smoking lapse [80].
These finding suggest a hypothesis that can be tested in treatment-seeking smokers. Smokers with reduced α5 subunit function and associated increased nicotine dependence might be expected to have more difficulty quitting. The observation in this analysis that smokers with reduced α5 subunit function treated with NRT have increased overall abstinence rates, and the increased direct effect of rs1051730 on abstinence in mediation analysis, reflect a mechanism that is distinct from the effects of rs1051730 on nicotine dependence, and of nicotine dependence upon abstinence. Prescribed NRT may improve cognitive performance that assists abstinent smokers to maintain normal brain functioning after quitting smoking, and this effect may be stronger for individuals carrying the risk allele of rs1051730. Retrospective analyses of RCT arms randomizing individuals to PLA or NRT, and/or a prospective genotype and NRT-stratified trial, with the appropriate genetic, behavioral and cognitive function data, could test this hypothesis.
Conti et al identified the association of rs2072661 with abstinence in analyses of a double-blind randomized controlled trial of placebo or active bupropion, e.g., a SNP OR (95% confidence interval) of 0.40 (0.25–0.67) at EOT and of 0.31 (0.18–0.55) at 6MO [46]. The associations of this SNP with a variety of smoking related phenotypes [35,43,54], including short-term cessation in a cross-over trial of NRT and placebo patch [53], suggested to us that rs2072661 might exhibit effects on abstinence with other PGs and that we might more accurately quantify its association in larger samples. However, we did not observe statistically significant association of rs2072661 with any PG when analyzing 24 arms of seven RCTs, i.e., excluding the two arms of the RCT in which the abstinence association was discovered [46]. If the main effect size of rs2072661 on abstinence in RCT participants is weaker than the effect observed by Conti et al, which is expected [81], analysis of additional RCT arms will be necessary to validate the original [46] or subsequent associations [53], or discover novel associations.
Limitations
Limitations of our analyses include sample size limitations on statistical power, RCT participant heterogeneity, and assumptions about variable effects required by our pooled regression analyses. Sample size limitations on statistical power (See Table, Supplemental Digital Content 11: Odds Ratio (OR) detectable with 80% power) may underlie our inability to make statements about chr15q25.1 nAChR SNP pharmacogenetic effects in individuals randomized to BUP, VAR or combined therapies. Increasing PG sample sizes in future analyses will increase power to detect pharmacogenetic effects, but will still require integrated data analysis choices to be made. While there are differences in baseline, treatment and outcome variables among the RCTs, ascertainment characteristics of the RCTs are similar and there are no significant differences in nAChR SNP allele or genotype frequencies among the RCTs. In the analyses reported here, we utilize one approach to performing integrated data analysis, namely, pooled regression analysis. Heuristically, all of the studies contributed to the estimation of the regression coefficients for each demographic and dependence variable, which were assumed to have the same value across arms; each individual arm contributed to estimation of an arm-specific level variable, allowing for different abstinence rates across arms, and each individual arm contributed to estimation of a pharmacotherapy-specific coefficient for the SNP variable, and, if present, pharmacotherapy-specific coefficients for covariate SNPs. This approach was implemented because many of the arms had insufficient observations for reliable estimation of SNP effects if all of the covariates had been included and regressions were performed separately by arm.
Summary
Treatment-seeking smokers with the minor alleles of chr15q25.1 SNPs rs588765 or rs1051730, versus those without these alleles, are less likely to achieve 6MO abstinence if prescribed PLA, and more likely to achieve 6MO abstinence if prescribed NRT. However, identification and characterization of biomarkers that support the personalization of smoking cessation therapy will be challenging. For example, differences in prediction of abstinence between ROC models with and without rs1051730 (Fig. 2) were a fraction (average of 10%) of the AUC change observed when nicotine dependence measures are added to the ROC models. The modest improvement in prediction attributable to genetic variables versus the larger impact of dependence measures on abstinence likelihood suggests that risk models will include multiple non-genetic and genetic variables [10]. The analysis of multiple randomized clinical trials in an integrated data analysis framework to validate the novel association of rs1051730 with abstinence in individuals randomized to NRT, and to discover, and then to validate, additional novel biomarker associations with abstinence, will be necessary to develop algorithms for smoking cessation treatment assignment, i.e., personalized medicine [82]. The goal of developing predictive models of treatment response to be implemented into clinical practice will require collaborative efforts from each of the domains of research, policy, industry, and healthcare.
Supplementary Material
Digital Content 1. Randomized clinical trial design characteristics.
Digital Content 2. Behavioral and demographic variables selected for analysis.
Digital Content 3. Inclusion and exclusion criteria for eight RCTs.
Digital Content 4. Pharmacotherapy and behavioral therapy to EOT and 6MO of eight RCTs by randomization arm.
Digital Content 5. nAChR SNPs counts and Hardy-Weinberg equilibrium P value, by arm.
Digital Content 6. Effects of rs2072661 on EOT and 6MO abstinence, 24 arms.
Digital Content 7. Effects of chr15q25.1 nAChR SNPs on abstinence, 26 arms.
Digital Content 8. Effects of chr15q25.1 nAChR SNPs on abstinence, excluding dependence measures, 26 arms.
Digital Content 9. Mediation of rs1051730 association with 6MO abstinence by nicotine dependence measures in individuals randomized to NRT and to PLA.
Digital Content 10. Area Under the Curve (AUC) mean and 95%CI estimates from PLA, NRT and All PG models.
Digital Content 11. Odds Ratio (OR) detectable with 80% power.
Acknowledgments
Funding and Role of Sponsor
We acknowledge the financial support of U01 DA020830 (CL, RFT), P50 CA143187 (CL), CA63562 (CL), CA071358 (GES), DA16752 (SH), DA18691 (SH), DA15732 (SH), DA9253 (SH), P50 CA84724 (TBB), K05 CA139871 (TBB) and P50 DA19706 (TBB). This work was supported in part by the Centre for Addiction and Mental Health (RFT) and a Canada Research Chair in Pharmacogenetics (RFT).
RCTs 3A, 3B, 5, 9A, 9B, and 9C were funded by the National Cancer Institute and RCTs 6A and 6B were funded by the National Institute of Drug Abuse. Pharmacia & Upjohn provided nicotine nasal spray for RCT 3A. GlaxoSmithKline provided study medication for RCT 3B. Varenicline and nominal support for recruiting RCT 5 participants was provided by Pfizer, Inc. GlaxoSmithKline provided medication for RCTs 9B and 9C. Neither the National Cancer Institute, National Institute on Drug Abuse, Pharmacia & Upjohn, GlaxoSmithKline or Pfizer had any role in the study design of the eight RCTs, the collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the report.
We thank the participants in the randomized clinical trials for their participation in research. We thank: Faith Allen (University of California San Francisco) for curation of PNAT data; Lisa Jack (SRI International) for curation of RCT 5 data; Robert M. Curley (University of Pennsylvania) for meta-data management; and Jonathan Woo (University of California San Francisco) for GoldenGate genotyping of RCTs 5, 6A, 6B, 9A, 9B, and 9C.
Footnotes
Dr. Bergen, Dr. Javitz, Ms. Krasnow and Dr. Swan had full access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis.
Conflicts of Interest
Dr. Bergen has received research support from Medco Health Solutions and Affymetrix. Dr. Javitz has conducted research sponsored by Pfizer, SmithKline Beecham, CV Therapeutics, Biogen, Berlex Laboratories, Johnson & Johnson, Ciba-Geigy, Angiotech, Merck & Co., Eli Lilly and the Pharmaceutical Researchers and Manufacturers Association. He has also been a paid expert witness in litigation against tobacco companies relating to advertising to minors. Mr. Edlund is currently employed by BioRealm LLC and the University of Southern California. Dr. Hall is the recipient of a materiel grant from Pfizer Pharmaceuticals. Dr. Benowitz has served as a consultant to several pharmaceutical companies that market smoking cessation products, including Pfizer, GlaxoSmithKline and Novartis. He has also been a paid expert witness in litigation against tobacco companies relating to nicotine addiction. Dr. Tyndale owns shares and participates in Nicogen Research Inc., a company focused on novel smoking cessation treatment approaches. No Nicogen funds were used in this work and no other Nicogen participants reviewed the manuscript. Dr. Tyndale has received financial support from Novartis and from McNeil to participate in one-day advisory meetings in 2008 and 2011 respectively. Dr. Lerman has served as a consultant and/or has received research funding from AstraZeneca, GlaxoSmithKline, Targacept, Pfizer, and Novartis. Dr. Swan received financial support from Pfizer to attend a one-day advisory meeting in 2008.
Ms. Krasnow, Ms. Nishita, Dr. Michel, Dr. Conti, Dr. Liu, Dr. Lee, Dr. Kwok and Dr. Baker report no conflicts of interest.
References
- 1.Rostron B. Smoking-attributable mortality in the United States. Epidemiology. 2011;22:350–355. doi: 10.1097/EDE.0b013e3182126729. [DOI] [PubMed] [Google Scholar]
- 2.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 3.Treating tobacco use and dependence: 2008 update U S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53:1217–1222. [PubMed] [Google Scholar]
- 4.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 5.Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagerstrom K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerstrom Test for Nicotine Dependence as a Predictor of Smoking Abstinence: A Pooled Analysis of Varenicline Clinical Trial Data. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- 7.Brown RA, Niaura R, Lloyd-Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine Tob Res. 2007;9:721–730. doi: 10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol Med. 2010;16:247–253. doi: 10.2119/molmed.2009.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ockene JK, Emmons KM, Mermelstein RJ, Perkins KA, Bonollo DS, Voorhees CC, et al. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19:17–31. doi: 10.1037/0278-6133.19.suppl1.17. [DOI] [PubMed] [Google Scholar]
- 12.Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 13.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh WY, Piper ME, Schlam TR, Fiore MC, Smith SS, Jorenby DE, et al. Should All Smokers Use Combination Smoking Cessation Pharmacotherapy? Using Novel Analytic Methods to Detect Differential Treatment Effects Over 8 Weeks of Pharmacotherapy. Nicotine Tob Res. 2011 doi: 10.1093/ntr/ntr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, et al. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol Genet. 2005;14:1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- 16.Greenbaum L, Kanyas K, Karni O, Merbl Y, Olender T, Horowitz A, et al. Why do young women smoke? I. Direct and interactive effects of environment, psychological characteristics and nicotinic cholinergic receptor genes. Mol Psychiatry. 2006;11:312–322. 223. doi: 10.1038/sj.mp.4001774. [DOI] [PubMed] [Google Scholar]
- 17.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, Dunn DM, et al. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11:785–796. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One. 2009;4:e4653. doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, et al. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010:6. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MD, Xu Q, Lou XY, Payne TJ, Niu T, Ma JZ. Association and interaction analysis of variants in CHRNA5/CHRNA3/CHRNB4 gene cluster with nicotine dependence in African and European Americans. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:745–756. doi: 10.1002/ajmg.b.31043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li MD, Yoon D, Lee JY, Han BG, Niu T, Payne TJ, et al. Associations of variants in CHRNA5/A3/B4 gene cluster with smoking behaviors in a Korean population. PLoS One. 2010;5:e12183. doi: 10.1371/journal.pone.0012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, et al. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010;9:741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wessel J, McDonald SM, Hinds DA, Stokowski RP, Javitz HS, Kennemer M, et al. Resequencing of nicotinic acetylcholine receptor genes and association of common and rare variants with the Fagerstrom test for nicotine dependence. Neuropsychopharmacology. 2010;35:2392–2402. doi: 10.1038/npp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamidovic A, Kasberger JL, Young TR, Goodloe RJ, Redline S, Buxbaum SG, Benowitz NL, Bergen AW, Butler KR, Franceschini N, Gharib SA, Hitsman B, Levy D, Meng Y, Papanicolaou GJ, Preis SR, Spring B, Styn MA, Tong EK, White WB, Wiggins KL, Jorgenson E. Genetic variability of smoking persistence in African Americans. Cancer Prev Res (Phila) 2011 May;4(5):729–734. doi: 10.1158/1940-6207.CAPR-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han S, Yang BZ, Kranzler HR, Oslin D, Anton R, Gelernter J. Association of CHRNA4 polymorphisms with smoking behavior in two populations. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:421–429. doi: 10.1002/ajmg.b.31177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie P, Kranzler HR, Krauthammer M, Cosgrove KP, Oslin D, Anton RF, et al. Rare nonsynonymous variants in alpha-4 nicotinic acetylcholine receptor gene protect against nicotine dependence. Biol Psychiatry. 2011;70:528–536. doi: 10.1016/j.biopsych.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen LS, Saccone NL, Culverhouse RC, Bracci PM, Chen CH, Dueker N, et al. Smoking and genetic risk variation across populations of European, Asian, and African American ancestry--a meta-analysis of chromosome 15q25. Genet Epidemiol. 2012;36:340–351. doi: 10.1002/gepi.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David SP, Hamidovic A, Chen GK, Bergen AW, Wessel J, Kasberger JL, et al. Genome-wide meta-analyses of smoking behaviors in African Americans. Transl Psychiatry. 2012;2:e119. doi: 10.1038/tp.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartz SM, Short SE, Saccone NL, Culverhouse R, Chen L, Schwantes-An TH, et al. Increased genetic vulnerability to smoking at CHRNA5 in early-onset smokers. Arch Gen Psychiatry. 2012;69:854–860. doi: 10.1001/archgenpsychiatry.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice JP, Hartz SM, Agrawal A, Almasy L, Bennett S, Breslau N, et al. CHRNB3 is more strongly associated with Fagerstrom Test for Cigarette Dependence-based nicotine dependence than cigarettes per day: phenotype definition changes genome-wide association studies results. Addiction. 2012;107:2019–2028. doi: 10.1111/j.1360-0443.2012.03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehringer MA, Clegg HV, Collins AC, Corley RP, Crowley T, Hewitt JK, et al. Association of the neuronal nicotinic receptor beta2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- 44.Hoft NR, Stitzel JA, Hutchison KE, Ehringer MA. CHRNB2 promoter region: association with subjective effects to nicotine and gene expression differences. Genes Brain Behav. 2011;10:176–185. doi: 10.1111/j.1601-183X.2010.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rigbi A, Yakir A, Sarner-Kanyas K, Pollak Y, Lerer B. Why do young women smoke? VI. A controlled study of nicotine effects on attention: pharmacogenetic interactions. Pharmacogenomics J. 2011;11:45–52. doi: 10.1038/tpj.2010.15. [DOI] [PubMed] [Google Scholar]
- 46.Conti DV, Lee W, Li D, Liu J, Van Den Berg D, Thomas PD, et al. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17:2834–2848. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heitjan DF, Guo M, Ray R, Wileyto EP, Epstein LH, Lerman C. Identification of pharmacogenetic markers in smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:712–719. doi: 10.1002/ajmg.b.30669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2011;37:641–650. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munafo MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 genotype and short-term smoking cessation. Nicotine Tob Res. 2011;13:982–988. doi: 10.1093/ntr/ntr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:275–284. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- 52.Chen LS, Baker TB, Piper ME, Breslau N, Cannon DS, Doheny KF, et al. Interplay of Genetic Risk Factors (CHRNA5-CHRNA3-CHRNB4) and Cessation Treatments in Smoking Cessation Success. Am J Psychiatry. 2012 doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perkins KA, Lerman C, Mercincavage M, Fonte CA, Briski JL. Nicotinic acetylcholine receptor beta2 subunit (CHRNB2) gene and short-term ability to quit smoking in response to nicotine patch. Cancer Epidemiol Biomarkers Prev. 2009;18:2608–2612. doi: 10.1158/1055-9965.EPI-09-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swan GE, Javitz HS, Jack LM, Wessel J, Michel M, Hinds DA, et al. Varenicline for smoking cessation: nausea severity and variation in nicotinic receptor genes. Pharmacogenomics J. 2011 doi: 10.1038/tpj.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103:1342–1346. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- 57.Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. Am J Prev Med. 2010;38:482–490. doi: 10.1016/j.amepre.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall SM, Humfleet GL, Gorecki JA, Munoz RF, Reus VI, Prochaska JJ. Older versus younger treatment-seeking smokers: differences in smoking behavior, drug and alcohol use, and psychosocial and physical functioning. Nicotine Tob Res. 2008;10:463–470. doi: 10.1080/14622200801901922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall SM, Humfleet GL, Munoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction. 2009;104:1043–1052. doi: 10.1111/j.1360-0443.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine Tob Res. 2007;9:947–954. doi: 10.1080/14622200701540820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine Tob Res. 2008;10:717–729. doi: 10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- 62.Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66:1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishita DM, Jack LM, McElroy M, McClure JB, Richards J, Swan GE, et al. Clinical trial participant characteristics and saliva and DNA metrics. BMC Med Res Methodol. 2009;9:71. doi: 10.1186/1471-2288-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas PD, Mi H, Swan GE, Lerman C, Benowitz N, Tyndale RF, et al. A systems biology network model for genetic association studies of nicotine addiction and treatment. Pharmacogenet Genomics. 2009;19:538–551. doi: 10.1097/FPC.0b013e32832e2ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 66.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 67.Ambler G, Omar RZ, Royston P. A comparison of imputation techniques for handling missing predictor values in a risk model with a binary outcome. Stat Methods Med Res. 2007;16:277–298. doi: 10.1177/0962280206074466. [DOI] [PubMed] [Google Scholar]
- 68.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 69.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 70.Mackinnon DP, Warsi G, Dwyer JH. A Simulation Study of Mediated Effect Measures. Multivariate Behav Res. 1995;30:41. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gauderman WJ, Morrison JM. QUANTO: A computer program for power and sample size calculations for genetic-epidemiology studies. 1.2 2006. [Google Scholar]
- 72.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- 75.Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, et al. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1448–1458. doi: 10.1002/ajmg.b.31126. [DOI] [PubMed] [Google Scholar]
- 79.Kleykamp BA, Jennings JM, Eissenberg T. Effects of transdermal nicotine and concurrent smoking on cognitive performance in tobacco-abstinent smokers. Exp Clin Psychopharmacol. 2011;19:75–84. doi: 10.1037/a0022417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19:640–648. doi: 10.1097/EDE.0b013e31818131e7. [DOI] [PubMed] [Google Scholar]
- 82.Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Cmaj. 2009;181:488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Digital Content 1. Randomized clinical trial design characteristics.
Digital Content 2. Behavioral and demographic variables selected for analysis.
Digital Content 3. Inclusion and exclusion criteria for eight RCTs.
Digital Content 4. Pharmacotherapy and behavioral therapy to EOT and 6MO of eight RCTs by randomization arm.
Digital Content 5. nAChR SNPs counts and Hardy-Weinberg equilibrium P value, by arm.
Digital Content 6. Effects of rs2072661 on EOT and 6MO abstinence, 24 arms.
Digital Content 7. Effects of chr15q25.1 nAChR SNPs on abstinence, 26 arms.
Digital Content 8. Effects of chr15q25.1 nAChR SNPs on abstinence, excluding dependence measures, 26 arms.
Digital Content 9. Mediation of rs1051730 association with 6MO abstinence by nicotine dependence measures in individuals randomized to NRT and to PLA.
Digital Content 10. Area Under the Curve (AUC) mean and 95%CI estimates from PLA, NRT and All PG models.
Digital Content 11. Odds Ratio (OR) detectable with 80% power.