Abstract
ARA 290 (a peptide designed to activate the innate repair receptor that arrests injury and initiates cytoprotection, antiinflammation and healing) reduces allodynia in preclinical neuropathy models. We studied the safety and efficacy of ARA 290 to reduce symptoms of small fiber neuropathy (SFN) in patients with sarcoidosis. A total of 22 patients diagnosed with sarcoidosis and symptoms of SFN were enrolled in a double-blind, placebo-controlled exploratory trial consisting of three times weekly intravenous dosing of ARA 290 (2 mg; n = 12) or placebo (n = 10) for 4 wks. Inclusion criteria were a diagnosis of neuropathy and a spontaneous pain score of ≥5 (Brief Pain Inventory [BPI]). Endpoints assessed were changes in pain intensity and the small fiber neuropathy screening list (SFNSL) score, quality of life (SF-36), depressive symptoms (Inventory of Depressive Symptomatology [IDS]) and fatigue (Fatigue Assessment Scale [FAS]). No safety concerns were raised by clinical or laboratory assessments. The ARA 290 group showed significant (p < 0.05) improvement at wk 4 in SFNSL score compared with placebo (Δ −11.5 ± 3.04 versus Δ −2.9 ± 3.34 [standard error of the mean]). Additionally, the ARA 290 group showed a significant change from baseline in the pain and physical functioning dimensions of the SF-36 (Δ −23.4 ± 5.5 and Δ −14.6 ± 3.9, respectively). The mean BPI and FAS scores improved significantly but equivalently in both patient groups. No change was observed in the IDS. ARA 290 appears to be safe in patients with sarcoidosis and can reduce neuropathic symptoms.
INTRODUCTION
Sarcoidosis is an inflammatory disease that targets many tissues. In common with a number of other conditions, for example, Sjogren disease (1), one prominent clinical manifestation is a dysfunction of small nerve fibers that occurs in a patchy, non–length-dependent manner (small fiber neuropathy [SFN]). Pathological investigation of sarcoid SFN has documented a loss of small myelinated (Aδ) and unmyelinated (C) fibers of the sensory and autonomic nervous systems (2), as well as both sensory and motor fibers (3). The clinical sequela of these changes is the development of sharp shock-like or burning pain, characterized by dysesthesia and allodynia, and loss of cutaneous sensation and autonomic function. These symptoms significantly reduce the quality of life and are often disabling and difficult to control (2).
SFN can be diagnosed in patients with neuropathic symptoms by using quantitative sensory testing or quantitative sudomotor axon testing and by performing skin biopsies that show a decreased density of intraepidermal sensory nerve fibers within affected body regions. Additionally, a questionnaire (4) was designed and validated in Dutch patients with sar-coidosis (the small fiber neuropathy screening list [SFNSL]) and is useful in following the clinical course of SFN.
Recent studies have shown that the prevalence of SFN is grossly underestimated. Unlike granulomatous, large neuron involvement of neurosarcoidosis, which has a prevalence of <10% (5), painful SFN is more common, with a prevalence of 40% (6) to 60% (7) of patients. The etiology of SFN is unknown, but inflammation is believed to play a prominent role in the generation and maintenance of the symptoms (8). Current therapy of sarcoidosis is primarily via immune suppression, which is generally ineffective for SFN (2).
In recent years, an endogenous system was identified that antagonizes the production and action of proinflammatory cytokines involved in promoting tissue injury, while simultaneously activating repair processes. The primary mediator of this system is locally produced hypo-glycosylated erythropoietin (EPO) that acts through a distinct receptor isoform, the innate repair receptor (IRR), which is a combination of EPO receptor and β common receptor subunits (9). EPO acting through the IRR was shown to improve recovery and function after nerve injury in a variety of preclinical models, including SFN caused by uncontrolled diabetes mellitus (10).
ARA 290 is a novel peptide modeled from the three-dimensional structure of EPO that specifically activates anti-inflammation and tissue protection through the innate repair receptor. Preclinical toxicology studies of ARA 290, as well as single and multiple ascending repeated dosing of human volunteers and patients with kidney disease, diabetes mellitus or sarcoidosis have raised no safety issues (11; unpublished data, Araim Pharmaceuticals).
ARA 290 is highly effective in preclinical models of neuropathic pain (12). We hypothesized that patients with symptomatic SFN would benefit from administration of ARA 290. The current trial was undertaken to determine the safety and activity of repeated intravenous dosing of ARA 290 in painful neuropathy.
MATERIALS AND METHODS
Study Design
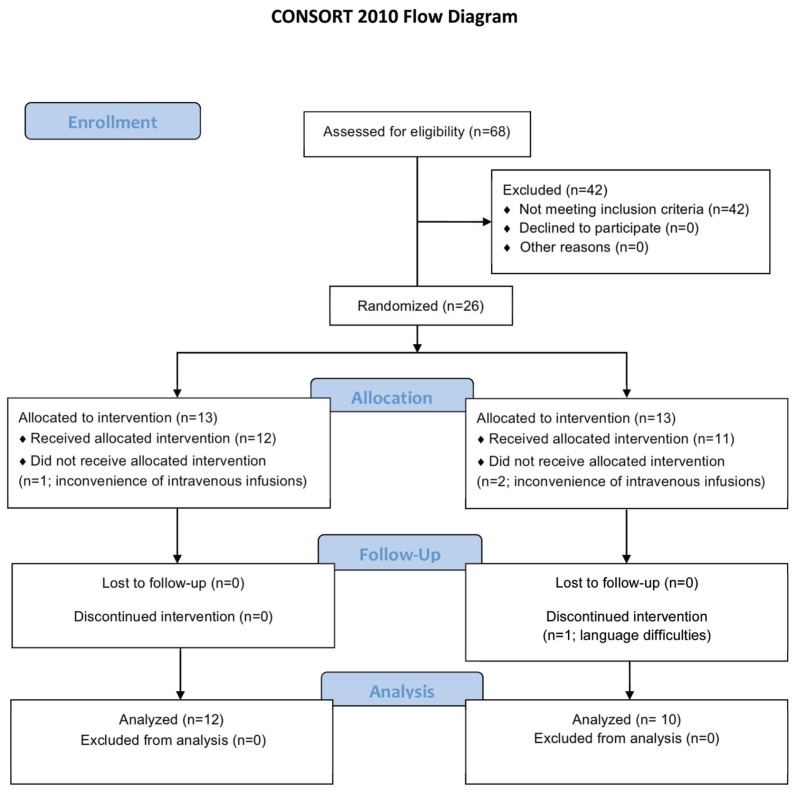
This study was a single-site, double-blind study carried out at Leiden University Medical Center (LUMC) and is summarized in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 1). A total of 26 patients (24 study and 2 alternates) diagnosed with sarcoidosis and as having chronic neuropathic symptoms consistent with SFN were recruited. The diagnosis of sar-coidosis was confirmed as being consistent with the criteria set out in the international guidelines previously reported (13). Only individuals with confirmed sarcoidosis were included. For inclusion, chronic neuropathic symptoms consistent with SFN required at least two of the following: (a) distal symmetrical dys-/paresthesias, (b) burning feet or (c) intolerance of sheets touching the legs or feet. Additionally, a patient’s spontaneous pain level was ≥5 (visual analog scale 0–10, with 10 being the worst pain imaginable). Patients also underwent quantitative sensory testing (QST) (Medoc Advanced Medical Systems, Ramat Yishai, Israel) according to the protocol of the German Research Network on Neuropathic Pain (14), with published reference values (15). The results showed a prominent loss of temperature and vibration detection thresholds (Table 1). Additional inclusion criteria were as follows: capable of reading Dutch (n = 1 excluded) and aged between 18 and 65 years, with a body mass index ≤34 kg/m2, since ARA 290 dosing was not scaled to body size. Women of childbearing potential (n = 1) were required to have a negative pregnancy test and use acceptable contraception for 2 months during the study. Exclusion criteria included the following: receiving a vaccination or immunization within the last month; participation in an investigational drug trial in the 3 months before administration of the initial dose of ARA 290 or more than four times per year; major surgery within 6 months before screening; or use of anti–tumor necrosis factor (TNF)-α or EPO or treatment with immunoglobulin drugs 6 months before or during ARA 290 administration and in the follow-up phase. The study was approved and monitored by the Ethics Committee of LUMC and is registered with the International Clinical Trials Registry (NCT 3081), Netherlands Trial Registry (trialregister.nl, NRT 3081) (EudraCT 2010-021518-45). All patients gave written informed consent before entering into the study.
Figure 1.
CONSORT flow diagram of the study of ARA 290 in sarcoidosis-associated chronic neuropathic pain.
Table 1.
Results of baseline quantitative sensory testing.
| ARA 290* | Placebo | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Variable | Nerve fibers involved | Change | Number of patients (%) | Change | Number of patients (%) |
| Cold detection threshold | Aδ and C | Decrease | 7 (64) | Decrease | 9 (90) |
| Warm detection threshold | Aδ and C | Decrease | 7 (64) | Decrease | 6 (60) |
| Thermal sensory limen | Aδ and C | Decrease | 3 (27) | Decreased, increased | 2 (20), 1 (10) |
| Paradoxical heat sensation | Aδ | Decrease | 1 (9) | — | 0 |
| Cold pain threshold | Aδ and C | — | 0 | Increase | 1 (10) |
| Heat pain threshold | C | Decrease, increase | 2 (18), 1 (9) | Decrease, increase | 1 (10), 2 (20) |
| Mechanical detection threshold | Aβ | Decrease, increase | 2 (18), 1 (9) | Decrease | 1 (10) |
| Mechanical pain threshold | Aβ | Decrease | 2 (18) | Decrease | 3 (30) |
| Mechanical pain sensitivity | Aβ + C | Decrease | 1 (9) | Increase | 2 (20) |
| Dynamic mechanical allodynia | Aβ | Increase | 1 (9) | Increase | 4 (40) |
| Windup ratio | Aδ and C | — | 0 | Increase | 1 (10) |
| Vibration detection threshold | Aβ | Decrease | 6 (55) | Decrease | 6 (60) |
| Pressure pain threshold | Aδ and C | Increase | 2 (18) | Increase | 8 (80) |
One patient in the ARA 290 treatment arm refused QST. Patients in both treatment groups showed functional impairment in the function of both small fibers (Aδ and C) as well as larger sensory fibers (Aβ). Data are expressed as either increased or decreased function in those patients deviating 2 or more standard deviations from measurements obtained from a normal population.
During the study, patients were maintained on a variable regimen of sarcoidosis therapeutics by their physicians, including oral glucocorticoids. Neuropathic symptom-directed agents (for example, tricyclic antidepressants or selective serotonin reuptake inhibitors) were also continued. Patients were randomly assigned (1:1) by the study pharmacist by using a computer-generated randomization code to either ARA 290 or matching placebo (vehicle only). All other study personnel were blinded to the treatment. ARA 290 (pyroglu-glu-gln-leu-glu-arg-ala-leu-asn-ser-ser) was manufactured by Bachem (Bubendorf, Switzerland) by using standard Fmoc solid-phase peptide synthesis. The characteristics of each patient group are summarized in Table 2.
Table 2.
Patient characteristics.
| Variable | ARA 290 | Placebo |
|---|---|---|
| n | 12 | 10 |
| M/F | 6/6 | 6/4 |
| Weight (kg) | 83.2 ± 3.7 | 85.5 ± 5.9 |
| Age | 48.1 ± 2.7 | 49.1 ± 2.7 |
| Height (cm) | 177.8 ± 2.8 | 177.4 ± 3.3 |
| Pulmonary involvement | 10/12 | 9/10 |
| Fatigue | 12/12 | 10/10 |
| Use of nonsteroidal antiinflammatory drugs (NSAIDs) | 2/12 | 1/10 |
| Use of psychological drugs | 2/12 | 2/10 |
| Use of oral steroids | 4/12 | 2/10 |
| Use of opioids | 1/12 | 0/10 |
| Use of analgesics | 2/12 | 2/10 |
| Use of anticonvulsants | 3/12 | 3/10 |
| Use of systemic antiinflammatory drug | 1/12 | 2/10 |
| Currently smoking | 2/12 | 2/10 |
| ARA 290 dose (μg/kg) | 24.6 ± 1.1 | 0 |
| ARA 290 dose (μg/m2) | 997.1 ± 26.9 | 0 |
| C-reactive protein (pretreatment versus posttreatment; NS) | 3.0 ± 1 versus 3.1 ± 1.2 | 3.7 ± 1.5 versus 4.1 ± 1.6 |
| SFNSL score (pretreatment versus posttreatment; p < 0.05) | 41.0 ± 4.6 versus 29.8 ± 3.5 | 30.6 ± 4.2 versus 26.2 ± 4.0 |
| BPI mean score (pretreatment versus posttreatment; NS) | 7.1 ± 0.2 versus 4.8 ± 0.4 | 6.2 ± 0.9 versus 4.1 ± 0.3 |
| SF-36 mean score (pretreatment versus posttreatment; NS) | 37.6 ± 2.8 versus 50.7 ± 3.1 | 44.5 ± 2.8 versus 52.3 ± 3.1 |
| FAS (pretreatment versus posttreatment; NS) | 37.9 ± 2.6 versus 33.3 ± 2.8 | 33.6 ± 2.3 versus 29.8 ± 3.3 |
| IDS (pretreatment versus posttreatment; NS) | 28.7 ± 4.9 versus 24.7 ± 4.2 | 24.1 ± 4.3 versus 22.1 ± 4.3 |
Data are mean ± SEM. BPI score consisted of most pain, average pain and pain now. NS, nonsignificant differences.
Baseline blood samples were obtained for routine chemistry, high-sensitivity C-reactive protein and hematology determinations. Repeat blood samples were obtained at wk 1 and also just before the last intravenous infusion (d 25). Samples were centrifuged, separated, stored and analyzed according to LUMC Clinical Laboratory protocols.
This study was carried out in the out-patient clinic of the Department of Anesthesiology, Leiden University Medical Center. ARA 290 (2 mg) or placebo was infused intravenously in 6 mL normal saline over 2 min by using a calibrated infusion pump on Monday, Wednesday and Friday for 4 consecutive weeks. Patients were monitored for 60 min after infusion for adverse effects and instructed to contact the research staff if delayed adverse effects were suspected. The endpoints of this exploratory study were change at wk 4 in (a) pain level, as assessed by the BPI and SF-36; (b) neuropathic symptoms, as assessed by the SFNSL (4); and (c) quality of life assessments by the SF-36, Inventory of Depressive Symptomatology (IDS) and the Fatigue Assessment Scale (FAS), which has been validated for sarcoidosis patients (16). These questionnaires were completed at baseline and then weekly for the 4 wks of dosing. Each patient independently completed the weekly questionnaires by using the Project Manager Internet Server maintained by LUMC, which provided a record that could not be modified.
One patient from the ARA 290 treatment group and two from the placebo group withdrew from the study because of the inconvenience of intravenous infusions and were replaced with the two alternates who had been previously recruited as backups for the study. A total of 12 ARA 290 patients and 10 placebo patients completed the study.
The trial sample size was chosen on the basis of the robust efficacy of ARA 290 in a rodent model of neuropathy (12) and from the observations derived from a prior small nonblinded trial of 20 patients with symptoms of SFN who received ARA 290 (2 mg intravenously) for three doses every 2 d over 1 wk (11). Responses from the weekly patient questionnaires were calculated as change from baseline values. Individual missing data points were assigned by using a last observation carried forward approach (number of missing data points summarized below for each variable). Normality of data distribution was assessed and confirmed by using the Kolmogorov-Smirnov test. Statistical significance (p < 0.05) of change from baseline value was calculated at wk 4 by using a two-sample t test comparing change at wk 4 over baseline (two-tailed distribution). Because no data points were missing from the SFNSL questionnaire, these data were analyzed by using repeated-measures analysis of variance (ANOVA). A cumulative proportional responders graph was constructed according to Farrar et al.(17).
RESULTS
There were no documented changes in concomitant drug treatment, including analgesic use, during the 4-wk dosing period. Patients tolerated repeated intravenous infusions of ARA 290 without adverse events noted by study personnel or self-reported. ARA 290 recipients and placebo patients exhibited no significant differences between chemistry and hematology values at baseline and wk 4. Hemoglobin concentrations (mmol/L) of ARA 290 patients compared with placebo were 8.9 ± 0.21 (standard error of the mean [SEM]) versus 8.6 ± 0.26 at baseline and 8.7 ± 0.19 versus 8.7 ± 0.34 at the end of dosing on wk 4.
The SFNSL score (no missing data points) showed a time-dependent, significant difference between treatment groups, reaching a maximum difference by wk 4 (Figure 2). The SFNSL data obtained at the end of dosing at wk 4 are expressed as a cumulative proportion of the responders plot in Figure 3. As illustrated, 60% of placebo patients experienced an improvement from baseline at wk 4 between 1 and 5 points, versus 83% of ARA 290 patients. In contrast, whereas none of the placebo patients experienced an improvement of 15 points or more, 42% of the ARA 290 recipients did. The SFNSL assesses both the frequency and the severity of symptoms. As shown in Figure 4A, the frequency of SFN symptoms decreased moderately in both groups to a similar extent. In contrast, the severity of neuropathic symptoms remained constant in the placebo group over the dosing period, whereas it significantly improved in the ARA 290 group (Figure 4B). The SFNSL can also be separated into components that assess symptoms referable to autonomic dysfunction (questions 2–5, 9 and 11–16) or to pain (questions 1, 6–8, 10 and 17–21). Patients who received ARA 290 reported a significant improvement in their autonomic symptoms, in contrast to the placebo group (Figure 5). Although a similar improvement was noted for the pain dimension, the magnitude was not significantly different from that observed for the placebo group. Assessing for change from baseline for individual questions showed that significant changes occurred in six questions for the ARA 290 group and one question for the placebo group (Table 3).
Figure 2.
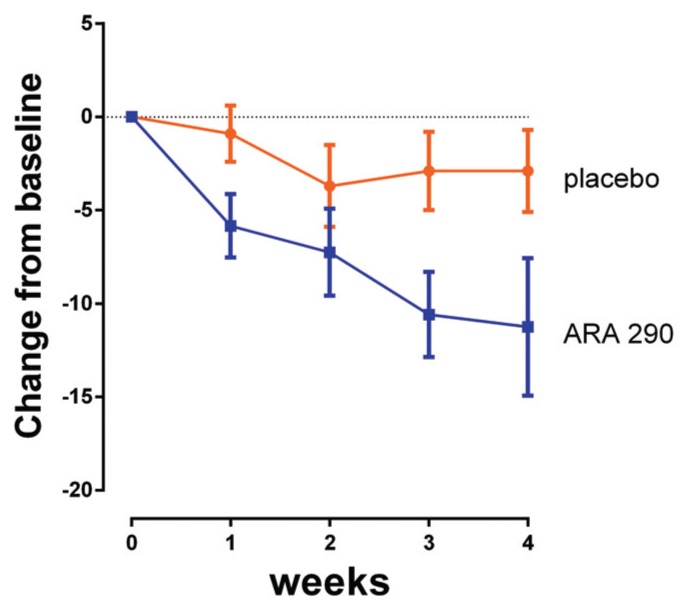
ARA 290 administration is associated with a time-dependent improvement of the SFNSL score. Reduction in SFNSL score from baseline (improvement) in the ARA 290 group occurs within the first week and continues for the whole duration of the dosing period. In contrast, a small, non-significant change in placebo patients appeared to reach a plateau. (Curves [mean ± SEM] are significantly different at the p < 0.05 level based on repeated-measures ANOVA).
Figure 3.
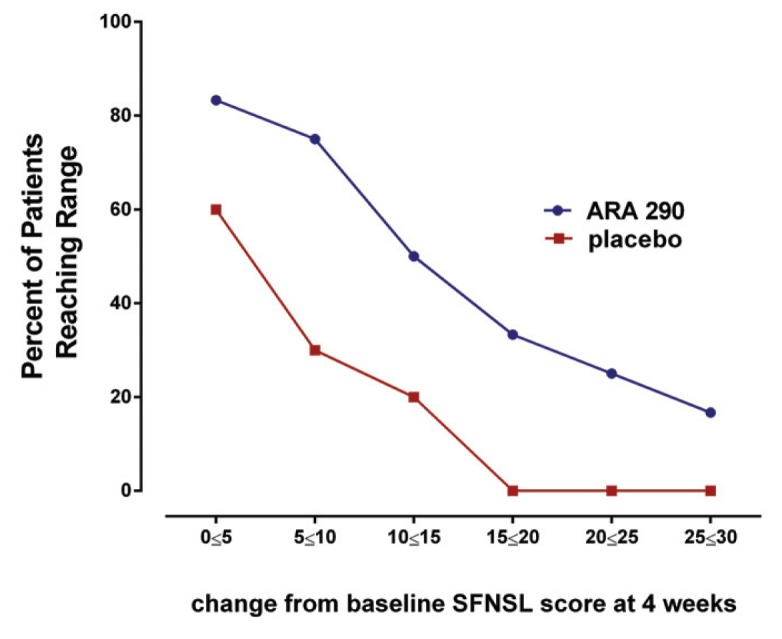
A higher proportion of ARA 290 patients exhibited a superior improvement than placebo patients in the SFNSL score at all levels of response. Cumulative proportion of responder analysis summarizes that across a wide range of score improvements a greater proportion of ARA 290 patients reached a specific level of reduction in the SFNSL score than placebo patients.
Figure 4.
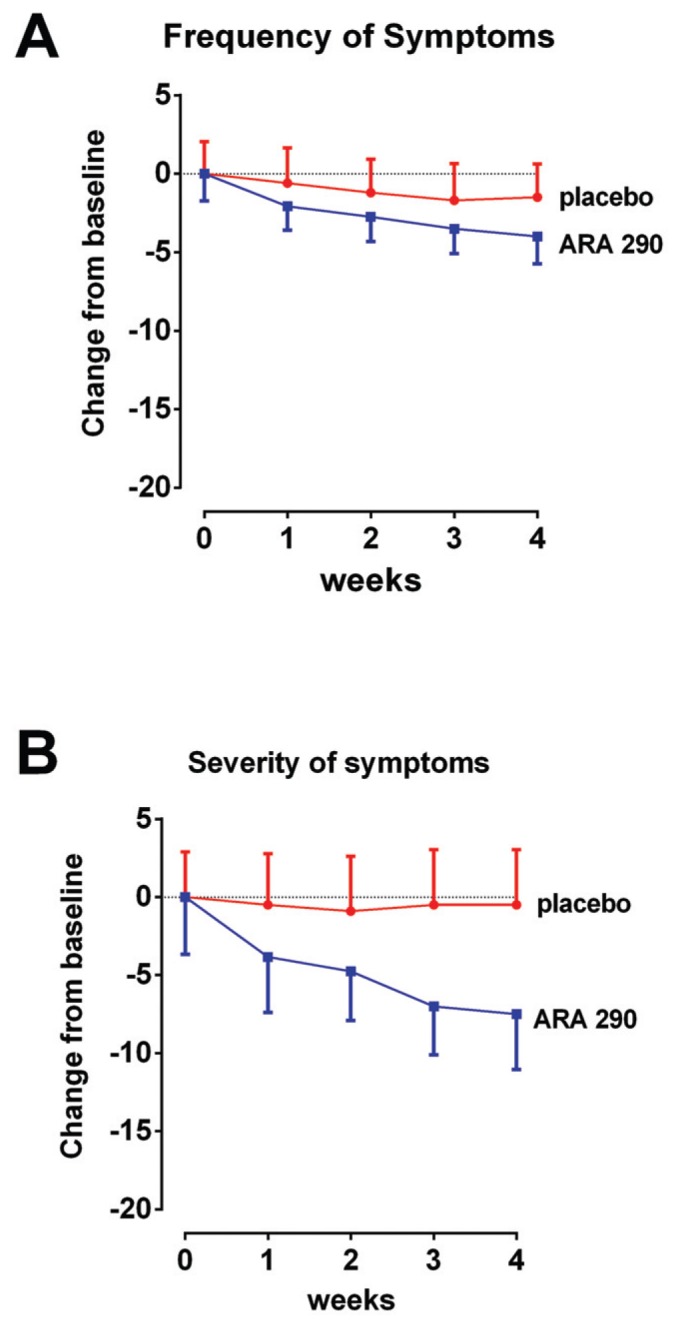
SFNSL subscore analysis. (A) A decrease in frequency of symptoms occurred in both groups over the dosing period, which did not differ significantly. (B) Severity of symptoms remained unchanged in the placebo group, whereas a decrease was noted in the ARA 290 (p < 0.05).
Figure 5.
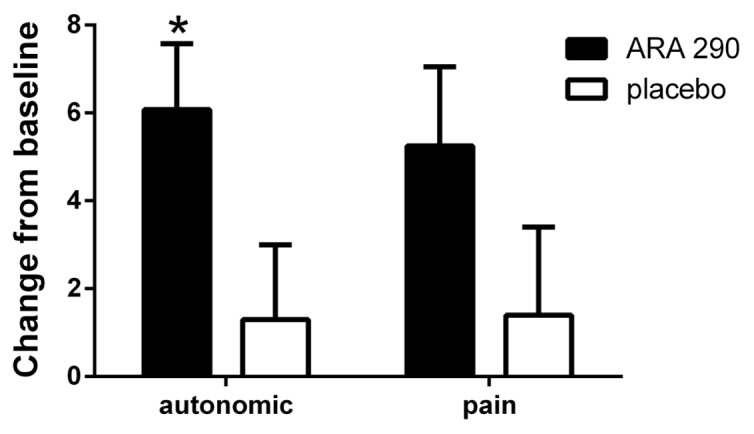
ARA 290 administration was associated with a significant improvement in the autonomic component of the SFNSL. ARA 290 administration was associated with a significant improvement in the autonomic component of the SFNSL at wk 4 compared with baseline (*p < 0.05). Although a similar change was noted in the pain subcategory, the magnitude did not differ significantly from that of the placebo group.
Table 3.
Significant changes in individual questions of the SFNSL.
| Patient group | Question | Symptom subgroup | Change from baseline (mean ± SEM) | Significance* |
|---|---|---|---|---|
| ARA 290 | 6 (muscle cramps) | Frequency | −1.1 ± 0.36 | 0.012 |
| ARA 290 | 8 (chest pain) | Frequency | −0.5 ± 0.19 | 0.026 |
| ARA 290 | 12 (dry eyes) | Severity | −1.33 ± 0.33 | 0.002 |
| ARA 290 | 13 (blurred vision) | Severity | −1.17 ± 0.32 | 0.004 |
| ARA 290 | 14 (dizzy when rising) | Severity | −0.83 ± 0.32 | 0.025 |
| ARA 290 | 21 (chest pain) | Severity | −0.58 ± 0.19 | 0.012 |
| Placebo | 6 (muscle cramps) | Frequency | −0.7 ± 0.30 | 0.045 |
Determined by two-tailed paired t test.
Similarly, quality of life, as assessed by the SF-36 (one patient had a total of four data points missing), showed significant improvements from baseline in the active treatment group for pain and physical functioning in contrast to the placebo group (Figure 6). Both groups showed significant improvements from baseline in general health (ARA 290: 35.4 ± 8.3; placebo: 22.7 ± 7.9). There were no significant changes from baseline between active and placebo groups in the remaining dimensions of SF-36. The mean pain score for the BPI (three patients had a total of five data points missing) and FAS (three patients had a total of one data point each missing) decreased to a similar extent for both ARA 290 and the placebo group (Table 2), which were not significantly different from each other. The IDS (four patients had a total of one data point each missing) did not change from baseline for either group (Table 2).
Figure 6.
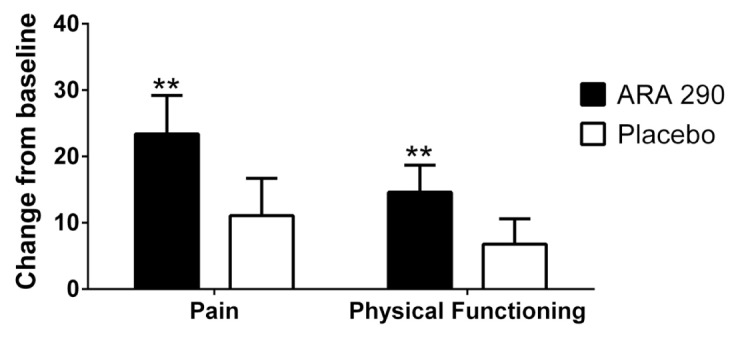
ARA 290 patients demonstrated a significant improvement from baseline in the pain and physical functioning dimensions of the SF-36 quality of life questionnaire. In contrast to the placebo patients, those receiving ARA 290 showed significant improvement from baseline in the dimension of pain and physical functioning at wk 4 (**p < 0.01).
DISCUSSION
This is the first study to demonstrate that ARA 290 appears to be safe when administered repeatedly over a 4-wk period to sarcoidosis patients with symptoms of SFN. During and after dosing, no abnormalities were noted in the laboratory or clinical evaluations, and the patients reported no potentially drug- related adverse effects. Notably, ARA 290 appears to improve symptoms of SFN, as assessed by the SFNSL, as well as on quality of life, as assessed by the pain and physical functioning dimensions of the SF-36. Pain, as assessed by the BPI, decreased significantly, but to the same degree in both patient groups.
Primary treatment of diseases complicated by SFN was reported to variably improve the symptoms of SFN. Sarcoidosis is a disease mediated by a complex interaction involving tissue injury and the responses of immune-competent cells. High-dose glucocorticoids have been the predominant therapeutic approach for all forms of this disease. However, use of glucocorticoids is frequently associated with unacceptable adverse effects and appears to be generally ineffective in improving the symptoms of SFN (2). Substitution of other immunosuppressants, for example, methotrexate, also fails to improve symptomatic SFN. Consequently, sarcoid patients who suffer from chronic symptomatic SFN currently lack effective treatment.
A recent development includes the availability of agents that directly inactivate the proinflammatory cytokine TNFα. Use of anti-TNFα therapy in refractive pulmonary sarcoidosis has been shown to directly reduce systemic proinflammatory cytokine concentrations and has successfully induced a sustained improvement in lung function in some patients (18,19). Case studies have also reported that direct antagonism of proinflammatory cytokines in patients with symptomatic SFN was associated with significant and sustained improvement in neuropathic symptoms, including dysautonomia (20), as well as with cognitive function and fatigue (18,21). The results of these studies directly support the concept that proinflammatory cytokines play a major etiologic role in the development of specific organ involvement in sarcoidosis and that antiproinflammatory cytokine therapy may constitute an effective treatment. However, the high costs and side effects of anti-TNFα therapy currently limit its widespread use. Finally, intravenous immunoglobulin infusions have been effective in a small number of patients with sarcoidosis and SFN (22), as well as in other diseases with associated SFN (23).
Since primary therapy of sarcoidosis does not generally treat SFN, alternative therapies used for for other peripheral neuropathies are emplyed. These include antidepressants, anticonvulsants, topical analgesics and/or opioids. Each of these classes of therapeutic agents has specific adverse effects, sometimes severe, and typically do not adequately treat the symptoms of SFN (24).
ARA 290 is a peptide designed to mitigate inflammation by activating the IRR, which in turn inhibits proinflammatory cytokine production and action. In pre-clinical models of injury, ARA 290 not only inhibits TNFα, but also other components of the proinflammatory cytokine cascade (25). Although ARA 290 has a short serum half-life of a few minutes (11), the peak plasma concentrations attained after the administration of 2 mg intravenously in normal human volunteers in pharmacokinetic studies reached ~50 ng/mL, exceeding the minimum effective peak concentration of ~1 ng/mL (26). ARA 290 at concentrations exceeding ~1 ng/mL activates the IRR, which functions as a molecular switch to provide long-lasting effects, similar to other effector molecules in the immune response. In this clinical trial, administration every other day for 1 month appears sufficient to produce a significant biological response, similar to the finding in a preclinical rodent model in which treatment every other day with ARA 290 is effective in preventing neuropathic pain (12). ARA 290 therefore constitutes an attractive candidate for treatment of symptomatic SFN.
The most robust effect of ARA 290 observed in this pilot trial was on the SFNSL score, which appeared to be primarily on symptom severity rather than frequency. It is notable that this instrument, which was specifically developed and validated in sarcoidosis patients (4), incorporates prominent symptoms of autonomic dysfunction as well as pain. In the current study, patients receiving ARA 290 appeared to improve specifically with respect to autonomic symptomatology, for example, with respect to dry eyes, blurred vision and orthostatic symptoms. In this regard, it is interesting to note that ARA 290 was observed to reverse neuropathic changes in the sympathetic ganglia of a mouse model of type 2 diabetes (27). Additionally, results of experiments performed on normal volunteers in whom intraepidermal nerve fibers were denervated by the application of capsaicin show that cutaneous autonomic nerve fibers regenerate much faster than sensory nerve fibers (28). This observation could explain, in part, the less robust effects that are noted in the pain dimension, which may require more prolonged or intensive dosing. The results also suggest that future studies in symptomatic SFN that focus on autonomic dysfunction may yield especially informative data.
The primary limitations of this trial are the small sample size, patient variability of neuropathic involvement and lack of skin biopsy or sudomotor testing evidence definitively establishing SFN. Also, a sizeable fraction of the study group also exhibited abnormalities in mechanoreception, as determined by QST in addition to the sensory and autonomic abnormalities attributable to SFN. Therefore, the observed reduction in the severity of symptoms as assessed by the SFNSL cannot be attributed with certainty only to effects of ARA 290 on small fiber function.
CONCLUSION
The acceptable safety profile noted for ARA 290 in this patient group with sarcoidosis, as well as an apparent reduction of symptoms of SFN, encourages a larger study of the potential effects of ARA 290 for this unmet medical need.
ACKNOWLEDGMENTS
The authors thank patients, and their families as well as F Breedveld, A Rabelink, L Aarts, E Lansink and E Sarton for support and assistance in making this study possible. This was an investigator-initiated study. ARA 290 was supplied by Araim Pharmaceuticals.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
A Dunne, M Brines and A Cerami are employees and officers of Araim Pharmaceuticals and own stock and/or stock options.
REFERENCES
- 1.Chai J, Herrmann DN, Stanton M, Barbano RL, Logigian EL. Painful small-fiber neuropathy in Sjogren syndrome. Neurology. 2005;65:925–7. doi: 10.1212/01.wnl.0000176034.38198.f9. [DOI] [PubMed] [Google Scholar]
- 2.Tavee J, Culver D. Sarcoidosis and small-fiber neuropathy. Curr Pain Headache Rep. 2011;15:201–6. doi: 10.1007/s11916-011-0180-8. [DOI] [PubMed] [Google Scholar]
- 3.Burns TM, Dyck PJ, Aksamit AJ. The natural history and long-term outcome of 57 limb sarcoidosis neuropathy cases. J Neurol Sci. 2006;244:77–87. doi: 10.1016/j.jns.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Hoitsma E, De Vries J, Drent M. The small fiber neuropathy screening list: construction and cross-validation in sarcoidosis. Respir Med. 2011;105:95–100. doi: 10.1016/j.rmed.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Lower EE, Weiss KL. Neurosarcoidosis. Clin Chest Med. 2008;29:475–92. ix. doi: 10.1016/j.ccm.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Bakkers M, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology. 2009;73:1142–8. doi: 10.1212/WNL.0b013e3181bacf05. [DOI] [PubMed] [Google Scholar]
- 7.Hoitsma E, et al. Small fibre neuropathy in sarcoidosis. Lancet. 2002;359:2085–6. doi: 10.1016/s0140-6736(02)08912-2. [DOI] [PubMed] [Google Scholar]
- 8.Uceyler N, et al. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology. 2010;74:1806–13. doi: 10.1212/WNL.0b013e3181e0f7b3. [DOI] [PubMed] [Google Scholar]
- 9.Brines M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–12. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchi R, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 2004;101:823–8. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niesters M, et al. The erythropoietin-analogue ARA 290 for treatment of sarcoidosis-induced chronic neuropathic pain. Exp Opin Orphan Drugs. 2013;1:77–87. [Google Scholar]
- 12.Swartjes M, et al. ARA290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: an experimental study in rats and beta-common receptor knockout mice. Anesthesiology. 2011;115:1084–92. doi: 10.1097/ALN.0b013e31822fcefd. [DOI] [PubMed] [Google Scholar]
- 13.Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735–7. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 14.Rolke R, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur. J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Magerl W, et al. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151:598–605. doi: 10.1016/j.pain.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 16.De Vries J, Michielsen H, Van Heck GL, Drent M. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS) Br J Health Psychol. 2004;9:279–91. doi: 10.1348/1359107041557048. [DOI] [PubMed] [Google Scholar]
- 17.Farrar JT, Dworkin RH, Max MB. Use of the cumulative proportion of responders analysis graph to present pain data over a range of cut-off points: making clinical trial data more understandable. J Pain Symptom Manage. 2006;31:369–77. doi: 10.1016/j.jpainsymman.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Erckens RJ, Mostard RL, Wijnen PA, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250:713–20. doi: 10.1007/s00417-011-1844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loza MJ, et al. Inflammatory profile and response to anti-tumor necrosis factor therapy in patients with chronic pulmonary sarcoidosis. Clin Vaccine Immunol. 2011;18:931–9. doi: 10.1128/CVI.00337-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoitsma E, et al. Improvement of small fiber neuropathy in a sarcoidosis patient after treatment with infliximab. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:73–7. [PubMed] [Google Scholar]
- 21.Elfferich MD, et al. Everyday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-alpha treatment. Respiration. 2010;80:212–9. doi: 10.1159/000314225. [DOI] [PubMed] [Google Scholar]
- 22.Parambil JG, Tavee JO, Zhou L, Pearson KS, Culver DA. Efficacy of intravenous immunoglobulin for small fiber neuropathy associated with sarcoidosis. Respir Med. 2011;105:101–5. doi: 10.1016/j.rmed.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Wakasugi D, et al. Extreme efficacy of intravenous immunoglobulin therapy for severe burning pain in a patient with small fiber neuropathy associated with primary Sjogren’s syndrome. Mod Rheumatol. 2009;19:437–40. doi: 10.1007/s10165-009-0180-2. [DOI] [PubMed] [Google Scholar]
- 24.Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med. 2009;76:297–305. doi: 10.3949/ccjm.76a.08070. [DOI] [PubMed] [Google Scholar]
- 25.Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405–32. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 26.Brines M, Cerami A. The receptor that tames the innate immune response. Mol Med. 2012;18:486–96. doi: 10.2119/molmed.2011.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt RE, et al. Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuritic dystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia. Exp Neurol. 2011;232:126–35. doi: 10.1016/j.expneurol.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbons CH, Wang N, Freeman R. Capsaicin induces degeneration of cutaneous autonomic nerve fibers. Ann Neurol. 2010;68:888–98. doi: 10.1002/ana.22126. [DOI] [PMC free article] [PubMed] [Google Scholar]