Abstract
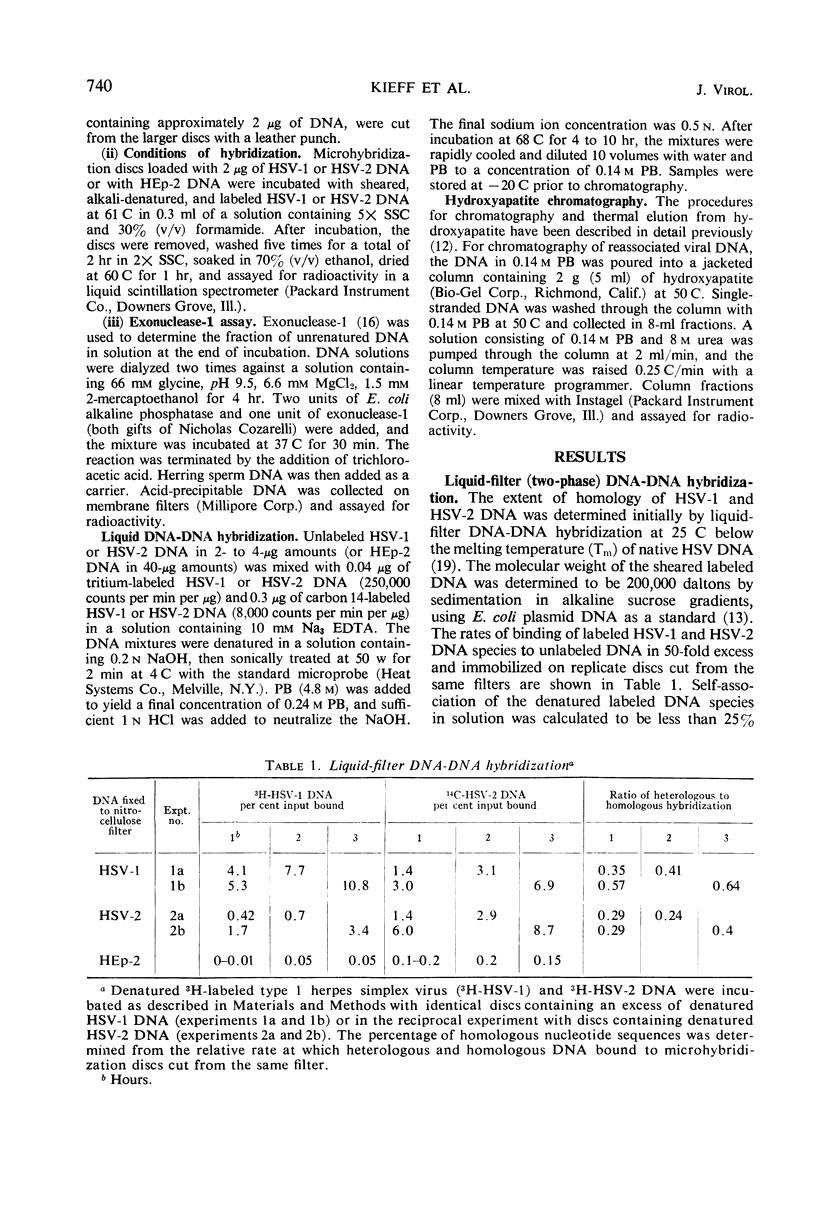
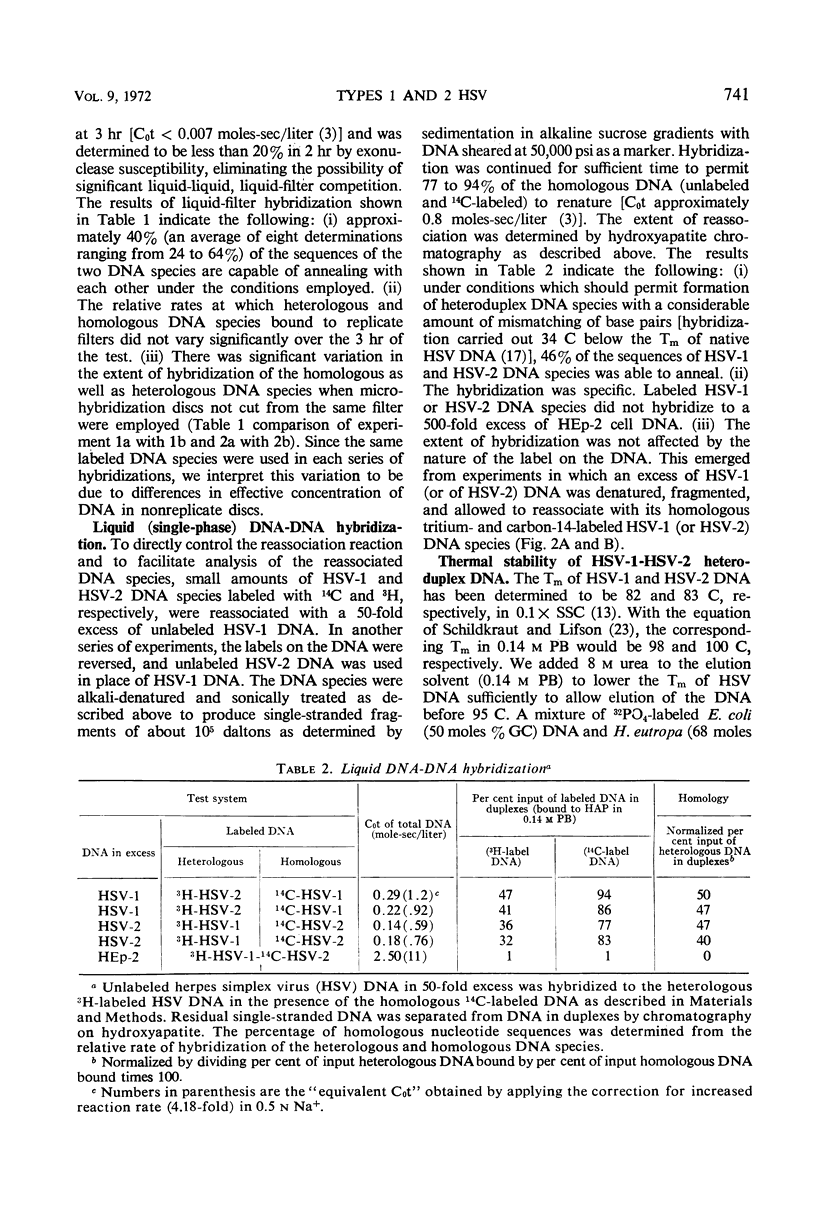
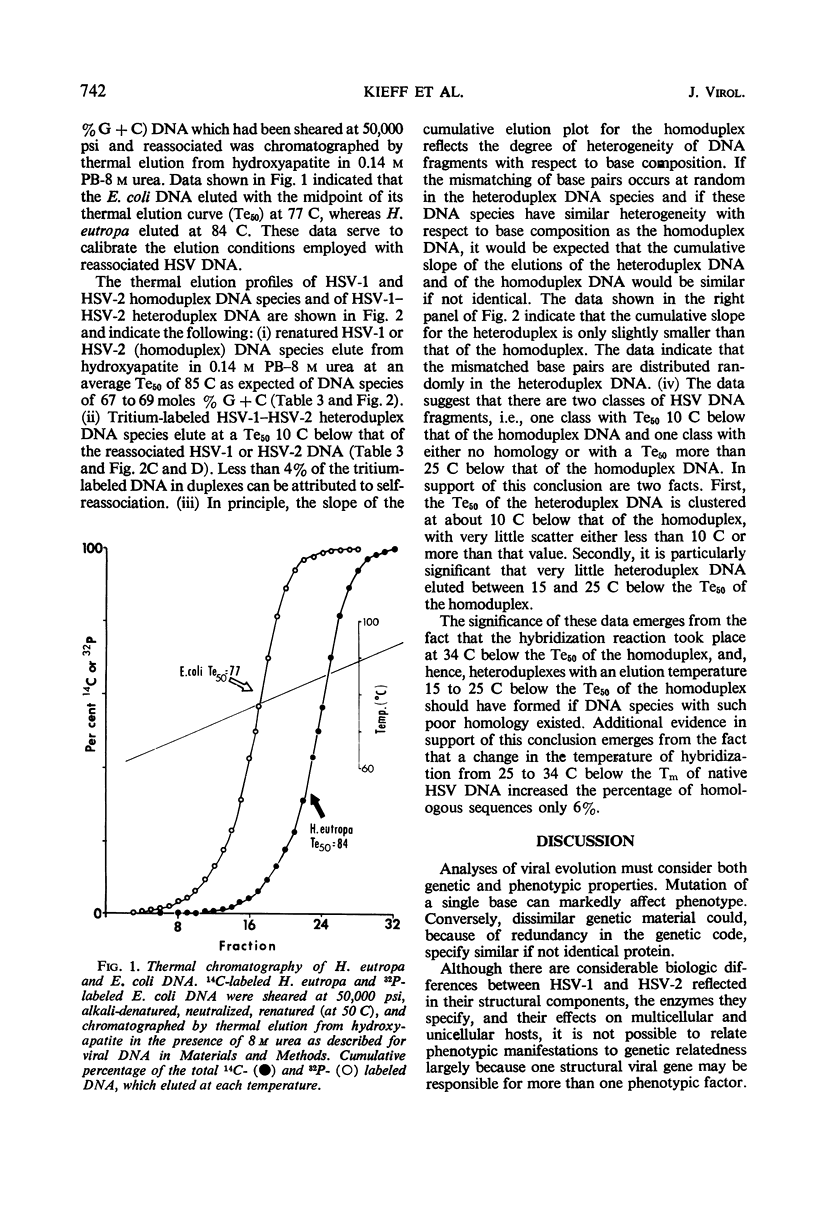
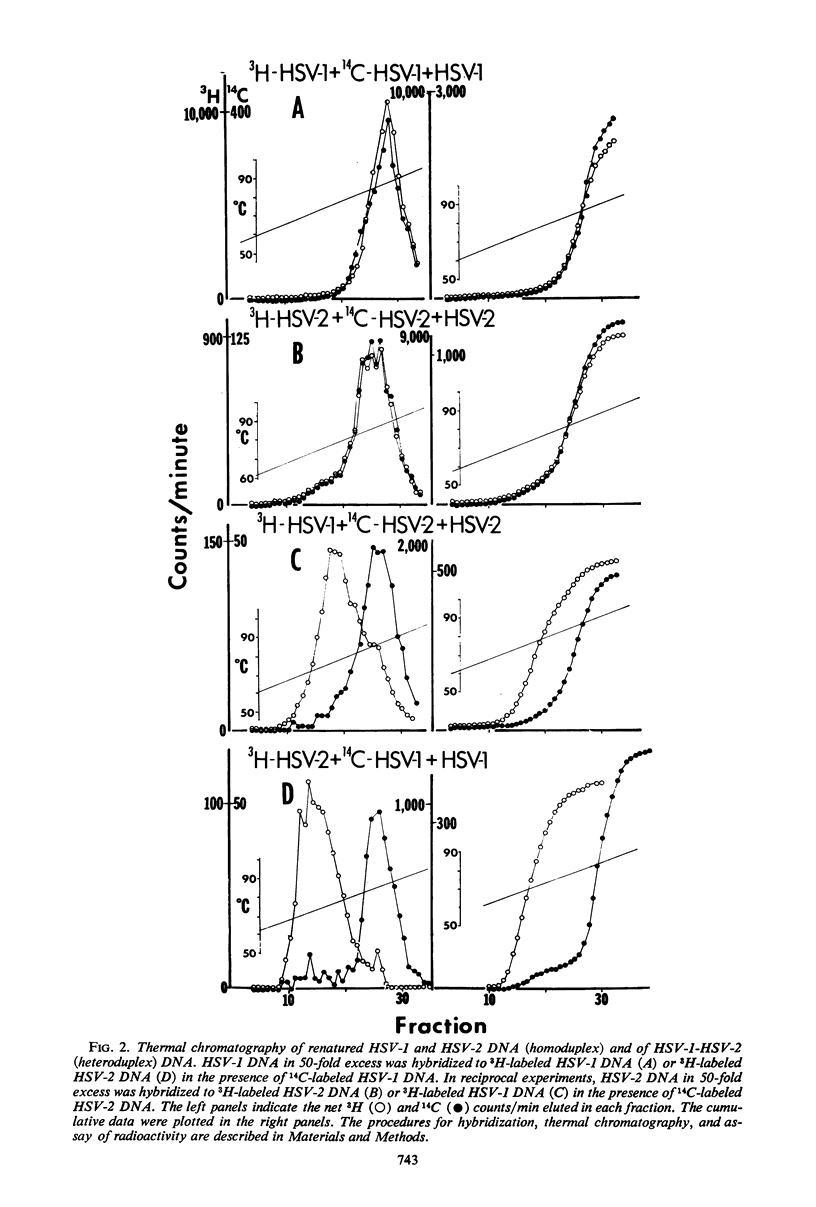
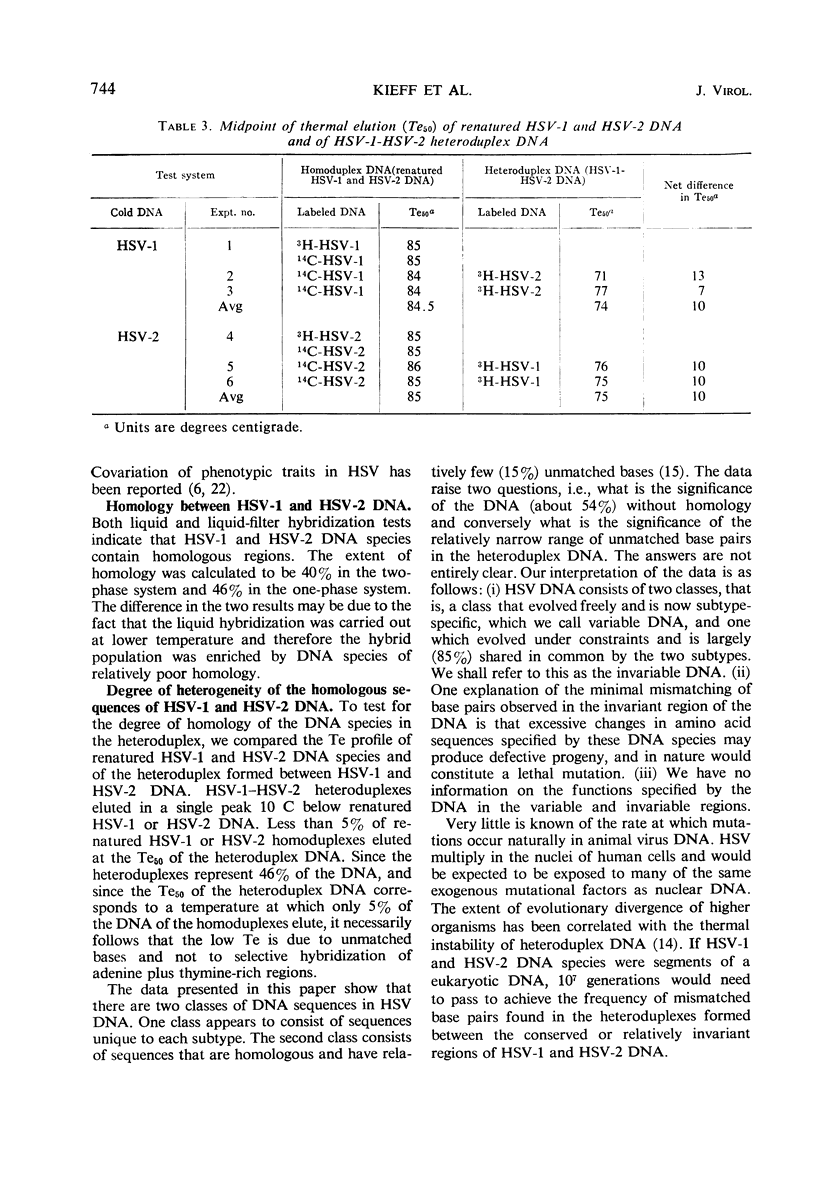
The extent of homology between herpes simplex virus1 and2 (HSV-1 and HSV-2) deoxyribonucleic acid (DNA) was measured in two ways: (i) by determination of the relative rate of hybridization of labeled HSV-1 and HSV-2 DNA to excess unlabeled HSV-1 or HSV-2 DNA immobilized on filters and (ii) by determination of the rate of hybridization of labeled HSV-1 and HSV-2 DNA to excess unlabeled HSV-1 or HSV-2 DNA in solution. Approximately 40% of HSV-1 and HSV-2 DNA is homologous at hybridization temperatures 25 C below the melting temperature (Tm) of HSV DNA (liquid-filter annealing). Lowering the temperature to 34 C below the Tm increased the extent of homology to 46% (liquid annealing). The extent of base-pairing in HSV-1-HSV-2 heteroduplex DNA was determined by thermal chromatography on hydroxyapatite. Heteroduplexes of HSV-1 and HSV-2 DNA eluted in a single peak whose midpoint (Te50) was 10 C below that of the homoduplex. Conspicuously absent were heteroduplexes that eluted at more than 15 C below the Te50 of the homoduplex. The data indicate the existence of a variable region of DNA (54%) with very little, if any, homology and an invariable region (46%) with relatively good (85%) matching of base pairs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker Y., Dym H., Sarov I. Herpes simplex virus DNA. Virology. 1968 Oct;36(2):184–192. doi: 10.1016/0042-6822(68)90135-9. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Bronson D. L., Graham B. J., Ludwig H., Benyesh-Melnick M., Biswal N. Studies on the relatedness of herpes viruses through DNA-RNA hybridization. Biochim Biophys Acta. 1972 Jan 18;259(1):24–34. doi: 10.1016/0005-2787(72)90470-4. [DOI] [PubMed] [Google Scholar]
- Dowdle W. R., Nahmias A. J., Harwell R. W., Pauls F. P. Association of antigenic type of Herpesvirus hominis with site of viral recovery. J Immunol. 1967 Nov;99(5):974–980. [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Figueroa M. E., Rawls W. E. Biological markers for differentiation of herpes-virus strains of oral and genital origin. J Gen Virol. 1969 Mar;4(2):259–267. doi: 10.1099/0022-1317-4-2-259. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Herpes vimplex virus: genome size and redundancy studied by renaturation kinetics. J Virol. 1971 Oct;8(4):591–593. doi: 10.1128/jvi.8.4.591-593.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geder L., Skinner G. R. Differentiation between type 1 and type 2 strains of herpes simplex virus by an indirect immunofluorescent technique. J Gen Virol. 1971 Aug;12(2):179–182. doi: 10.1099/0022-1317-12-2-179. [DOI] [PubMed] [Google Scholar]
- Goodheart C. R., Plummer G., Waner J. L. Density difference of DNA of human herpes simplex viruses, types I and II. Virology. 1968 Jul;35(3):473–475. doi: 10.1016/0042-6822(68)90225-0. [DOI] [PubMed] [Google Scholar]
- Hoyer B. H., McCullough N. B. Polynucleotide homologies of Brucella deoxyribonucleic acids. J Bacteriol. 1968 Feb;95(2):444–448. doi: 10.1128/jb.95.2.444-448.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- Laird C. D., McConaughy B. L., McCarthy B. J. Rate of fixation of nucleotide substitutions in evolution. Nature. 1969 Oct 11;224(5215):149–154. doi: 10.1038/224149a0. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E., Kramer J. H. Genital herpetic infection. Association with cervical dysplasia and carcinoma. Cancer. 1969 Apr;23(4):940–945. doi: 10.1002/1097-0142(196904)23:4<940::aid-cncr2820230432>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., AURELIAN L. ABORTIVE INFECTION OF CANINE CELLS BY HERPES SIMPLEX VIRUS. I. CHARACTERIZATION OF VIRAL PROGENY FROM CO-OPERATIVE INFECTION WITH MUTANTS DIFFERING IN CAPACITY TO MULTIPLY IN CANINE CELLS. J Mol Biol. 1965 Mar;11:528–538. doi: 10.1016/s0022-2836(65)80008-0. [DOI] [PubMed] [Google Scholar]
- Roizman B. The herpesviruses--a biochemical definition of the group. Curr Top Microbiol Immunol. 1969;49:3–79. [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Schneweis K. E., Nahmias A. J. Antigens of Herpes simplex virus type 1 and 2-immunodiffusion and inhibition passive hemagglutination studies. Z Immunitatsforsch Exp Klin Immunol. 1971 Jun;141(5):471–487. [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Similarities and Differences in the Development of Laboratory Strains and Freshly Isolated Strains of Herpes Simplex Virus in HEp-2 Cells: Electron Microscopy. J Virol. 1969 Dec;4(6):879–889. doi: 10.1128/jvi.4.6.879-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYBURN-MASON R. Malignant change following herpes simplex. Br Med J. 1957 Sep 14;2(5045):615–616. doi: 10.1136/bmj.2.5045.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]



