Abstract
Sensorineural hearing loss is associated with gradual degeneration of spiral ganglion neurons (SGNs), compromising hearing outcomes with cochlear implant use. Combination of neurotrophin delivery to the cochlea and electrical stimulation from a cochlear implant protects SGNs, prompting research into neurotrophin-eluting polymer electrode coatings. The electrically conducting polypyrrole/para-toluene sulfonate containing neurotrophin-3 (Ppy/pTS/NT3) was applied to 1.7 mm2 cochlear implant electrodes. Ppy/pTS/NT3-coated electrode arrays stored 2 ng NT3 and released 0.1 ng/day with electrical stimulation. Guinea pigs were implanted with Ppy/pTS or Ppy/pTS/NT3 electrode arrays two weeks after deafening via aminoglycosides. The electrodes of a subgroup of these guinea pigs were electrically stimulated for 8 hr/day for 2 weeks. There was a loss of SGNs in the implanted cochleae of guinea pigs with Ppy/pTS-coated electrodes indicative of electrode insertion damage. However, guinea pigs implanted with electrically stimulated Ppy/pTS/NT3-coated electrodes had lower electrically-evoked auditory brainstem response thresholds and greater SGN densities in implanted cochleae compared to non-implanted cochleae and compared to animals implanted with Ppy/pTS-coated electrodes (p<0.05). Ppy/pTS/NT3 did not exacerbate fibrous tissue formation and did not affect electrode impedance. Drug-eluting conducting polymer coatings on cochlear implant electrodes present a clinically viable method to promote preservation of SGNs without adversely affecting the function of the cochlear implant.
INTRODUCTION
Cochlear implants provide auditory perception to profoundly deaf individuals with a sensorineural hearing loss by electrically stimulating spiral ganglion neurons (SGNs) via an electrode array implanted into the scala tympani of the cochlea. Biphasic current pulses of varying amplitude, pulse width and rate are sent to the electrodes arranged according to frequency in the cochlea to provide loudness, pitch and temporal cues for speech understanding. SGNs in the vicinity of the stimulated electrode depolarize in response to the delivered charge. However, the loss of hair cells responsible for the sensorineural hearing loss leads to secondary degeneration and apoptosis of SGNs [1]. This is due in part to the loss of neurotrophic support that hair cells would normally provide. It is anticipated that extensive SGN degeneration would limit the functionality of the cochlear implant since fewer neurons would be available to transmit the information to the brain [2]. Further exacerbating the problem, cochlear implantation itself can cause the loss of residual hair cells that may otherwise have provided supplementary acoustic hearing and improved speech recognition after cochlear implantation, especially in the presence of background noise [3].
Experimentally, direct slow-rate administration of the neurotrophins brain-derived neurotrophic factor (BDNF) and/or neurotrophin-3 (NT3) into the scala tympani of the cochlea over the course of several weeks preserved SGNs after sensorineural hearing loss induced by aminoglycosides or noise [4–8]. Neuroprotective effects were limited to the period of neurotrophin delivery and were lost once treatment ceased [9]. Electrical stimulation via a cochlear implant has also been reported to maintain SGN survival after hearing loss [10–14] but other studies find that electrical stimulation alone does not provide enough trophic support for SGN survival [15, 16]. However, simultaneous co-administration of electrical stimulation and neurotrophins provided greater SGN survival after sensorineural hearing loss than either treatment alone [16, 17]. Furthermore, continuation of electrical stimulation beyond the period of neurotrophin delivery to the cochlea maintained the protective effects on SGNs [18]. Therefore, improved SGN survival after sensorineural hearing loss may be achieved via a device that provides prolonged neurotrophin treatment in addition to electrical stimulation.
Neurotrophin delivery via a cannula and electrical stimulation via a cochlear implant have previously been incorporated into a single device to examine the effect of combined treatment on SGN survival [19]. However, the device had a limited drug reservoir that would require frequent changes if drug delivery were to be continuous or surgical removal of the device if treatment were to cease. Furthermore, the drug delivery cannula may also have provided an additional route for bacteria to enter the cochlea. Safer drug delivery options that reduce the risk of infection by providing slow-release neurotrophic support from a single application need to be considered. Recent interest has focussed on drug eluting polymers, such as polypyrrole (Ppy), which may be used to coat cochlear implant electrodes. Ppy is an electroactive polymer consisting of pyrrole monomers held together by negatively charged dopants. Additional factors such as enzymes, DNA or cells can be incorporated and released from Ppy [20]. The main advantage of Ppy over other polymers is that release of incorporated molecules can be induced and controlled with electrical stimulation. For example, dexamethasone was released by cyclic voltammetry from Ppy/dexamethasone-coated neural recording electrodes with the aim of inhibiting scar formation at the electrode insertion site in the brain [21]. Likewise, electrical stimulation enhanced the release of heparin from Ppy/heparin two-fold for anticoagulation applications in implanted devices [22].
We previously created Ppy films using para-toluene sulfonate (pTS) as the negatively charged dopant with or without incorporated NT3 (Ppy/pTS and Ppy/pTS/NT3). NT3 slowly diffused from the polymer in the absence of electrical stimulation, but a large increase in NT3 release occurred when a physiologically relevant electrical stimulus was applied [23]. To test the biological properties of Ppy/pTS and Ppy/pTS/NT3, auditory nerve explants were cultured from rat cochleae and grown directly on the two polymers. Explants grown on Ppy/pTS/NT3 sprouted significantly more neurites than explants grown on Ppy/pTS, demonstrating that the released NT3 was biologically active. Electrical stimulation of Ppy/pTS/NT3 boosted the release of NT3 and neurite outgrowth from explants even further [24].
This paper describes the chronic implantation of Ppy/pTS/NT3-coated electrodes into deafened guinea pig (GP) cochleae to observe effects on SGNs. This study provides the first report of a conducting polymer coating on cochlear implant electrodes being used to preserve SGNs and provide activation of central auditory pathways in vivo.
MATERIALS AND METHODS
In vitro NT3 release kinetics
General kinetics of 125I-labelled NT3 release from Ppy/pTS/125I NT3 polymers were determined as described previously [23]. Briefly, Ppy/pTS/NT3 was synthesised for 60 min on 1 cm2 strips of gold-coated Mylar sheets using 125I-labelled NT3 (ProSearch International, Australia). The polymers were rinsed several times in MilliQ water and placed in a gamma counter for initial 125I NT3 incorporation readings. The polymers were then incubated in saline solution for 7 days at room temperature. Electrodes were divided into two groups; no electrical stimulation or continuous electrical stimulation (1 mA/cm2 biphasic current pulses) using a stainless steel mesh counter electrode. The saline was collected from each well on each day and replaced with new saline. At the end of 7 days, all samples were read in a gamma counter to assay the amount of 125I NT3 present.
Electrode arrays
Four-ring platinum electrode arrays specially designed for implantation in GPs were produced in-house using materials obtained from Cochlear Ltd. All electrode rings were 0.3 mm in width and had tapering diameters from 0.47 mm at the base (E4) to 0.41 mm at the tip of the electrode array (E1). Total electrode surface area for the four electrodes was 1.658 mm2. Electrode rings were set in a silastic carrier with individual stainless steel lead wires and gold pin connectors (Fig 1a). Electrodes were cleaned by sonication and sterilised before polymer coating and implantation.
Figure 1. Four-ring platinum electrode array for implantation in GPs.
(a) The electrode array consisted of four active electrodes individually wired for stimulation as electrode pairs and an extracochlear electrode as a marker for insertion depth. Diagram is not drawn to scale. (b) An electrode array coated with Ppy/pTS implanted into a GP cochlea. The fourth electrode can be seen protruding from the cochleostomy in this example. The fifth uncoated platinum extracochlear electrode is also visible.
Impedance measurements
The impedance of all electrode arrays was measured prior to Ppy-coating or implantation. The four platinum electrodes were electrically shorted and placed in a 50 ml solution of sterile phosphate buffered saline together with a return electrode that had a larger total surface area. A biphasic current pulse (3 mA current, 50-μs phase and 25-μs interphase gap) was passed between the two electrodes and the resulting voltage waveform was recorded on an isolated digital oscilloscope (see Fig 3a). Voltage was recorded from two points: 7 μs from the beginning of the first phase and the end of the first phase (total voltage; Vt). Extrapolation of a line between these two points was used to predict the voltage at 0 μs (access voltage; Va). Polarisation voltage (Vp) was calculated using Vp=Vt−Va. These values were then used to calculate total impedance (Zt), access impedance (Za), and polarisation impedance (Zp) using Ohm’s law (Z=V/I). After coating with Ppy/pTS or Ppy/pTS/NT3, the impedance values of each electrode array were measured again in the same way.
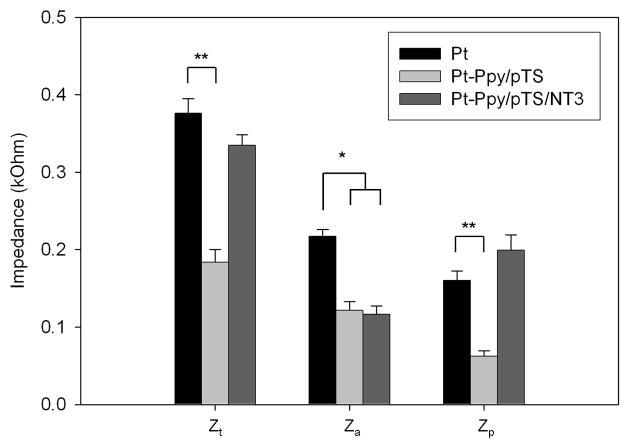
Figure 3. Impedances of uncoated, Ppy/pTS-coated and Ppy/pTS/NT3-coated platinum electrode arrays.
(a) The voltage waveform arising from electrical stimulation of each platinum (Pt) electrode array was used to calculate total impedance (Zt), access impedance (Za) and polarization impedance (Zp), before and after coating with Ppy/pTS or Ppy/pTS/NT3. (b) Zt and Zp of Ppy/pTS-coated electrodes were significantly lower than uncoated electrodes (**p<0.001). The Za of both Ppy/pTS- and Ppy/pTS/NT3-coated platinum electrodes was significantly lower than uncoated platinum electrodes (*p<0.01).
Synthesis of Ppy on electrode arrays
To coat the 4-ring platinum electrode arrays with Ppy/pTS or Ppy/pTS/NT3, the electrode arrays were placed in a 2-electrode set-up in which the platinum rings were electrically connected to form one electrode and a ring of stainless steel mesh was placed around the array as a common counter electrode. Under sterile conditions, the electrode array was placed in a solution of 0.2 M pyrrole, 0.05 M pTS and 2 μg/ml NT3. A constant current was applied at 2 mA/cm2 for 40 min. After polymerization, coated electrodes were rinsed several times in MilliQ water and stored in MilliQ water at 4°C for no longer than 4 days before use.
Guinea pigs
Male or female adult pigmented Dunkin-Hartley GPs averaging 491 ± 19 g were used. National Institutes of Health (NIH) guidelines for the care and use of laboratory animals were observed (NIH Publication #85-23 Rev. 1985). The Animal Research Ethics Committee of the Royal Victorian Eye and Ear Hospital approved the care and use of the animals in this study (ethics #06/131A). GPs were randomly assigned to 4 groups (Table 1).
Table 1.
Groups for in vivo analysis of effects of polymer-coated electrodes on SGNs after hearing loss.
| Group name | Implant coating | Electrical stimulation | n |
|---|---|---|---|
| Ppy | Ppy/pTS | None | 4 |
| PpyNT3 | Ppy/pTS/NT3 | None | 6 |
| Ppy-stim | Ppy/pTS | 2 wk, 8 hr/day | 4 |
| PpyNT3-stim | Ppy/pTS/NT3 | 2 wk, 8 hr/day | 4 |
Auditory brainstem responses
At least one week prior to deafening, an auditory brainstem response (ABR) was performed under injected anaesthesia induced by intramuscular 3:1 ketamine/xylazine mixture (60 mg/kg ketamine; Parnell Laboratories, Australia and 4 mg/kg ilium xylazil-20; Troy Laboratories, Australia). Computer generated acoustic rarefaction clicks were delivered to the anaesthetised GPs via a loudspeaker placed 10 cm from the pinna. ABRs were recorded differentially using subcutaneous needle electrodes placed at the vertex and the nape of the neck, with an additional needle electrode in the thorax as a ground. Normal hearing was defined by a threshold <43 dB peak equivalent sound pressure level (SPL). Another ABR was performed after deafening just prior to the implantation procedure to confirm deafness, defined as threshold shifts of >50 dB.
Deafening
Under gaseous anaesthetic (induced with 2% isofluorane and 1% oxygen and maintained with 1.5% isofluorane and 0.5% oxygen), the jugular vein was exposed and cannulated for the intravenous administration of the loop diuretic frusemide (100 mg/kg; Troy Laboratories, Australia). This was followed by a subcutaneous injection of the aminoglycoside kanamycin sulphate (400 mg/kg; Sigma-Aldrich, Australia). The wound was closed with tissue adhesive. The health and weight of the GP were carefully monitored over the following weeks. Animals were deafened two weeks before implantation.
Cochlear implantation
Using injected anaesthesia as described for ABRs, a post-auricular incision was made to expose the tympanic bulla. The bulla was opened using a 2 mm cutting drill-bit and a cochleostomy performed with a 0.6 mm diamond drill-bit. The polymer-coated electrode was inserted into the cochlea through the cochleostomy (Fig 1b). Connective tissue was placed around the insertion site to promote sealing of the entry site. The lead wire of the electrode array was secured in the bulla with dental cement then directed subcutaneously towards the skull where it was secured with a titanium clip and a screw. The lead wire was then directed towards the nape of the neck where it exited the skin for connection to stimulators. The wounds were then closed in layers with internal and external sutures.
Electrically-evoked auditory brainstem responses
Immediately following implantation and under the same anaesthesia, electrically-evoked auditory brainstem responses (EABRs) were measured using recording needles placed as previously described for ABRs. Biphasic stimuli (100 μs/phase) were delivered in bipolar configuration to electrode pairs, E1/E2 and E3/E4. The resultant biological signals were differentially amplified and recorded using a custom designed data acquisition system (National Instruments DAQ board; Igor Pro software). Electrical stimuli with intensities up to 2 mA were randomly presented and an average response was generated for each stimulus intensity from 100 repetitions. At least two average response traces were recorded for each current level. The peak to peak wave III responses that were present 1.5–2.5 ms following electrical stimulation were measured and used to generate EABR threshold growth functions. EABR threshold was defined as the current level that produced a peak to peak response above baseline levels. The EABR threshold data were used to set the levels of chronic electrical stimulation to ensure activation of the auditory pathway. At the conclusion of the two week implantation period EABR thresholds were again measured to determine changes in EABR thresholds over time.
Electrical stimulation
Electrodes were stimulated as bipolar pairs. Electrodes 1 and 2, the electrodes at the tip of the array (hence the most deeply inserted electrodes) were stimulated as an electrode pair (E1/E2). Electrodes 3 and 4, the electrodes closer to the base of the array (hence those closest to the cochleostomy) were stimulated as the second electrode pair (E3/E4).
On the day following implantation, charge-balanced biphasic current pulses (pulse width 100μs, 250 Hz) were delivered to the two electrode pairs for 8 hr/day using in-house portable stimulators placed in pockets of specially designed jackets worn by the GPs. Current amplitude was set at 3 dB above EABR threshold determined for each implanted GP. However, behavioural cues were also used to fine-tune the current amplitude. For example, if GPs did not respond to the stimulus, the current amplitude was slowly increased until a response such as an ear twitch was observed. If GPs showed signs of distress at stimulus switch-on, the current amplitude was decreased until a comfortable level was found. Similarly, over the two weeks of electrical stimulation, GPs that no longer responded to the stimulus switch-on (for example with an ear twitch), had their stimulus current amplitude slowly increased. Actual stimulating currents ranged between 350 μA and 825 μA.
Impedance of implanted electrodes
On each day of electrical stimulation of electrodes implanted in GP cochleae, impedance measurements were recorded just after switch-on and just before switch-off. As the charge-balanced biphasic current pulses were applied to the electrodes, the resulting voltage waveform was displayed on an analogue oscilloscope. Vt and Va were measured from the oscilloscope display and Zt, Za and Zp were calculated as described earlier.
Perfusion, histology and SGN counts
Two weeks post-implantation GPs were euthanised with 1.5 ml pentobarbitone and intracardially perfused with 0.9% (w/v) saline containing 0.1% (v/v) heparin sodium and 0.025% (w/v) sodium nitrite, followed by 10% (v/v) neutral buffered formalin (NBF). The bullae were removed and the cochleae dissected out. Cochleae were placed in 10% (v/v) NBF for a further 24 hr and then decalcified over 7–14 days in 10% (w/v) EDTA in 0.1 M phosphate buffer. Cochleae were embedded in OCT (Tissue–Tek) and sectioned on a cryostat at 12μm through the mid-modiolar orientation and mounted onto Menzel-Glaser Superfrost-Plus slides [25].
Sections were rated 1–4 for insertion damage or fibrous tissue response to the implants. Rating 1 was given to sections in which there was little or no fibrous tissue reaction, rating 2 to sections with fibrous tissue that occupied less than 10% of the scala tympani, 3 to sections with fibrous tissue occupying 10–50% of the scala tympani and 4 to sections with fibrous tissue that occupied 50–100% of the scala tympani. In cases where the electrode footprint was visible, the upper basal turn or adjacent sections were used to help assess the fibrous tissue rating.
For SGN density and soma area measurements, four non-consecutive sections from every cochlea were dehydrated through three lots of 100% (v/v) ethanol and stained with haematoxylin and eosin. The areas of Rosenthal’s canal in the basal, middle and apical turns were measured using a Zeiss Axioplan II microscope and Axiovision software. The number of SGN soma within the measured areas was counted in a blinded manner and expressed as a density. Ten SGN somas were randomly selected (using a random number generator and overlaid grid) for cross-sectional soma area measurement.
Statistical analysis
Cumulative release of NT3 from stimulated and non-stimulated polymers in vitro was compared using a t-test. Impedances of platinum and coated platinum electrodes prior to implantation were compared using one way ANOVAs and Bonferroni post-hoc analyses. Pairwise t-tests were used to compare SGN densities and SGN soma areas of implanted and non-implanted cochleae of GPs in each group. Comparisons between groups were performed using one-way ANOVAs and Tukey post-hoc analyses. Confidence levels were set at 95%. All data are presented as mean ± standard error.
RESULTS
1. In vitro studies
Kinetics of NT3 release from Ppy/pTS/NT3-coated electrodes
An average of 128.7 ± 12.5 ng NT3 was incorporated into 1 cm2 Ppy/pTS/NT3-coated gold Mylar electrodes (n=6). In the absence of electrical stimulation, NT3 was released at an average rate of 1.3% of total incorporated NT3 per day over 7 days (1.7 ng per day; n=3). When biphasic electrical stimulation was applied, release of NT3 was enhanced 3.5 fold to 4.6% per day over 7 days (5.9 ng per day; n=3) (Fig 2). The difference between the cumulative NT release over 7 days is statistically significant (p<0.001). Converting these figures to 1.658 mm2 implantable electrode arrays, it is estimated they would contain 2.13 ng NT3 and release 0.03 ng NT3 per day when not stimulated and approximately 0.1 ng NT3 per day during electrical stimulation.
Figure 2. 125I NT3 release kinetics from stimulated and non-stimulated Ppy/pTS/125I NT3-coated electrodes.
Cumulative release of 125I NT3 (as a percent of total NT3 incorporated) was 3.5 fold greater from stimulated 1 cm2 Ppy/pTS/125I NT3-coated electrodes compared to non-stimulated Ppy/pTS/125I NT3-coated electrodes over 7 days. Error bars indicate the standard error of the mean.
Pre-implantation impedance of Ppy-coated platinum electrode arrays
Impedances of implantable platinum electrode arrays were measured before and after coating with Ppy/PTS or Ppy/pTS/NT3. Zt and Zp of Ppy/pTS-coated platinum electrode arrays, but not Ppy/pTS/NT3-coated electrode arrays, were significantly lower than uncoated platinum electrode arrays (p<0.001). Za of both Ppy/pTS and Ppy/pTS/NT3-coated arrays were significantly lower than that of uncoated platinum arrays (p<0.01) (Fig 3b).
2. In vivo studies
Effects of deafening
Before deafening, GPs had click-evoked auditory brainstem response thresholds at 34.2 ± 0.8 dB peak-equivalent SPL. After deafening, auditory thresholds were 92.8 ± 0.2 dB SPL giving an average threshold shift of 58.8 ± 0.9 dB.
Effects of Ppy/NT3 coating ± electrical stimulation – SGN survival
Figure 4 shows examples of photomicrographs of SGNs in Rosenthal’s canal of the basal turn of the implanted cochlea in each group. Comparing SGN densities in implanted cochleae to their corresponding contralateral non-implanted cochlea, GPs in the Ppy group had significantly fewer SGNs in their implanted cochlea (968 ± 72 SGNs/mm2) compared to their non-implanted cochlea (1122 ± 79 SGNs/mm2) (p<0.05; pairwise t-test). Conversely, there was a significantly greater density of SGNs in the implanted cochlea of GPs in the PpyNT3-stim group (1130 ± 42 SGNs/mm2) compared to the non-implanted cochlea in the basal turn (995 ± 33 SGNs/mm2) (p=0.05 paired t-test). There were no significant differences between implanted and non-implanted cochleae in the PpyNT3 and Ppy-stim groups.
Figure 4. Examples of Rosenthal’s canals in the basal turn of implanted cochleae.
Micrographs of Rosenthal’s canals such as these were used for SGN density and soma area measurements. Examples are shown from the basal turn of each group.
The difference in SGN density between implanted and non-implanted cochleae was calculated for all turns and compared across groups. There was an overall difference between groups in the basal turn (p=0.001; one-way ANOVA), with a Tukey post-hoc analysis finding a significant difference between Ppy and PpyNT3-stim groups (p<0.01) and the PpyNT3 and PpyNT3-stim groups (p<0.01) (Fig 5a).
Figure 5. SGN survival in the basal, middle and apical turns of implanted cochleae.
(a) In the basal turn, the difference in SGN densities between implanted and non-implanted cochleae in the PpyNT3-stim group was significantly greater than Ppy and Ppy/NT3 groups (* p<0.01). (b) In the middle turn, there were also significant differences in SGN survival between PpyNT3stim and PpyNT3 groups (* p<0.05) as well as between Ppy-stim and both Ppy and PpyNT3 groups (* p<0.01). (c) No significant differences between groups were detected in the apical turns. Error bars indicate the standard error of the mean.
In the middle turn, the difference in SGN densities between implanted and non-implanted cochleae was significantly greater in the PpyNT3-stim group compared to the PpyNT3 group (p<0.05) and in the Ppy-stim group compared to the Ppy and PpyNT3 groups (p<0.001) (Fig 5b). There were no significant differences between groups in the apical turns (Fig 5c).
SGN soma areas
The areas of SGN somas were measured in the basal turns of implanted and non-implanted cochleae in each GP. Compared to non-implanted contralateral cochleae, the area of SGN somas in implanted cochleae were significantly larger in the PpyNT3 group (132 ± 9 μm2 vs 113 ± 6 μm2) and the Ppy-stim group (121 ± 8 μm2 vs 106 ± 6 μm2) (p<0.001). In Ppy and PpyNT3-stim groups, the implanted and non-implanted cochleae were not significantly different to each other. The difference between implanted and non-implanted cochleae of each GP was calculated and compared across groups using a one-way ANOVA. Significant differences were identified between the following groups: Ppy and PpyNT3 (p<0.001), Ppy and Ppy-stim (p<0.05), and PpyNT3 and PpyNT3-stim (p<0.01) (Fig 6).
Figure 6. Cross-sectional area of SGN somas.
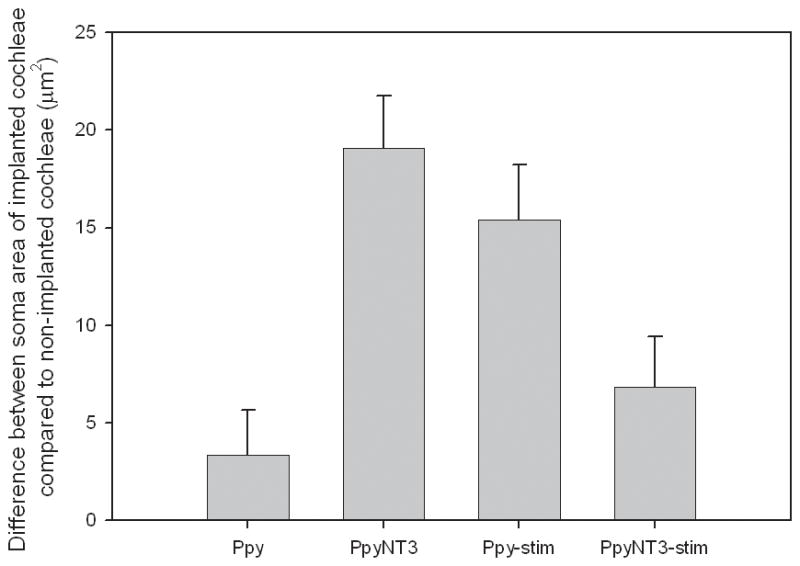
The difference between implanted and non-implanted SGN soma areas was calculated for each group and compared using one-way ANOVA. The difference in SGN soma area was greater in PpyNT3 and Ppy-stim groups compared to the Ppy group (p<0.05). The PpyNT3 group also had a significantly larger SGN somas in implanted cochleae with respect to non-implanted cochleae compared to the PpyNT3-stim group (p<0.01). Error bars represent standard error of the mean.
Pathology of implanted cochleae
Out of 18 implanted GPs, 13 had minor or no fibrous tissue present in histological sections (rating 1–2) (Table 2; Fig 7 a–b). The remaining 5 GPs had fibrous tissue occupying 10% or more of the scala tympani in cross section (rating 3–4) (Table 2) (Fig 7 c–e). These 5 GPs were spread across all implanted groups. There was histological evidence of minor insertion damage to the osseous spiral lamina in 2 GPs, one in each of the PpyNT3 and Ppy-stim groups (Table 2). No other cochlea showed evidence of insertion trauma. There was no evidence of additional loss of SGNs in GPs identified with minor insertion damage.
Table 2.
Fibrous tissue reaction and insertion damage in implanted GPs
1- Little or no fibrous tissue reaction
2- Fibrous tissue occupying less than 10% of the scala tympani in cross section
3- Fibrous tissue occupying 10–50% of the scala tympani in cross section
4- Fibrous tissue occupying 50–100% of the scala tympani in cross section
-Minor insertion damage
Figure 7. Examples of fibrous tissue formation in implanted cochleae.
(a) A section in which there was little or no fibrous tissue reaction (rating 1). (b) A section in which fibrous tissue occupied less than 10% of the scala tympani in cross section (arrows) (rating 2). (c) A section in which fibrous tissue occupied 10–50% of the scala tympani in cross section (rating 3). (d) A section in which fibrous tissue occupied 50–100% of the scala tympani in cross section (rating 4). In this example, the electrode ‘footprint’ can be seen; the upper basal turn of the same cochlea was completely filled with fibrous tissue (e). The site of cochleostomy is sometimes visible in the outer wall of the scala tympani (b, d). ST=scala tympani
Functional effects - EABR thresholds
EABR thresholds for both electrode pairs were determined immediately post-implantation and at the completion of the two-week implantation period for GPs that received chronic electrical stimulation. EABR thresholds were 15.2% higher for the basal E3/E4 electrode pair than for the apical E1/E2 electrode pair (data not shown). EABR thresholds for E1/E2 rose significantly over the two week period for GPs in the Ppy-stim group (54.4% increase, p<0.05, paired t-test), whereas the rise in thresholds in the PpyNT3-stim group was not significant (28.6% increase) (Fig 8).
Fig 8. EABR threshold measurements in Ppy-stim and PpyNT3-stim groups.
Average EABR thresholds for the apical electrode pair (E1/E2) were measured pre and post stimulation for the Ppy-stim and PpyNT3-stim groups and the percentage change was calculated. Thresholds were higher for the Ppy-stim group and following the two week stimulation period.
Impedances of electrodes in implanted GPs
Impedances of implanted electrodes were measured twice daily at switch-on and switch-off in Ppy-stim and PpyNT3-stim groups. In both groups there was a gradual increase in impedance over the 2 week period of implantation (99 ± 30% in Ppy-stim group and 141 ± 56% in PpyNT3-stim group). There was no significant difference in the change in impedance over two weeks between the Ppy-stim and PpyNT3-stim groups.
DISCUSSION
When neurotrophins are delivered directly to the cochlea in high concentrations after sensorineural hearing loss, SGN survival is near 100% for the duration of drug delivery [5, 26–28]. However, it is not possible to translate this research directly to the clinic for a number of reasons; SGN survival drops off significantly after drug delivery has ceased, suggesting that neurotrophin delivery must be provided continuously [9]; neurotrophin delivery at high concentrations cannot be sustained over long periods due to potential side effects in the cochlea and in the brain where substances are known to spread after delivery to the cochlea [29–35]; there are also technical difficulties of delivering neurotrophins locally to the inner ear over long periods [36]. To investigate a clinically relevant method for delivering neurotrophins to the cochlea after sensorineural hearing loss, this study used Ppy/pTS/NT3 coated platinum electrode arrays implanted into deafened GPs for preservation of SGNs. Prior to implantation, the impedances of electrode arrays and the kinetics of NT3 release were analysed. Post-implantation, EABR thresholds, impedances, SGN density, SGN soma area and tissue response were examined.
Drug elution from the cochlear implant has been explored previously, using a mini-osmotic pump or neurotrophin-releasing cells in a gel surrounding the implant [18, 19, 37]. The conducting polymer coating investigated in this study ensures that the function of the cochlear implant is not affected making obsolete the need for cannulae that may increase the infection risk.
Kinetics of NT3 release from Ppy/pTS/NT3
In vitro analysis of 125I NT3 release from stimulated and non-stimulated Ppy/pTS/125I NT3 electrodes revealed some leakiness of the polymer in the absence of stimulation and enhanced NT3 release with electrical stimulation. It was estimated that 2.13 ng NT3 was incorporated into 1.658 mm2 Ppy/pTS/NT3-coated electrode arrays. The rate of release of neurotrophins from non-stimulated Ppy/pTS/NT3-coated electrodes (estimated to be 0.03 ng/day) was not sufficient to overcome degeneration of SGNs. At a rate of release of 4.6% of total incorporated NT3 per day during electrical stimulation, 0.1 ng NT3 would be released per day from Ppy/pTS/NT3-coated arrays. This is very low compared to 360–700 ng/day in many studies using mini-osmotic pumps to deliver neurotrophins to the cochlea [4, 27, 38, 39]. Nevertheless, there was SGN survival in cochleae implanted with stimulated Ppy/pTS/NT3-coated electrodes. At the stimulated rate of NT3 release, the polymer would be depleted of neurotrophin by 21 days with continuous electrical stimulation or slightly longer with 8 hr/day stimulation. This suggests that Ppy/pTS/NT3-coated electrodes will only be useful to help protect SGNs during the first few weeks after implantation. In one study, however, it was found that combined drug delivery and electrical stimulation, followed by electrical stimulation alone promoted extended SGN survival beyond the period of drug delivery [18]. Therefore, short-term slow-rate neurotrophin delivery to the cochlea can provide an extended survival benefit to SGNs if cochlear electrical stimulation is continued.
Degenerative changes associated with deafness and implantation
The mean ABR threshold of GPs in this study was almost 60 dB greater at the time of implantation (two weeks after deafening) than pre-deafening. However, four weeks after deafening, SGN densities were not significantly lower than those measured from normal hearing GPs despite loss of hair cells and partial loss of structure of the organ of Corti. Cochleae implanted with non-stimulated Ppy/pTS- and Ppy/pTS/NT3-coated electrodes showed loss of SGNs with respect to contralateral non-implanted cochleae in the basal turn. This could be attributed to implantation trauma or, alternatively, toxicity from the Ppy coatings. Only two cochleae showed visible evidence of minor trauma (one in the Ppy-stim group and one in the PpyNT3 group), ruling out loss of SGNs due to obvious physical trauma in the cochlea. However, we cannot rule out loss of SGNs due to other factors relating to cochlear implantation e.g. drilling, cochleostomy, and increased fluid pressure during insertion. It also cannot be ruled out that Ppy/pTS is toxic to SGNs in vivo, perhaps due to excess pyrrole or pTS remaining from synthesis, or being released from the Silastic electrode carrier following absorption during polymerisation, despite the fact that polymers are thoroughly washed before use. However, SGNs from post-natal rats have been cultured on Ppy/pTS as well as in media containing pyrrole (Ppy monomer) and pTS without apparent influence on SGN survival [24]. Furthermore, biocompatibility of Ppy made with various dopants has been extensively studied in vitro and in vivo with no reports of major toxicity issues [40–42]. Para-toluene sulfonate was chosen as the dopant for Ppy as it was the best characterised polymer at the time of the study in terms of NT3 release and stability of the coatings over electrodes. Toxicity arising specifically from Ppy/pTS can be eliminated by replacing the dopant with other negatively charged dopants without significantly affecting NT3 release [23].
Combination of neurotrophins and electrical stimulation
This study found that SGN preservation after hearing loss and cochlear implantation was greater when the implant was coated with Ppy/pTS/NT3 and when electrical stimulation was provided compared to other conditions tested. This is in agreement with previous studies in which it was shown that the combination of neurotrophins and electrical stimulation provides greater SGN preservation than either treatment alone [16–18]. However, it was not possible in this experiment to distinguish whether the greater SGN survival resulted from the enhanced release of NT3 from the Ppy/pTS/NT3-coated electrodes due to electrical stimulation or an interacting trophic effect on SGNs from exposure to a combination of neurotrophins and electrical stimulation. Electrical stimulation has been shown to up-regulate neurotrophin receptors on neurons [43, 44]. There is also evidence in vitro that greater neurite outgrowth occurred when SGN explants were grown on stimulated Ppy/pTS/NT3 than would be expected from the amount of NT3 that was released [24], which may be due to enhanced responsiveness of SGNs to neurotrophins after depolarisation. A possible way to distinguish these factors is to release of NT3 from the polymers using sub-threshold stimulation, such that a greater amount of NT3 is released than non-stimulated polymers without depolarizing the SGNs.
Each GP received different stimulation currents as the stimulation current was set at 3 dB over EABR threshold, hence NT3 release would have been slightly different for each GP in the PpyNT3-stim group. However, the average stimulating currents used over the two weeks in the PpyNT3-stim group were remarkably similar (516μA, 500μA, 575 μA and 595 μA) and would not have dramatically affected the amount of NT3 released.
Effects on SGNs were also observed in the middle turns of the cochlea, especially in the two stimulated groups, but not the apical turns. Effects of current spread to middle and even apical turns have been observed in other studies [14, 45], whereas effects of neurotrophins are usually local to the delivery site [46].
SGN soma area data did not correlate with SGN survival data in this study. Previous studies demonstrate larger SGN somas in neurotrophin-treated cochleae [16, 47] but not with stimulation alone [16, 48]. However, in cochleae where SGN survival is evident, there is usually a corresponding increase in soma area. The variability in the data, low animal numbers or the use of frozen sections for histological analysis may have contributed to this lack of correlation.
EABRs
After implantation, EABR thresholds are known to gradually increase over time as a consequence of the gradual loss of neural tissue in the cochlea after sensorineural hearing loss [49]. Formation of a fibrous tissue capsule around the implant can also have a minor impact on EABR threshold by affecting the current distribution. In this study, EABRs were measured in the two stimulated groups. Only EABRs from the E1/E2 electrode pairs have been presented here as these were always fully implanted, whereas E4 in the E3/E4 electrode pair was often not completely internalised in the cochlea (see Fig 1b). GPs in the Ppy-stim group exhibited a significant increase in EABR threshold over 2 weeks of implantation, whereas GPs in the PpyNT3-stim group did not. The thickness of the fibrous tissue capsule did not correlate with changes in EABR threshold over 2 weeks. Furthermore, GPs in the PpyNT3-stim group had greater SGN density than GPs in the Ppy-stim group. Taken together, the EABR changes over 2 weeks of implantation in this study may reflect greater preservation of SGNs due to NT3 release or electrical stimulation or both. Lower EABR thresholds equate to lower stimulating voltages necessary to deliver set levels of current and improved battery life of the cochlear implant speech processor.
Impedance changes with Ppy-coating
Zt, Za and Zp are commonly measured in cochlear implantees and in vivo experiments when entire electrode impedance spectroscopy cannot be performed on an implanted electrode (Ni 1992; Xu 1997). The total impedance of Ppy-coated platinum electrodes was reduced compared to the same platinum electrodes prior to coating, while Ppy/NT3-coated electrodes were not significantly different to uncoated platinum electrodes. The drop in impedance after Ppy/pTS coating was attributed to decreases in both Za and Zp. Za decreased after electrodes were coated with either Ppy or Ppy/NT3. Since Za represents changes to the electrolyte, it is unknown why this occurred, although it was consistently observed. Za measurement of clean electrodes in solutions in which Ppy/pTS or Ppy/pTS/NT3 were stored were not affected, hence there was no leakage or breakdown of components of the Ppy coatings that increased the conductivity of the solution. Zp was reduced after coating of platinum electrodes with Ppy/pTS but not Ppy/pTS/NT3. The Ppy/pTS or Ppy/pTS/NT3 coatings on platinum electrodes are rough and porous, effectively increasing the surface area of the electrode array with conductive elements [50, 51]. It is possible that the electron conduction in Ppy/pTS/NT3 electrodes is impeded by the presence of the non-conductive NT3, or that the polymer strand connectivity or entanglement is modified during polymerisation due to the presence of the protein, reducing the conductive surface area and bringing the impedance of the coated electrode back to the level of platinum. It is also possible that NT3 in the polymerisation solution may have bio-fouled the platinum electrodes prior to polymerisation, leading to decreased electron transfer between the platinum and Ppy layers. No difference in electrode impedance was detected between Ppy- and Ppy/pTS/NT3-coated electrodes after implantation.
It is important for cochlear implant applications that electrode impedance remains low for battery life and optimal SGN stimulation. For this reason, Ppy/pTS/NT3 coatings on cochlear implant electrodes are superior to other drug-eluting electrode coatings as they do not impede the current flow from the electrode.
CONCLUSIONS
Neurotrophin-eluting conducting polymer coatings for cochlear implant electrodes have been described. When implanted into deafened GP cochleae, stimulated Ppy/pTS/NT3-coated electrodes promoted a slight improvement to neural density, as indicated from histology and lower EABR thresholds, without affecting fibrous tissue formation. Polymer coated electrodes have similar impedance to plain platinum electrodes, hence the coating does not detract from the original purpose of the cochlear implant; that is to deliver electrical current to SGNs for auditory perception. NT3 release from Ppy/pTS/NT3-coated electrodes was greater when electrical stimulation was applied to the electrodes compared to non-stimulated coated electrodes; hence these polymers enable control over the rate of release of NT3. This work demonstrates the use of the cochlear implant deliver trophic agents to SGNs in a safe and controlled manner over the short-term in addition to electrical stimulation for enhanced preservation of SGNs after hearing loss.
Acknowledgments
The authors would like to thank the following funding institutions associated with this research: Stavros S. Niarchos Foundation, John T Reid Charitable Trusts, Royal National Institute for Deaf People, Pierce Armstrong Foundation, The University of Melbourne, Department of Otolaryngology, NIH contract HHS-N-263-2007-00053-C and ARC Centre of Excellence for Electromaterials Science. The authors are also grateful for the help of Dr Carrie Newbold in collection of impedance data and research engineer Rodney Millard for helpful discussions.
References
- 1.Shepherd RK, Hardie NA. Deafness-induced changes in the auditory pathway: implications for cochlear implants. Audiol Neurootol. 2001;6:305–18. doi: 10.1159/000046843. [DOI] [PubMed] [Google Scholar]
- 2.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–20. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 3.Di Nardo W, Cantore I, Melillo P, Cianfrone F, Scorpecci A, Paludetti G. Residual hearing in cochlear implant patients. Eur Arch Otorhinolaryngol. 2007;264:855–60. doi: 10.1007/s00405-007-0270-8. [DOI] [PubMed] [Google Scholar]
- 4.Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nature Medicine. 1996;2:463–7. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 5.Staecker H, Gabaizadeh R, Federoff H, Van De Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- 6.Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA, Miller JM. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000;146:134–42. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 7.McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–72. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–65. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. Journal of Neuroscience Research. 2003;71:785–90. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- 10.Lousteau R. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope. 1987;97:836–42. [PubMed] [Google Scholar]
- 11.Hartshorn D, Miller J, Altschuler R. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngology Head and Neck Surgery. 1991;104:311–9. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- 12.Leake P, Hradek GT, Rebscher SJ, Snyder RL. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hearing Research. 1991;54:251–71. doi: 10.1016/0378-5955(91)90120-x. [DOI] [PubMed] [Google Scholar]
- 13.Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Chronic intracochlear electrical stimulation in neonatally deafened cats: effects of intensity and stimulating electrode location. Hear Res. 1992;64:99–117. doi: 10.1016/0378-5955(92)90172-j. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens Hear Res. 1994;81:150–66. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–58. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–60. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242:100–9. doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172:92–8. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 20.Wallace GG, Kane-Maguire LAP. Manipulating and monitoring biomolecular interactions with conducting electroactive polymers. Advanced Materials. 2002;14:953–60. [Google Scholar]
- 21.Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J Control Release. 2006;110:531–41. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Neoh KG, Kang ET. Controlled release of heparin from polypyrrole-poly(vinyl alcohol) assembly by electrical stimulation. J Biomed Mater Res A. 2005;73:171–81. doi: 10.1002/jbm.a.30286. [DOI] [PubMed] [Google Scholar]
- 23.Thompson BC, Moulton SE, Ding J, Richardson R, Cameron A, O’Leary S, Wallace GG, Clark GM. Optimising the incorporation and release of a neurotrophic factor using conducting polypyrrole. J Control Release. 2006;116:285–94. doi: 10.1016/j.jconrel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Richardson RT, Thompson B, Moulton S, Newbold C, Lum MG, Cameron A, Wallace G, Kapsa R, Clark G, O’Leary S. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials. 2007;28:513–23. doi: 10.1016/j.biomaterials.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Coleman B, Hardie NA, de Silva MG, Shepherd RK. A protocol for cryoembedding the adult guinea pig cochlea for fluorescence immunohistology. J Neurosci Methods. 2008 doi: 10.1016/j.jneumeth.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–94. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 27.Miller JM, Chi DH, O’Keefe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–43. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–60. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalwani AK, Walsh BJ, Reilly PG, Muzyczka N, Mhatre AN. Development of in vivo gene therapy for hearing disorders: introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588–92. [PubMed] [Google Scholar]
- 30.Lalwani AK, Han JJ, Walsh BJ, Zolotukhin S, Muzyczka N, Mhatre AN. Green fluorescent protein as a reporter for gene transfer studies in the cochlea. Hear Res. 1997;114:139–47. doi: 10.1016/s0378-5955(97)00151-2. [DOI] [PubMed] [Google Scholar]
- 31.Lalwani AK, Walsh BJ, Carvalho GJ, Muzyczka N, Mhatre AN. Expression of adeno-associated virus integrated transgene within the mammalian vestibular organs. Am J Otol. 1998;19:390–5. [PubMed] [Google Scholar]
- 32.Stover T, Yagi M, Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7:377–83. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- 33.Kho ST, Pettis RM, Mhatre AN, Lalwani AK. Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Mol Ther. 2000;2:368–73. doi: 10.1006/mthe.2000.0129. [DOI] [PubMed] [Google Scholar]
- 34.Shoji F, Yamasoba T, Magal E, Dolan DF, Altschuler RA, Miller JM. Glial cell line-derived neurotrophic factor has a dose dependent influence on noise-induced hearing loss in the guinea pig cochlea. Hear Res. 2000;142:41–55. doi: 10.1016/s0378-5955(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 35.Tateya I, Nakagawa T, Iguchi F, Kim TS, Endo T, Yamada S, Kageyama R, Naito Y, Ito J. Fate of neural stem cells grafted into injured inner ears of mice. Neuroreport. 2003;14:1677–81. doi: 10.1097/00001756-200309150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Richardson RT, Wise AK, Andrew JK, O’Leary SJ. Novel drug delivery systems for inner ear protection and regeneration after hearing loss. Expert Opinion in Drug Delivery. 2008;5:1059–76. doi: 10.1517/17425247.5.10.1059. [DOI] [PubMed] [Google Scholar]
- 37.Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180–7. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staecker H, Galinovic-Schwartz V, Liu W, Lefebvre P, Kopke R, Malgrange B, Moonen G, Van De Water TR. The role of the neurotrophins in maturation and maintenance of postnatal auditory innervation. Am J Otol. 1996;17:486–92. [PubMed] [Google Scholar]
- 39.Ruan RS, Leong SK, Mark I, Yeoh KH. Effects of BDNF and NT-3 on hair cell survival in guinea pig cochlea damaged by kanamycin treatment. Neuroreport. 1999;10:2067–71. doi: 10.1097/00001756-199907130-00014. [DOI] [PubMed] [Google Scholar]
- 40.George PM, Lyckman AW, Lavan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials. 2005;26:3511–9. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Gu X, Yuan C, Chen S, Zhang P, Zhang T, Yao J, Chen F, Chen G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2004;68:411–22. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 42.Ramanaviciene A, Kausaite A, Tautkus S, Ramanavicius A. Biocompatibility of polypyrrole particles: an in-vivo study in mice. J Pharm Pharmacol. 2007;59:311–5. doi: 10.1211/jpp.59.2.0017. [DOI] [PubMed] [Google Scholar]
- 43.Kingsbury TJ, Murray PD, Bambrick LL, Krueger BK. Ca(2+)-dependent regulation of TrkB expression in neurons. J Biol Chem. 2003;278:40744–8. doi: 10.1074/jbc.M303082200. [DOI] [PubMed] [Google Scholar]
- 44.Du J, Feng L, Yang F, Lu B. Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol. 2000;150:1423–34. doi: 10.1083/jcb.150.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang DH, Lusted HS, White RL. The nerve-electrode interface of the cochlear implant: current spread. IEEE Trans Biomed Eng. 1999;46:35–43. doi: 10.1109/10.736751. [DOI] [PubMed] [Google Scholar]
- 46.Richardson RT, Noushi F, O’Leary S. Inner Ear Therapy for Neural Preservation. Audiol Neurootol. 2006;11:343–56. doi: 10.1159/000095896. [DOI] [PubMed] [Google Scholar]
- 47.Richardson RT, O’Leary S, Wise A, Hardman J, Clark G. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res. 2005;204:37–47. doi: 10.1016/j.heares.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Araki S, Kawano A, Seldon H, Shepherd R, Funasaka S, Clark G. Effects of Chronic Electrical Stimulation on Spiral Ganglion Neuron Survival and Size in Deafened Kittens. The Laryngoscope. 1998;108:687–95. doi: 10.1097/00005537-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–44. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 50.Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56:261–72. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 51.Fonner JM, Forciniti L, Nguyen H, Byrne JD, Kou YF, Syeda-Nawaz J, Schmidt CE. Biocompatibility implications of polypyrrole synthesis techniques. Biomed Mater. 2008;3:034124. doi: 10.1088/1748-6041/3/3/034124. [DOI] [PMC free article] [PubMed] [Google Scholar]