Abstract
Trastuzumab is a monoclonal antibody targeted against the HER2 tyrosine kinase receptor. Although trastuzumab is a very active agent in HER2-overexpressing breast cancer, the majority of patients with metastatic HER2-overexpressing breast cancer who initially respond to trastuzumab develop resistance within 1 year of initiation of treatment and, in the adjuvant setting, progress despite trastuzumab-based therapy. The antibody-drug conjugate trastuzumab-DM1 (T-DM1) was designed to combine the biological activity of trastuzumab with the targeted delivery of a highly potent antimicrotubule agent, DM1 (N-methyl-N-[3-mercapto-1-oxopropyl]-l-alanine ester of maytansinol), a maytansine derivative, to HER2-overexpressing breast cancer cells. T-DM1 is the first antibody-drug conjugate with a nonreducible thioether linker in clinical trials. Phase I and II clinical trials of T-DM1 as a single agent and in combination with paclitaxel, docetaxel and pertuzumab have shown clinical activity and a favorable safety profile in patients with HER2-positive metastatic breast cancer. Two randomized phase III trials of T-DM1 are awaiting final results; the EMILIA trial is evaluating T-DM1 compared with lapatinib plus capecitabine, and early positive results have been reported. The MARIANNE trial is evaluating T-DM1 plus placebo versus T-DM1 plus pertuzumab versus trastuzumab plus a taxane. Here, we summarize evidence from clinical studies and discuss the potential clinical implications of T-DM1.
INTRODUCTION
Cytotoxic drugs and therapeutic monoclonal antibodies represent two major classes of agents currently used for cancer treatment. The therapeutic activity of cytotoxic drugs is, in general, limited by their narrow therapeutic window. Methods to enhance and improve the selectivity of cytotoxic drugs and significantly improve the therapeutic index are currently being pursued. One particular method is targeting drug carriers such as antibodies; this is the objective of antibody-drug conjugates (ADCs), in which cytotoxic drugs are attached via chemical linkers to antibodies that recognize cancer cell antigens and deliver the cytotoxic drug only to the cells of interest.
ADCs can be viewed as sophisticated delivery systems for antitumor cytotoxic drugs. Critical parameters for ADC development include target antigen selection, conjugate internalization by tumor cells, drug potency and stability of the linker between drug and antibody. Other important considerations include the conjugation methods, drug-to-antibody ratio and the effects of drug conjugation on antibody properties.
To date, two ADCs approved by the U.S. Food and Drug Administration (FDA) include gemtuzumab ozogamicin (approved in 2000 for treating relapsed CD33-positive acute myeloid leukemia in older patients) and brentuximab vedotin (approved in 2011 for treating patients with Hodgkin lymphoma after failure of autologous stem cell transplant or after failure of at least two prior multi-agent chemotherapy regimens) (1–3). Gemtuzumab ozogamicin was recently withdrawn from clinical use when post-approval studies showed no benefit of adding this ADC to chemotherapy for the treatment of acute myeloid leukemia (4).
The rationale for the development of ADCs was defined in a number of previous reviews (5–7). The underlying principle is to combine the selectivity, favorable pharmacokinetics, biodistribution and functional activity of antibodies with high agents of cytotoxic potency. The antimitotic drug maytansine was chosen for use in the targeted delivery approach because of its high in vitro potency (8). The maytansinoids bind tubulin competitively with vinca alkaloids and are approximately 100 times more potent than vincristine (9,10). Maytansine is too toxic to use alone, but an analog of the clinically studied drug maytansine was linked to trastuzumab (T-DM1) is an ADC. As such, T-DM1 offers a delivery system to target HER2-positive breast cancer. T-DM1 has been administered safely at therapeutically effective doses (11,12). In this report, we summarize evidence from clinical studies and discuss the potential impact of the use of an HER2 ADC in the treatment of HER2-overexpressing breast cancer.
TRASTUZUMAB AND DM1
Trastuzumab
The design of an ADC centers on the selection of an antigen that is relatively tumor specific and accessible to an antibody binding to the tumor cell. A validated antibody target for breast cancer is HER2, a 185-kDa transmembrane receptor protein encoded by the proto-oncogene HER2/neu, that when overexpressed, can be as dense as 1.5 million copies per cell, making HER2 an ideal target for an ADC (13). By protein expression or genomic analysis, approximately 20–30% of breast tumors overexpress this surface antigen (13–15). HER2-positive breast cancer was reported to be a poor prognostic factor and a predictive risk factor for decreased disease-free survival and overall survival rates (14,16).
Trastuzumab, a humanized monoclonal antibody, targets the extracellular domain of the HER2 receptor. As adjuvant treatment, trastuzumab reduces the recurrence of HER2-positive breast cancer by approximately 50% and mortality by 30% (17–20). Given its prognostic, predictive and therapeutic implications, an accurate HER2 assessment for breast cancer patients is crucial; only patients with HER2 overexpression are eligible for trastuzumab treatment.
Interlaboratory reproducibility of the assessment of HER2 status continues to be a concern as well as intratumoral HER2 heterogeneity, which occurs in 5–15% of tumors (21–23). Accurate HER2 testing is critical, and evaluating molecular markers and further defining the signaling pathways in HER2 disease are the next apparent steps in advancing the understanding of this subtype of invasive breast cancer.
Trastuzumab was approved in 1998 for the treatment of metastatic breast cancer and in 2006 for the adjuvant treatment of HER2-overexpressing breast cancer (24). The exact mechanisms by which trastuzumab causes regression of HER2-overexpressing tumors are still under investigation, but several effects have been reported in the literature. These mechanisms include antibody-dependent cellular cytotoxicity, angiogenesis inhibition and reduced downstream signaling through phosphatidylinositol 3-kinase and mitogen-activated protein kinase, which ultimately results in cell-cycle arrest and apoptosis (25–27).
Mature data from four large phase III trials, in which trastuzumab was evaluated in the adjuvant setting, have demonstrated significant improvements in both disease-free and overall survival for patients with HER2-positive breast cancer (17–20). However, most patients with HER2-positive metastatic breast cancer will eventually have disease progression during anti-HER2 therapy, while the tumor continues to overexpress HER2. Various treatment strategies have demonstrated that continuous HER2 blockade can provide significant clinical benefit to patients who have previously received trastuzumab. One clinical strategy is to continue trastuzumab therapy but combine it with either an alternative chemotherapy regimen or an alternative anti-HER2 drug (13,28,29). Another clinical strategy is to combine an alternative anti-HER2 drug with an alternative chemotherapy regimen (30). Hence, HER2 remains a viable therapeutic target despite progression on trastuzumab, and there is great need for additional or enhanced HER2 targeted agents.
DM1
Preclinical and clinical studies indicate that trastuzumab has enhanced anti-tumor effects when combined with microtubule-directed agents (31,32). Thus, a maytansinoid, which is an extremely potent microtubule-directed drug, is attractive for combination with trastuzumab. Maytansine was originally isolated from members of the plant families Rhamnaceae and Euphorbiaceae, as well as some mosses (9,33). The maytansinoids bind tubulin at a site that binds vinca alkaloids and are approximately 100 times more potent than vincristine. To link maytansinoids to antibodies through disulfide bonds, a thiol-containing maytansinoid, DM1 (N-methyl-N-[3-mercapto-1-oxopropyl]-l-alanine ester of maytansinol), was synthesized, which demonstrated an advantage over disulfide and thioether linkers (34).
T-DM1
DM1 is linked to trastuzumab to make T-DM1 by using the bifunctional reagent SMCC (N-succinimidyl-4-[maleimidomethyl] cyclohexanecarboxy-late). SMCC is first added to lysine residues on the protein to produce a linker modified antibody. The thiol group in DM1 is then reacted with the maleimide group of the linker to form a nonreducible thioether bond (Figure 1). An average of 3.5 DM1 moieties is linked to each trastuzumab molecule in T-DM1 (35).
Figure 1.
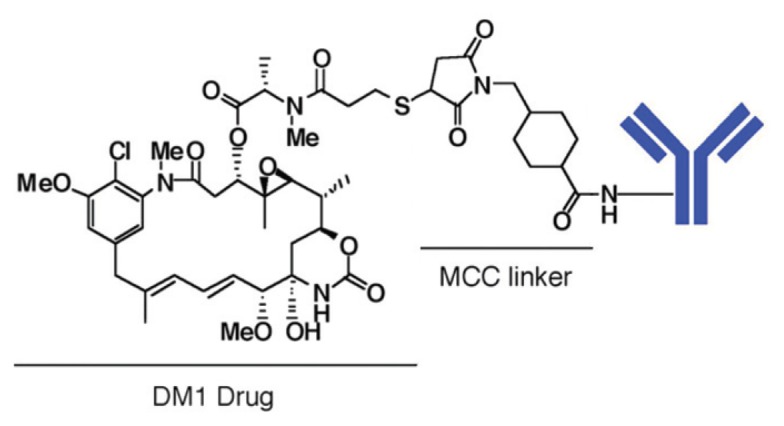
Illustration of T-DM1. An average of 3.5 DM1 moieties is linked to each trastuzumab molecule in T-DM1. T-DM1 is the first ADC with a nonreducible thioether linker in clinical trials.
The biological effects of disulfide linkers were investigated by constructing various T-DM1 conjugates, since the stability of the linker not only compromises potency but can also exacerbate toxicity. Trastuzumab conjugated to DM1 through a nonreducible thioether linkage displayed better efficacy and lower toxicity than trastuzumab conjugated with reducible disulfide linkers (34,35). T-DM1 is internalized upon binding to HER2-overexpressing tumor cells. It is believed that maytansine is released secondary to intracellular proteolytic degradation (36).
The pharmacokinetics of T-DM1 were assessed in nonclinical and clinical studies. Preliminary results demonstrated that T-DM1 exhibits dose-proportional pharmacokinetics in species that do not overexpress the HER2 oncogene (mice and rats) and a dose-dependent decrease in clearance associated with an increasing dose in species that do overexpress the HER2 oncogene (monkeys and humans) (35,37). Results from a preclinical absorption, distribution, metabolism and excretion study of T-DM1 in rats suggest that T-DM1 nonspecifically distributes to tissues without accumulation (38). The DM1 component of T-DM1 is primarily metabolized through the biliary route, mainly by CYP3A4 and, to a lesser extent, by CYP3A5 (39). T-DM1 is neither an inducer nor an inhibitor of major CYP isoforms, which is consistent with the lack of evidence of drug-drug interactions observed in phase I and II clinical studies of T-DM1 combined with taxanes and pertuzumab (14,40). Pertuzumab is a humanized monoclonal antibody that binds to subdomain II of the HER2 extra-cellular domain, whereas trastuzumab binds to subdomain IV (18).
CLINICAL TRIALS
T-DM1 is the first HER2-targeted ADC with a stable and unique SMCC linker to enter the clinic. A phase I study in patients (N = 24) with HER2 metastatic breast cancer whose disease had progressed on earlier trastuzumab-based therapy assessed the safety and tolerability of T-DM1 administered every 3 wks by intravenous infusion over 30–90 min (Table 1) (41). T-DM1 was well tolerated. There were no cardiac events requiring treatment discontinuation or dose modification. Local review of multiple-gated acquisition scans and echocardiograms demonstrated no reduction in left ventricular ejection function of >10 percentage points. Dose-limiting grade 4 thrombocytopenia was observed in cycle 1 for two of three patients treated at 4.8 mg/kg. The maximum tolerated dose of T-DM1 every 3 wks was 3.6 mg/kg. Thrombocytopenia at the maximum tolerated dose was generally grade 1–2 and rapidly reversible. Declines in platelet levels occurred in almost all patients receiving T-DM1 at doses >1.2 mg/kg, and nadirs were observed on approximately d 8. Platelet recovery was generally rapid by d 15.
Table 1.
Data from clinical trials of single agent T-DM1, administered every 3 wks, in HER2-positive metastatic breast cancer.
| Trial | N | Study design | Study population | Overall response rate | Progression-free survival | Grade ≥3 AE |
|---|---|---|---|---|---|---|
| Krop et al.(41) | 24 | Phase I single arm | Treated with chemotherapy and progressed on T | 25%a | NR | Thrombocytopenia |
| Dieras et al.(40) | 77 | Phase Ib/II single arm, T-DM1 and pertuzumab | Treated with chemotherapy and progressed on T | Relapsed patients: 35%; first-line patients: 58% | NR | Relapsed patients: fatigue, thrombocytopenia, cellulitis; first-line patients: thrombocytopenia, increased LFTS |
| Burris et al.(42) | 112 | Phase II single arm | Treated with chemotherapy and progressed on HER2 therapy | 26% | 4.6 months | Hypokalemia, thrombocytopenia, fatigue |
| Krop et al.(43) | 110 | Phase II single arm | Treated with A, TX, C and L and/or T | 35% | 7 months | Thrombocytopenia |
| Perez et al.(29,49) | 137 | Phase II randomized T-DM1 versus T + D | No prior chemotherapy for metastatic disease | T-DM1: 64%; T + D: 58.0% | T-DM1: 14.2 months; T + D: 9.2 months | T-DM1: thrombocytopenia, increased LFTS; T + D: neutropenia, leukopenia |
AE, Adverse events; NR, not reported; T, trastuzumab; A, anthracycline; TX, taxane; C, capecitabine; L, lapatinib; D, docetaxel.
Patients treated with the maximum tolerated dose of 3.6 mg/kg (N = 9): 44%.
Of the 24 patients enrolled to the every-3-wks schedule, 6 had a partial response. These patients had previously been treated with a median of 3.5 chemotherapy cycles in addition to trastuzumab for metastatic breast cancer. All patients had received at least one previous antimicrotubule agent (that is, paclitaxel, docetaxel or vinorelbine), and four patients had received more than two antimicrotubule agents. Nine of fifteen patients who received the maximum tolerated dose of 3.6 mg/kg every 3 wks had measurable disease, and four (44%) of these patients had a confirmed partial response. The maximum tolerated dose of T-DM1, 3.6 mg/kg every 3 wks, was selected for phase II trials of patients with HER2-positive metastatic breast cancer.
Two phase II single-arm studies of T-DM1 were completed, and one phase II study of a T-DM1 single-arm study is ongoing (42–44). In a study of 112 patients who had received previous chemotherapy and had tumor progression after HER2-directed therapy, T-DM1 was associated with an objective response rate of 25.9% on the basis of an independent review (42). The median duration of response was 9.4 months by investigator assessment. The median progression-free survival was 4.6 months. T-DM1 was well tolerated with most adverse events of grade 1–2. The most common grade 3 adverse events were hypokalemia (8.9%), thrombocytopenia (8.0%) and fatigue (4.5%). There was no dose-limiting cardiotoxicity. Trastuzumab-induced cardiotoxicity is a major concern and varies according to the definition used in different studies, but has been reported to be as high as 30% when associated with anthracyclines (45–47).
In a study of 110 patients with HER2-overexpressing metastatic breast cancer who had received previous anthracy-cline, trastuzumab, taxane, capecitabine and lapatinib therapy with evidence of progressive disease on their last regimen, T-DM1 monotherapy led to an objective response rate of 34.5%, a median duration of response of 7.2 months and a median progression-free survival of 6.9 months (43). T-DM1 was well tolerated in this heavily pretreated population, that is, median of seven agents for metastatic disease with no cardiac events requiring dose reductions or additional new safety concerns.
Preliminary results from a randomized phase II trial of T-DM1 versus trastuzumab plus docetaxel in first-line, HER2-positive metastatic breast cancer were recently updated and presented (48,49). In 137 patients, with a median follow-up of 13.5 months, a significant difference in the progression-free survival was seen in patients receiving T-DM1 (14.2 versus 9.2 months, hazard ratio [HR] 0.59, p = 0.035) (48).
On the basis of the efficacy and safety profile of T-DM1 in the phase I and II studies, three confirmatory, randomized, phase III trials were initiated (Table 2) (50–52). The EMILIA trial (a randomized, multicenter, phase III open-label study of the efficacy and safety of trastuzumab-MCC-DM1 versus capecitabine plus laptinib in patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy) evaluated the safety and efficacy of T-DM1 compared with lapatinib plus capecitabine in patients with HER2-positive advanced breast cancer after prior trastuzumab-and taxane-based chemotherapy (Table 3) (50). The investigators have preliminarily reported that the median progression-free survival was 9.6 months in the T-DM1 cohort compared with 6.4 months in the capecitabine and lapatinib cohort (HR 0.650, 95% confidence interval [CI] 0.55–0.77; p < 0.0001). The objective response rate was significantly higher in the T-DM1 cohort at 43.6% compared with 30.8% in the capecitabine plus lapatinib cohort (95% CI 6.0–19.4, p = 0.0002). The overall survival seemed to be improved for patients receiving T-DM1, but the median overall survival was not reached at time of reporting.
Table 2.
Phase III clinical trials evaluating T-DM1.
| Trial | N (estimated enrollment) | Schema | Status |
|---|---|---|---|
| NCT00829166: EMILIA Study (50) | 991 | Two arms: T-DM1 alone versus L + C after progression on T and TX | Closed to accrual |
| NCT01120184: MARIANNE Study (51) | 1,092 | Three arms: T-DM1 with P or T-DM1 with P placebo (blinded for P), versus T + TX in untreated metastatic breast cancer; may have had chemotherapy and T or L in adjuvant setting | Closed to accrual |
| NCT01419197: TH3RESA Study (52) | 795 | Two arms: T-DM1 versus treatment choice; may have received two lines of HER2 therapy | Recruiting patients |
L, lapatinib; C, capecitabine; T, trastuzumab; TX, taxane; P, pertuzumab.
Table 3.
EMILIA trial evaluated the safety and efficacy of T-DM1 compared with lapatinib plus capecitabine in patients with HER2-positive, locally advanced breast cancer or metastatic breast cancer after prior trastuzumab- and taxane-based chemotherapy.
| T-DM1 | Lapatinib + capecitabine | ||
|---|---|---|---|
| Progression-free survival | 9.6 months | 6.4 months | HR 0.650, 95% CI 0.55–0.77; p < 0.0001 |
| Objective response rate | 43.6% | 30.8% | 95% CI 6.0–19.4, p = 0.0002 |
| Median time to symptom progression | 7.1 months | 4.6 months | HR 0.80, 95% CI 0.67–0.95, p = 0.0121 |
| Dose reduction | 16.3% | Lapatinib 53.4%, capecitabine 27.3% | NR |
| ≥ Grade 3 AE | 40.8% | 57% | NR |
| AE resulting in treatment discontinuation | 5.9% | 10.7% | NR |
| Death due to toxicity | 1 | 5 | NR |
NR, not reported.
Preliminary results were presented at the annual American Society of Clinical Oncology conference, 2012 (50).
Dose reduction was necessary for 16.3% of patients randomized to T-DM1. The capecitabine dose and the lapatinib dose were reduced for 53.4% and 27.3% of patients, respectively. The most common grade 3 or greater adverse events for T-DM1 were thrombocytopenia and increased liver function tests for the T-DM1 cohort and diarrhea, palmar plantar erythrodysesthesia and emesis for the capecitabine plus lapatinib cohort.
The incidence of grade 3 or greater adverse events was lower in patients randomized to T-DM1 (40.8%) compared with patients randomized to capecitabine plus lapatinib (57.0%), as was the incidence of adverse events resulting in treatment discontinuation (5.9% versus 10.7%, respectively). Cardiac toxicity was not increased. There was one death due to toxicity in the T-DM1 group and five deaths due to toxicity in the capecitabine plus lapatinib group.
The MARIANNE study (a randomized, three-arm, multicenter, phase III study to evaluate the efficacy and safety of T-DM1 combined with pertuzumab or T-DM1 combined with pertuzumab-placebo [blinded for pertuzumab], versus the combination of trastuzumab plus taxane, as first-line treatment in HER2-positive progressive or recurrent locally advanced or metastatic breast cancer) is comparing the efficacy and safety of single-agent T-DM1 to T-DM1 plus pertuzumab to trastuzumab plus a taxane for the first-line treatment of HER2-positive, metastatic or locally recurrent breast cancer (51). The planned enrollment is 1,092 patients. The primary endpoint is progression-free survival. Secondary endpoints include safety, overall response rate, overall survival, duration of response and quality of life (51).
The Trastuzumab Emtansine in Comparison With Treatment of Physician’s Choice in Patients With HER2-Positive Breast Cancer Who Have Received at Least Two Prior Regimens of HER2-Directed Therapy (TH3RESA) Study is comparing third-line T-DM1 to physician’s choice of treatment in HER2-positive metastatic breast cancer (52). This randomized, two-arm study is evaluating the efficacy and safety of T-DM1 compared with treatment of the physician’s choice in patients with metastatic or unresectable locally advanced or recurrent HER2-positive breast cancer. Time on study treatment is until disease progression or unacceptable toxicity occurs. The primary outcomes are objective response rate by independent review and overall survival. Secondary outcomes include progression-free survival, the duration of objective responses, safety and clinical benefit rate, defined as the proportion of patients achieving an objective response or stable disease for at least 6 months from randomization.
CLINICAL IMPLICATIONS
T-DM1 demonstrates significant progress in the development of ADCs as anticancer agents. In an attempt to improve the efficacy and potency of trastuzumab therapy, the ADC T-DM1 was designed to use the antibody to deliver cytotoxic therapy to overexpressing tumors. In the nearly 1,000 women studied in the EMILIA trial, all of whom had previously received a taxane and trastuzumab, T-DM1 reduced the risk of progression-free survival events by 35% and the risk of death by 38% when compared with capecitabine plus lapatinib (50). In addition, the rate of grade 3 or higher adverse events was about one-third lower with T-DM1 than with the combination therapy.
The success of the EMILIA trial raises new opportunities for further exploration. The European Organization for Research and Treatment of Cancers (EORTC) Elderly Task Force and Breast Cancer Group is evaluating T-DM1 in elderly patients, defined as women over the age of 70 years or over the age of 60 years with comorbidities (53). This phase II trial uses T-DM1 after progression on pertuzumab plus trastuzumab or pertuzumab plus trastuzumab with metronomic chemotherapy. The aim is to define HER2-targeted regimens with minimal toxicity to delay or avoid the use of classic chemotherapy because of competing risks of death in this patient group. There are also plans to investigate T-DM1 in combination with chemotherapy and pertuzumab in the neoadjuvant setting (54).
In 2010, the FDA declined a biologic license application for T-DM1 under the Accelerated Approval Regulations, which require single-arm trials to demonstrate efficacy in patients who are unresponsive to all available therapies (55). T-DM1 has now demonstrated significant therapeutic effects in phase III trials and may set a new standard for anti-cancer therapy as a drug with minimal toxicity and significant efficacy in previously treated HER2-overexpressing breast cancer patients.
T-DM1 may eventually have a role as a single agent as well as a role in early-stage breast cancer. Data from ongoing phase II and phase III studies of T-DM1 are eagerly anticipated.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Bross PF, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–6. [PubMed] [Google Scholar]
- 2.Gopal AK, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;2012;120:560–8. doi: 10.1182/blood-2011-12-397893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pro B, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–6. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 4.Mylotarg (gemtuzumab ozogamicin): market withdrawal [Internet] Silver Spring, (MD): FDA; [updated 2010 Jun 21; cited 2013 Jan 11]. Available from: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm216458.htm. [Google Scholar]
- 5.Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol. 2009;13:235–44. doi: 10.1016/j.cbpa.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2010;21:5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 7.Carter PJ, Senter PD. Antibody-drug conjugates for cancer therapy. Cancer J. 2008;14:154–69. doi: 10.1097/PPO.0b013e318172d704. [DOI] [PubMed] [Google Scholar]
- 8.Higashide E, et al. Ansamitocin, a group of novel maytansinoid antibiotics with antitumour properties from Nocardia. Nature. 1977;270:721–2. doi: 10.1038/270721a0. [DOI] [PubMed] [Google Scholar]
- 9.Yu TW, et al. The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnema pretiosum. Proc Natl Acad Sci U S A. 2002;99:7968–73. doi: 10.1073/pnas.092697199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Amphlett G, Blattler WA, Lambert JM, Zhang W. Structural characterization of the maytansinoid-monoclonal antibody immuno-conjugate, huN901-DM1, by mass spectrometry. Protein Sci. 2005;14:2436–46. doi: 10.1110/ps.051478705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeram M, et al. A phase I study of trastuzumab-DM1 (T-DM1), a first-in-class HER2 antibody-drug conjugate (ADC), in patients (pts) with advanced HER2+ breast cancer (BC) J. Clin. Oncol. 2008;26(15 Suppl) doi: 10.1200/JCO.2009.26.2071. [May 20, 2008]. Abstr nr 1028. [DOI] [PubMed] [Google Scholar]
- 12.Burris HA, et al. A phase II study of trastuzumab-DM1 (T-DM1), a HER2 antibody-drug conjugate (ADC), in patients (pts) with HER2+ metastatic breast cancer (MBC). Abstract presented at: ASCO 2008 Breast Cancer Symposium; 2008. Abstr nr 155. [Google Scholar]
- 13.Carter P, Smith L, Ryan M. Identification and validation of cell surface antigens for antibody targeting in oncology. Endocr Rel Cancer. 2004;11:659–87. doi: 10.1677/erc.1.00766. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ. Proto-oncogenes and human cancers. N Engl J Med. 1987;317:955–7. doi: 10.1056/NEJM198710083171509. [DOI] [PubMed] [Google Scholar]
- 15.Sjogren S, Inganas M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–9. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 16.Arteaga CL, et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 17.Piccart-Gebhart MJ, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 18.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 19.Slamon DJ, Eiermann W, Robert N, et al. Second interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2neu positive early breast cancer patients. Breast Cancer Res. Treat. 2006;100(1 Suppl) Abstr nr LBA 53. 94. [Google Scholar]
- 20.Joensuu H, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 21.Tubbs RR, et al. Fluorescence in situ hybridization (FISH) as primary methodology for the assessment of HER2 status in adenocarcinoma of the breast: a single institution experience. Diagn Mol Path. 2007;16:207–10. doi: 10.1097/PDM.0b013e318064c72a. [DOI] [PubMed] [Google Scholar]
- 22.Ohlschlegel C, Zahel K, Kradolfer D, Hell M, Jochum W. HER2 genetic heterogeneity in breast carcinoma. J Clin Path. 2011;64:1112–6. doi: 10.1136/jclinpath-2011-200265. [DOI] [PubMed] [Google Scholar]
- 23.Yang YL, et al. Genetic heterogeneity of HER2 in breast cancer: impact on HER2 testing and its clinicopathologic significance. Breast Cancer Res Treat. 2012;134:1095–102. doi: 10.1007/s10549-012-2046-0. [DOI] [PubMed] [Google Scholar]
- 24.Drugs [Internet] Silver Spring, (MD): FDA; [updated 2013 Jan 10; cited 2013 Jan 11]. Available from: http://www.fda.gov/Drugs/default.htm. [Google Scholar]
- 25.Yamauchi C, et al. E-cadherin expression on human carcinoma cell affects trastuzumab-mediated antibody-dependent cellular cytotoxicity through killer cell lectin-like receptor G1 on natural killer cells. Int J Cancer. 2011;128:2125–37. doi: 10.1002/ijc.25803. [DOI] [PubMed] [Google Scholar]
- 26.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahta R. Pharmacological strategies to overcome HER2 cross-talk and Trastuzumab resistance. Curr Med Chem. 2012;19:1065–75. doi: 10.2174/092986712799320691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Minckwitz G, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47:2273–81. doi: 10.1016/j.ejca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 29.von Minckwitz G, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 30.Geyer CE, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–31. [PubMed] [Google Scholar]
- 32.Pegram M, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemo-therapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18:2241–51. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 33.Schechter AL, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–6. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 34.Lewis Phillips GD, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 35.Girish S, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69:1229–40. doi: 10.1007/s00280-011-1817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erickson HK, et al. The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates. Mol Cancer Ther. 2012;11:1133–42. doi: 10.1158/1535-7163.MCT-11-0727. [DOI] [PubMed] [Google Scholar]
- 37.Holden SN, et al. A phase I study of weekly dosing of trastuzumab-DM1 (T-DM1) in patients (pts) with advanced HER2+ breast cancer (BC) J. Clin. Oncol. 2008;26(15 Suppl) [May 20, 2008]). Abstr nr 1029. [Google Scholar]
- 38.LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17:6437–47. doi: 10.1158/1078-0432.CCR-11-0762. [DOI] [PubMed] [Google Scholar]
- 39.Wong S, et al. Abstract A136: Nonclinical disposition, metabolism, and in vitro drug-drug interaction assessment of DM1, a component of trastuzumab emtansine (T-DM1) Mol. Cancer Ther. 2011;2011;10(11 Suppl 1) [Google Scholar]
- 40.Dieras V, et al. Abstract P3-14-01: A phase Ib/II trial of trastuzumab-DM1 with pertuzumab for patients with HER2-positive, locally advanced or metastatic breast cancer: interim efficacy and safety results. Cancer Res. 2010;70(24 Suppl 2) [Google Scholar]
- 41.Krop IE, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 42.Burris HA, 3rd, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 43.Krop IE, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2012;30:3234–41. doi: 10.1200/JCO.2011.40.5902. [DOI] [PubMed] [Google Scholar]
- 44.Dang C, et al. Cardiac safety in a phase II study of trastuzumab emtansine (T-DM1) following anthracycline-based chemotherapy as adjuvant or neoadjuvant therapy for early-stage HER2-positive breast cancer. J. Clin. Oncol. 2012;30(15 Suppl) [May 20, 2012]. Abstr nr 532. [Google Scholar]
- 45.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525–33. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 46.Seidman A, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 47.Guglin M, Hartlage G, Reynolds C, Chen R, Patel V. Trastuzumab-induced cardiomyopathy: not as benign as it looks? A retrospective study. J Card Fail. 2009;15:651–7. doi: 10.1016/j.cardfail.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Hurvitz S, et al. Trastuzumab emtansine (T-DM1) vs trastuzumab plus docetaxel (H+T) in previously-untreated HER2-positive metastatic breast cancer (MBC): primary results of a randomized, multicenter, open-label phase II study (TDM4450g/B021976) Eur. J. Cancer. 2011;47(Suppl 1):S330. Abstr nr 5001. [Google Scholar]
- 49.Perez EA, et al. Efficacy and safety of trastuzumab-DM1 versus trastuzumab plus doxetaxel in HER2-positive metastatic breast cancer patients with no prior chemotherapy for metastatic disease: preliminary results of a randomized, multicenter, open-label phase 2 study (TDM4450G) Ann. Oncol. 2010;21(Suppl 8):viii2. Abstr nr LBA3. [Google Scholar]
- 50.Blackwell KL, et al. Primary results from EMILIA, a phase III study of trastuzumab emtansine (T-DM1) versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC) previously treated with trastuzumab (T) and a taxane. J. Clin. Oncol. 2012;30(15 Suppl) [May 20, 2012]. Abstr nr LBA1. [Google Scholar]
- 51.Ellis PA, et al. MARIANNE: A phase III, randomized study of trastuzumab-DM1 (T-DM1) with or without pertuzumab (P) compared with trastuzumab (H) plus taxane for first-line treatment of HER2-positive, progressive, or recurrent locally advanced or metastatic breast cancer (MBC) J. Clin. Oncol. 2011;29(15 Suppl) [May 20, 2011]. Abstr nr TPS102. [Google Scholar]
- 52.A Study of Trastuzumab Emtansine in Comparison With Treatment of Physician’s Choice in Patients With HER2-Positive Breast Cancer Who Have Received at Least Two Prior Regimens of HER2-Directed Therapy (TH3RESA) [Internet] Bethesda (MD): NIH; [study updated 2012 Dec 3; cited 2013 Jan 11]. Available from: http://clinicaltrials.gov/show/NCT01419197. [Google Scholar]
- 53.Elderly Metastatic Breast Cancer: Pertuzumab-Herceptin vs Pertuzumab-Herceptin-Metronomic Chemotherapy, Followed by T-DM1 [Internet] Bethesda (MD): NIH; [study updated 2012 Jun 1; cited 2013 Jan 11]. Available from: http://clinicaltrials.gov/show/NCT01597414. [Google Scholar]
- 54.ImmunoGen, Inc. announces development of trastuzumab emtansine for early stage HER2-positive breast cancer [Internet] Waltham (MA): ImmunoGen, Inc; 2012. Jun 3, [cited 2013 Jan 11]. Available from: http://investor.immunogen.com/releasedetail.cfm?ReleaseID=679372. [Google Scholar]
- 55.Roche provides update on FDA application for T-DM1: Roche expects a global regulatory submission mid 2012 [Internet] Basel: F. Hoffmann-La Roche Ltd; 2010. Aug 10, [cited 2013 Jan 18]. Available from: http://www.roche.com/media/media_releases/med-cor-2010-08-27.htm. [Google Scholar]


