Abstract
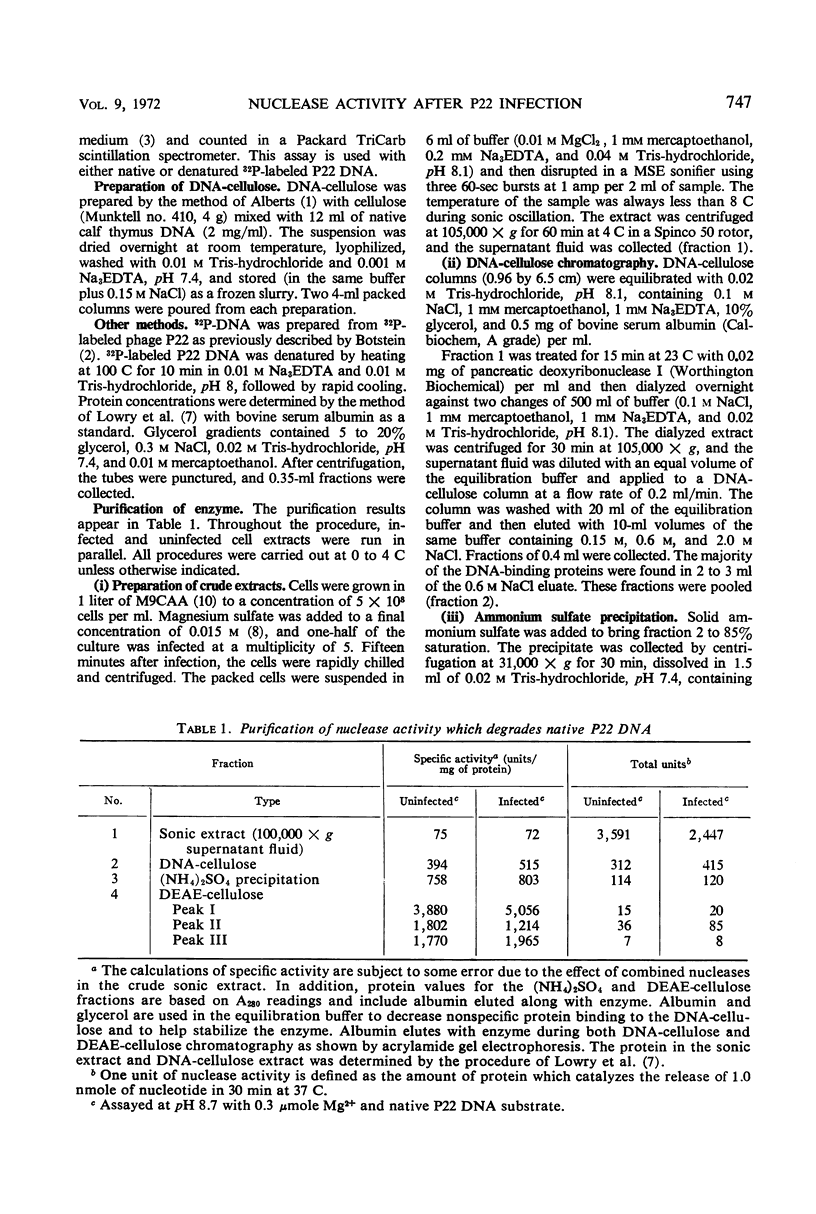
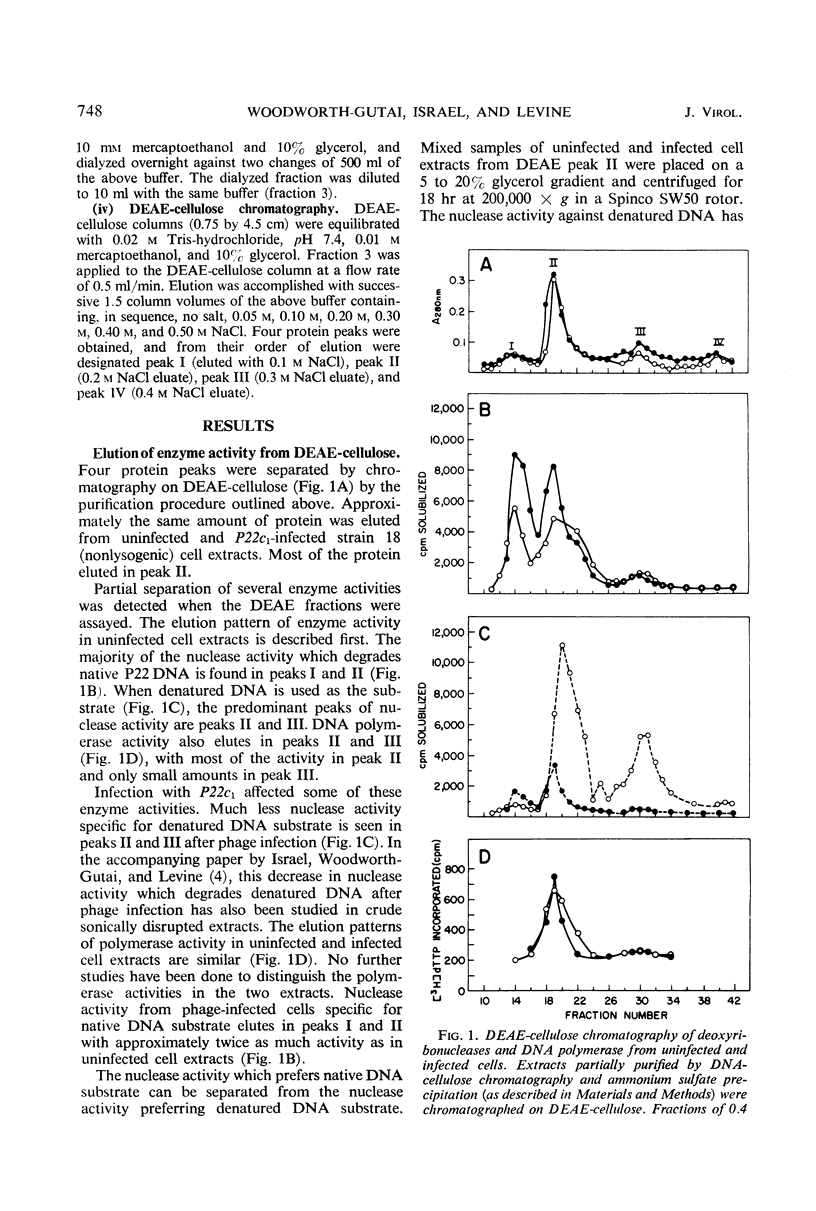
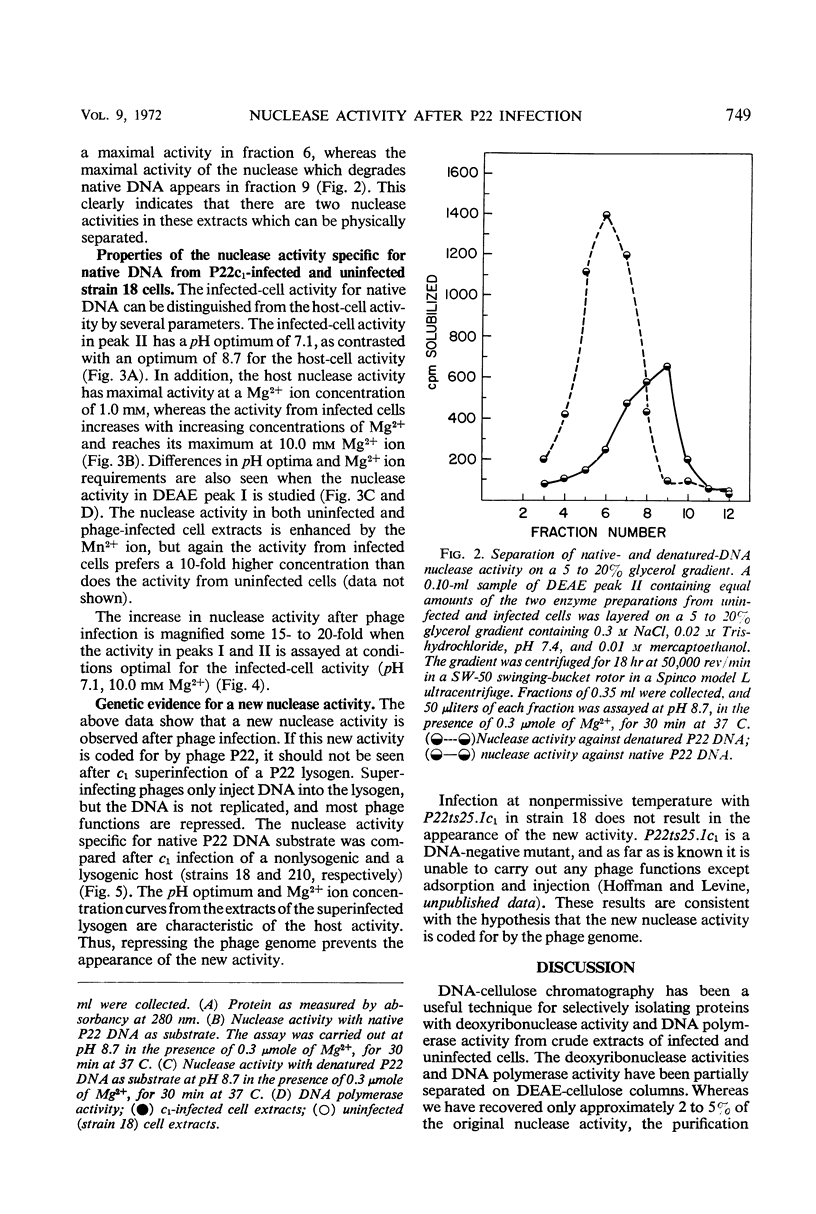
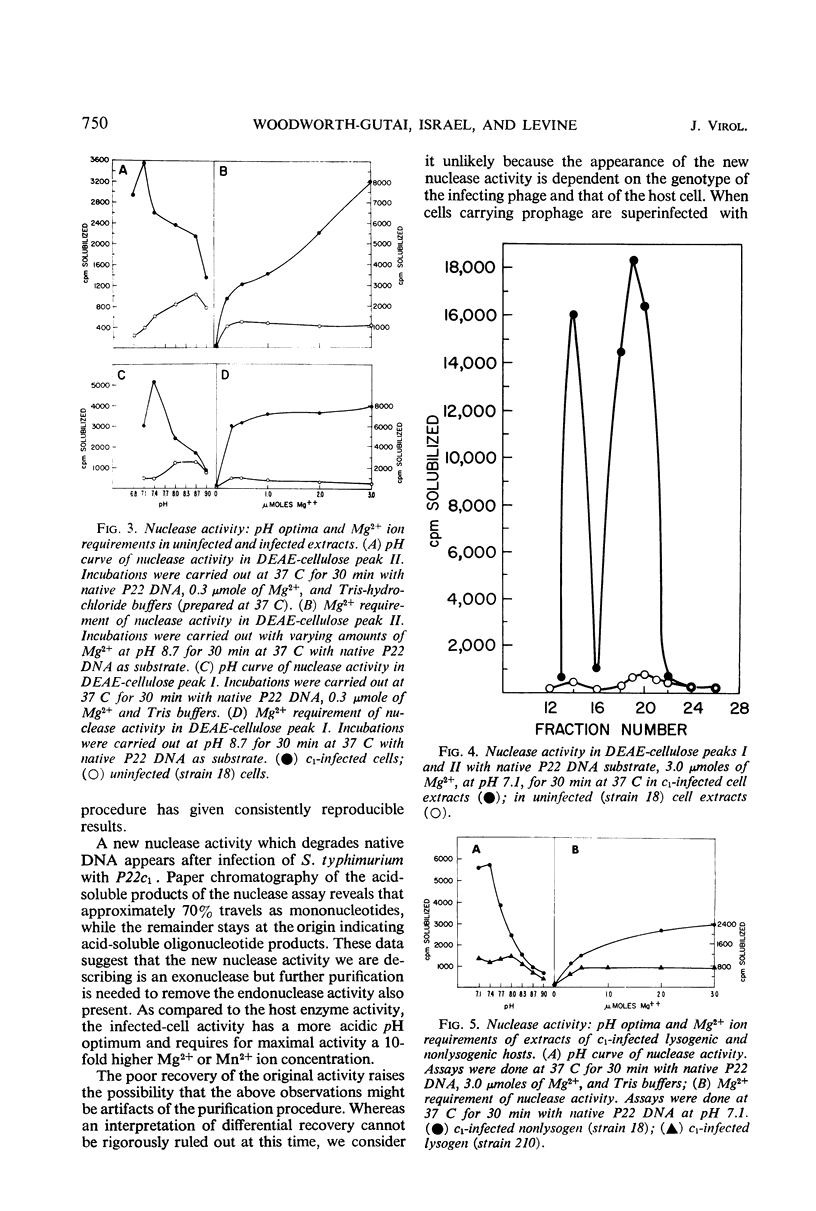
Extracts from P22-infected and uninfected cultures of Salmonella typhimurium were subjected to deoxyribonucleic acid (DNA)-cellulose and diethylaminoethyl-cellulose chromatography. Comparison of the elution patterns revealed that in infected cells there is a decrease in the amount of nuclease activity specific for denatured DNA and an increase in the amount of nuclease activity specific for native DNA. The latter activity was shown to differ from a similar host enzyme in Mg2+, Mn2+, and pH optima. This new activity is not found after infection of a lysogen with a nonvirulent phage or after infection under nonpermissive conditions with P22ts25.1 (a mutant in gene 25 that carries out no known functions other than adsorption and injection) and thus appears to be specified by the phage genome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M., Chakravorty M., Bronson M. J. Control of the replication complex of bacteriophage P22. J Virol. 1970 Oct;6(4):400–405. doi: 10.1128/jvi.6.4.400-405.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N. Bacteriophage P22 controlled exclusion in Salmonella typhimurium. J Mol Biol. 1968 Aug 14;35(3):607–622. doi: 10.1016/s0022-2836(68)80017-8. [DOI] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. TWO SEQUENTIAL REPRESSIONS OF DNA SYNTHESIS IN THE ESTABLISHMENT OF LYSOGENY BY PHAGE P22 AND ITS MUTANTS. Proc Natl Acad Sci U S A. 1964 Aug;52:356–363. doi: 10.1073/pnas.52.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J., Meynell G. G. The isolation of non-excluding mutants of phage P22. J Gen Virol. 1967 Oct;1(4):581–582. doi: 10.1099/0022-1317-1-4-581. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]



