Abstract
Intracerebral hemorrhage (ICH) is a devastating stroke subtype characterized by severe brain edema formation leading to cerebral blood flow compromise and parenchymal damage. Arginine vasopressin (AVP), a non-peptide antidiuretic hormone, has recently been implicated as a modulator of brain edema following injury. In this study, we investigated the effects of SR49059, a highly specific AVP V1a receptor antagonist, on brain injury outcomes following ICH, specifically assessing the ability of SR49059 in reducing brain edema and improving neurobehavioral deficits. Male CD1 mice (n = 35) were randomly assigned to the following groups: sham, ICH, ICH with SR49059 at 0.5 mg/kg, and ICH with SR49059 at 2 mg/kg. ICH was induced by using the collagenase injection model, and treatment was given 1 h after surgery. Post-assessment was conducted at 24 and 72 h after surgery, and included brain water content and neurobehavioral testing. The study found that SR49059 significantly reduced cerebral edema at 24 and 72 h post-ICH injury and improved neurobehavioral deficits at 72 h. Our study suggests that blockage of the AVP V1a receptor is a promising treatment target for improving ICH-induced brain injury. Further studies will be needed to confirm this relationship and determine future clinical direction.
Keywords: Intracerebral hemorrhage (ICH), Arginine vasopressin (AVP), SR49059
Introduction
Intracerebral hemorrhage (ICH) is a devastating stroke subtype victimizing over 120,000 Americans each year. One of the major reasons for its severity is the development of perihematomal edema – on average, close to 40% of victims have a 1–2% increase, resulting in an increased intracranial pressure and heightened risk for brain herniation. Today, despite extensive research focusing on ameliorating or reducing the development of edema, research has not been effective in developing a treatment option for this ICH-induced outcome.
Arginine vasopressin (AVP) is a non-peptide antidiuretic hormone responsible for regulating water and electrolyte homeostasis in the body. Normally, AVP is produced in the hypothalamus and then released into the circulation by the posterior pituitary. However, recent studies have suggested an alternate pathway for AVP, specifically suggesting that AVP can be released directly into the brain where it acts as a neurotransmitter, regulating water permeability, ion homeostasis, and cerebrospinal fluid production [1]. In an acute ischemic stroke model, AVP injection directly into the brain exacerbated already accumulating brain edema, while injection of an AVP anti-serum reduced edema formation [2].
Accordingly, in the present study we investigated the role of AVP inhibition and its effects on cerebral edema formation and neurobehavioral functioning. We hypothesize that SR49059, a highly specific AVP V1a receptor competitive antagonist, could reduce brain edema accumulation and improve neurobehavioral functioning following an ICH brain injury in mice.
Materials and Methods
This study was in accordance with the guidelines of the National Institute of Health for the treatment of animals and was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Male CD1 mice (weight 35–45 g, Charles River, MA) were housed in a 12-h light/dark cycle at a controlled temperature and humidity with free access to food and water. Mice were divided into the following groups: sham (n = 5), ICH (n = 5), ICH treated with low-dose antagonist (SR49059 at 0.5 mg/kg; n = 5), and ICH treated with high-dose antagonist (SR49059 at 2 mg/kg; n = 5).
Operative Procedure
The collagenase-induced intracerebral hemorrhage model [3] was adapted as previously described in mice [4]. Briefly, mice were anesthetized intrapertioneally with a ketamine (100 mg/kg)/xylazine (10 mg/kg) cocktail and positioned prone in a stereotaxic head frame (Stoelting, Wood Dale, IL). An electronic thermostat-controlled warming blanket was used to maintain the core temperature at 37°C. The calvarium was exposed by a midline scalp incision from the nose to the superior nuchal line, and the skin was retracted laterally. With a variable speed drill (Fine Scientific Tools, Foster City, CA), a 1.0-mm burr hole was made 0.9 mm posterior to the bregma and 1.45 mm right-lateral to the midline. A 26-G needle on a Hamilton syringe was inserted with stereotaxic guidance 4.0 mm into the right deep cortex/basal ganglia at a rate 1 mm/min. Collagenase (0.075 units in 0.5 μL saline; Sigma, St Louis, MO) was then infused into the brain at a rate of 0.25 μL/min over 2 min using an automatic infusion pump (Stoelting, Wood Dale, IL). The needle was left in place for an additional 10 min after injection to prevent the possible leakage of collagenase solution. After removal of the needle, the incision was sutured closed, and mice were allowed to recover. Sham operation was performed with needle insertion only.
Treatment Method
SR49059 (Tocris Bioscience, Ellisville, MO) was dissolved in 0.5% DMSO and administered one time intraperitoneally approximately 1 h after ICH induction.
Brain Water Content
Brain water content was measured as previously described [5]. Briefly, rats were sacrificed at 24 h and 72 h post ICH, and brains were immediately removed and divided into five parts: ipsilateral frontal, contralateral frontal, ipsilateral parietal, contralateral parietal, and cerebellum. The cerebellum was used as an internal control for brain water content. Tissue samples were then weighed on an electronic analytical balance (APX-60, Denver Instrument; Arvada, CO) to the nearest 0.1 mg to obtain the wet weight (WW). The tissue was then dried at 105°C for 48 h to determine the dry weight (DW). The percent brain water content was calculated as [(WW – DW)/WW] × 100.
Assessment of Neurobehavioral Deficits
Neurological outcomes were assessed by a blind observer at 24 h and 72 h post ICH using the Modified Garcia Score [6].The Modified Garcia Score is a 21-point sensorimotor assessment system consisting of seven tests with scores of 0–3 for each test (maximum score = 21). These seven tests included: (1) spontaneous activity, (2) side stroking, (3) vibris touch, (4) limb symmetry, (5) climbing, (6) lateral turning, and (7) forelimb walking.
Additionally, beam balance and wire hang testing was performed. Both the beam (590 cm in length by 51 cm in width) and wire (550 cm in length by 51 mm in width) were constructed and held in place by two platforms on each side. Mice were put on the center of the beam or wire and allowed to reach the platform. Mice were observed for both their time and behavior until they reached one platform and scored according to six grades. The test was repeated three times, and an average score was taken [minimum score 0; maximum score (healthy mouse)] [2].
Statistical Analysis
Quantitative data were expressed as the mean ± SEM. One-way ANOVA and Tukey test were used to determine significance in differences between the means. Neurological scores were evaluated using the Dunn method. A p-value < 0.05 was considered statistically significant.
Results
SR49059 decreases cerebral edema formation after ICH injury in mice
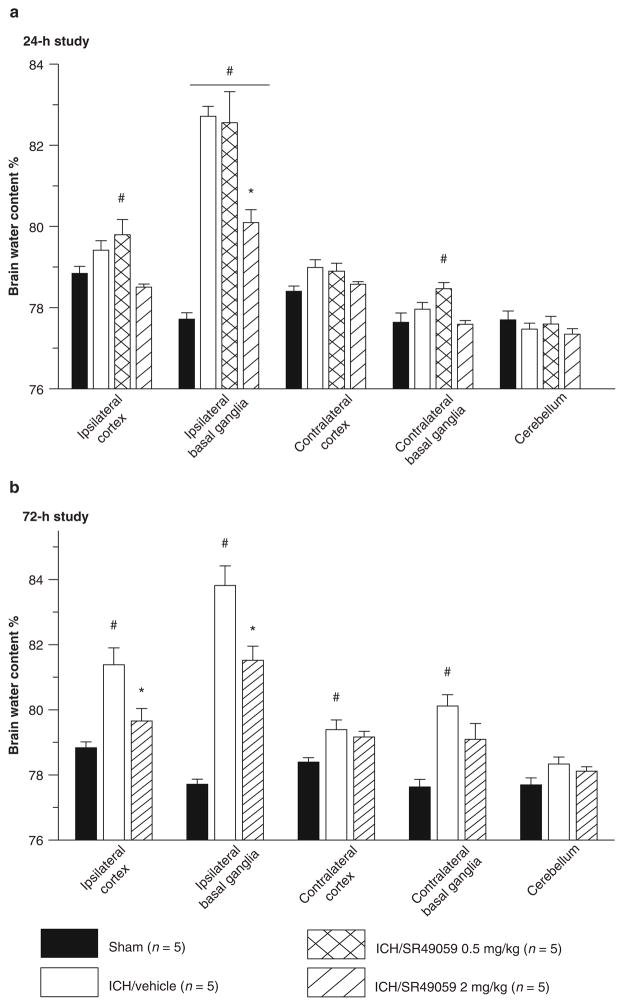
Vehicle groups demonstrated a consistently elevated level of cerebral edema in the ipsilateral basal ganglia. Inhibition of the AVP V1a receptor using high-dose SR49059 was able to significantly reduce the cerebral edema at 24 and 72 h post injury (p < 0.05) (Fig. 1). Low dose SR49059 failed to reduce cerebral edema at both time points.
Fig. 1.
High-dose SR49059 significantly reduced ICH-induced brain edema at 24 (a) and 72 h (b) *Significant difference vs. sham (p < 0.05); #significant difference vs. vehicle (p < 0.05); (a) 24 h: sham = 5; (vehicle) = 5; (ICH + 0.5 mg/kg SR49059) = 5; (ICH + 2 mg/kg SR49059) = 5. (b) 72 h: sham = 5; (vehicle) = 5; (ICH + 0.5 mg/kg SR49059) = 5; (ICH + 2 mg/kg SR49059) = 5
SR49059 improves neurobehavioral deficits
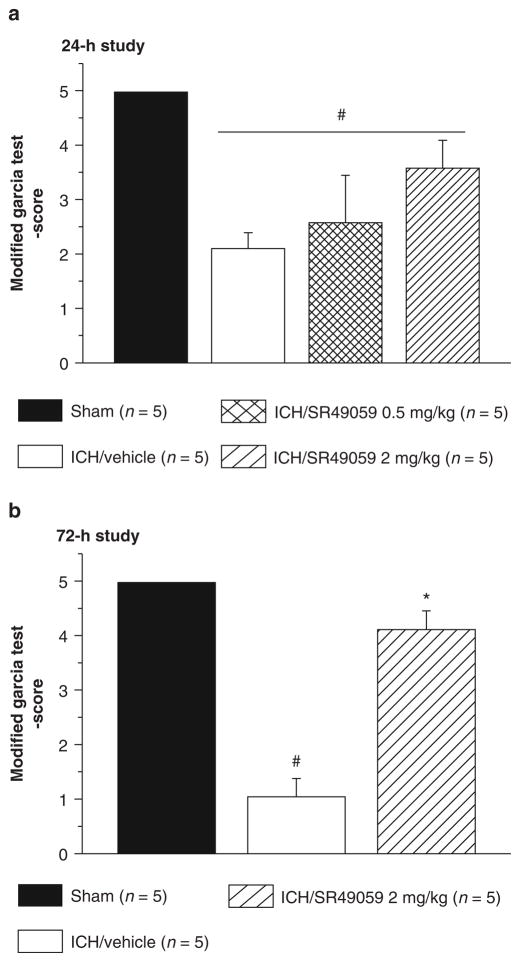
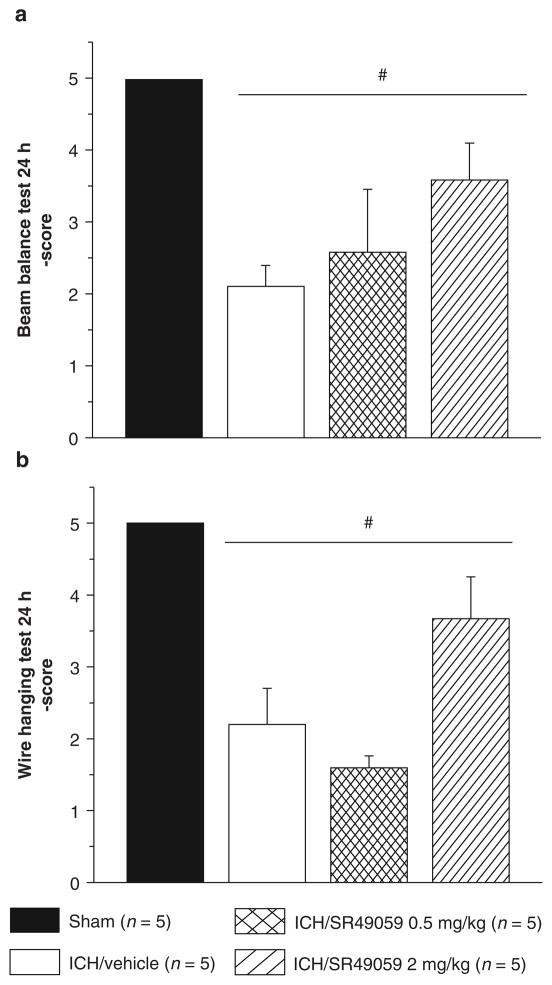
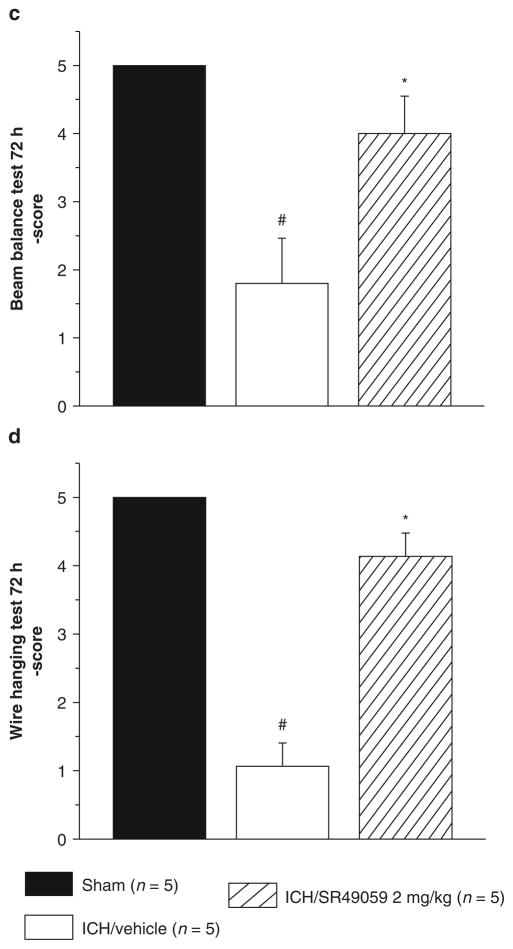
Neurobehavioral deficits were present in all animals after collagenase injection. At 24 h, treatment with SR49059 failed to improve deficits. However, at 72 h post-injury, high-dose SR49059 significantly improved neurobehavioral deficits according to the Modified Garcia test, beam balance, and wire hang tests (Figs. 2 and 3). Additionally, improvements were not seen with low-dose SR49059.
Fig. 2.
High-dose SR49059 has no effect on ICH-induced neurological deficit at 24 h (a), however improved it at 72 h (b). *Significant difference vs. sham (p < 0.05); #significant difference vs. vehicle (p < 0.05); (a) 24 h: sham = 5; (vehicle) = 5; (ICH + 0.5 mg/kg SR49059) = 5; (ICH + 2 mg/kg SR49059) = 5; (b) 72 h: sham = 5; (vehicle) = 5; (ICH + 2 mg/kg SR49059) = 5
Fig. 3.
High-dose SR-49059 is ineffective at 24 h (a and b), however at 72 h improves neurobehavioral deficits as assessed by wire hang (b) and beam balance (d) tests. *Significant difference vs. sham (p < 0.05); #significant difference vs. vehicle (p < 0.05); (a) beam balance, 24 h: sham = 5; (vehicle) = 5; (ICH + 0.5 mg/kg SR49059) = 5; (ICH + 2 mg/kg SR49059) = 5; (b) wire hang, 24 h: sham = 5; (vehicle) = 5; (ICH + 0.5 mg/kg SR49059) = 5; (ICH + 2 mg/kg SR49059) = 5; (c) beam balance, 72 h: sham = 5; (vehicle) = 5; (ICH + 2 mg/kg SR49059) = 5; (d) wire hang, 72 h: sham = 5; (vehicle) = 5; (ICH + 2 mg/kg SR49059) = 5
Discussion
The aim of this study was to determine the effects of AVP V1a receptor inhibition on ICH-induced brain injury in mice. Using high-dose SR49059, a highly specific AVP V1a receptor competitive antagonist, we found there was a strong reduction in brain edema accumulation and improvement in neurobehavioral deficits in our treated versus untreated mice groups. This suggests a key role for AVP in ICH-induced brain injury outcomes. To the best of our knowledge, this is the first study to elucidate the benefits of AVP inhibition in ICH injured mice.
Brain injury following an ICH involves a wide array of consequences, including cerebral edema formation, blood-brain barrier (BBB) disruption, apoptotic cell death, direct injury from the expanding hematoma, and secondary changes from hemoglobin breakdown products and inflammation. Of these, cerebral edema formation seems to be the most devastating – responsible for increasing intracranial pressure that can lead to brain herniation and death [7]. In our bodies, the BBB prevents the movement of large molecules from the blood to the brain, allowing for proper water and ion homeostasis. During an ICH injury, the BBB gets disrupted by various mediators – including inflammatory cells, thrombin, hemoglobin breakdown products, and enzyme activation – which no longer permit the BBB from carrying out its functions [8]. In our study, we found a significant increase in brain edema with ICH injury and subsequent reduction with high-dose SR49059 treatment. This was similar to previous studies in other brain injury models that have looked at the effects of SR49059 on brain edema.
To date, many brain injury studies, including traumatic brain injury (TBI) and middle cerebral artery occlusion (MCAO), have demonstrated AVP’s role in mediating brain edema formation [9, 10]. Although no formal mechanism has been determined, we speculate that AVP may in fact play a role in activating aquaporin channels in the brain, specifically, AQP4. In previous studies, upregulation of AQP4 has been observed in surrounding tissue injury sites in MCAO injury models and focal cerebral ischemic rat models [11]. This relationship could possibly explain the increase in brain edema formation following ICH injury. We acknowledge the fact that more studies will be needed, specifically to look at the relationship between these two proteins.
Conclusion
We conclude that AVP V1a receptor inhibition using SR49059, a specific receptor competitive antagonist, can in fact reduce brain edema and improve neurobehavioral deficits following an ICH injury in mice.
Acknowledgments
This study is partially supported by NIH NS053407 to J.H. Zhang and NS060936 to J. Tang.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
Anatol Manaenko, Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Nancy Fathali, Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Nikan H. Khatibi, Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA
Tim Lekic, Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Kenneth J. Shum, Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Loma Linda, CA, USA
Robert Martin, Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA.
John H. Zhang, Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA and Department of Neurosurgery, Loma Linda University Medical Center, Loma Linda, CA, USA
Jiping Tang, Email: jtang@llu.edu, Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Neurosurgery, Loma Linda University Medical Center, 11234 Anderson Street, Room 2562B, 92354, Loma Linda, CA, USA.
References
- 1.Vakili A, Hiroharu K, Plesnila N. Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:1012–1019. doi: 10.1038/sj.jcbfm.9600097. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Jin Y, Zheng H, Chen G. Arginine vasopressin gene expression in supraoptic nucleus and paraventricular nucleus of hypothalamus following cerebral ischemia and reperfusion. Chin Med Sci J. 1996;15:157–161. [PubMed] [Google Scholar]
- 3.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 4.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Pinsky D. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- 5.Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- 6.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 7.Diringer MN. Intracerebral hemorrhage: pathophysiology and management. Crit Care Med. 1993;2:159–1603. doi: 10.1097/00003246-199310000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Keep RF, Xi G, Hua Y, Hoff JT. The deleterious or beneficial effects of different agents in intracerebral hemorrhage: think big, think small, or is hematoma size important? Stroke. 2005;6:1594–1596. doi: 10.1161/01.STR.0000170701.41507.e1. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Nakayama S, Ottersen OP, Bhardwaj A. Arginine vasopressin V1 but not V2 receptor antagonism modulates infarct volume, brain water content, and aquaporin-4 expression following experimental stroke. Neurocrit Care. 2000;12 (1):124–131. doi: 10.1007/s12028-009-9277-x. [DOI] [PubMed] [Google Scholar]
- 10.Trabold R, Krieg S, Scholler K. Role of vasopressin v1a and v2 receptors for the development of secondary brain damage after traumatic brain injury in mice. J Neurotrauma. 2008;25:1459–1465. doi: 10.1089/neu.2008.0597. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Gao F, Liu H, Yu WH, Sun SQ. Temporal changes in expression of aquaporin-3, -4, -5 and -8 in rat brains after permanent focal cerebral ischemia. Brain Res. 2009;1290:121–132. doi: 10.1016/j.brainres.2009.07.018. [DOI] [PubMed] [Google Scholar]