Abstract
We hypothesize that isoflurane and ketamine impact ventilatory pattern variability (VPV) differently. Adult Sprague-Dawley rats were recorded in a whole-body plethysmograph before, during and after deep anesthesia. VPV was quantified from 60-s epochs using a complementary set of analytic techniques that included constructing surrogate data sets that preserved the linear structure but disrupted nonlinear deterministic properties of the original data. Even though isoflurane decreased and ketamine increased respiratory rate, VPV as quantified by the coefficient of variation decreased for both anesthetics. Further, mutual information increased and sample entropy decreased and the nonlinear complexity index (NLCI) increased during anesthesia despite qualitative differences in the shape and period of the waveform. Surprisingly mutual information and sample entropy did not change in the surrogate sets constructed from isoflurane data, but in those constructed from ketamine data, mutual information increased and sample entropy decreased significantly in the surrogate segments constructed from anesthetized relative to unanesthetized epochs. These data suggest that separate mechanisms modulate linear and nonlinear variability of breathing.
1. Introduction
General anesthesia is employed routinely in clinical practice, both in healthy patients undergoing surgical procedures as well as severely ill patients with respiratory disease requiring mechanical ventilation. Analgesics and sedatives are used to relieve pain or anxiety in a wide range of medical problems and interventional procedures. These medications influence breathing and its variability (Icaza et al., 2009; Jaspar et al., 1983; Morel, et al., 1986; Teppema and Baby, 2011; Vanini, et al., 2008), whether they are used in patients with or without underlying respiratory pathology. Because of their widespread use, they may be a confounding factor during medical interventions.
Breathing patterns are altered in different states of health and disease. For instance, decrease of variability in temporal patterning can be a sign of disease. Changes in complex temporal relationships in the ventilatory pattern leads to a decrease in variability in restrictive lung disease (Brack et al., 2002), respiratory failure (Bien et al., 2004; Casaseca-de-la-Higuera et al., 2006; Giraldo et al., 2004; Wysocki et al., 2006), and sepsis (Askanazi et al., 1979). On the other hand, asthma (Kuratomi et al., 1985), panic disorder (Yeragani et al., 2002), and sleep apnea syndrome (Ibrahim et al., 2008) increase respiratory variability. While the association of these conditions with these changes in breathing pattern is appreciated, the impact of breathing variability on disease state is not well understood. Respiratory variability is not widely used to track clinical progress or deterioration, but assessing respiratory patterning may improve risk stratification of critically ill patients or facilitate weaning patients off mechanical ventilation (Bien et al., 2004; Casaseca-de-la-Higuera et al., 2006; Giraldo et al., 2004; Jacono et al., 2010).
Anesthetics are also routinely employed in basic research. They are most often used as part of the preparation for an intervention, e.g. surgery or mechanical ventilation. Our study examines the effect of two anesthetics on respiratory variability in rats: isoflurane and ketamine, which are commonly used in research. These sedatives are frequently used in experiments employing animal models of human disease, but their potential ability to confound the results by their own direct effect on breathing is not well established ((Icaza et al., 2009; Jaspar et al., 1983). Detailed analyses of the impact of isoflurane and ketamine on VPV have not been performed. To address this knowledge gap, we tested the hypothesis that these medications would impact VPV differently.
Respiratory control is a complex system that exhibits deterministic behavior in the normal as well as the pathologic state (Brack et al., 2002; Bien et al., 2004; Casaseca-de-la-Higuera et al., 2006; Askanazi et al., 1979; Kuratomi et al., 1985; Yeragani et al. 2002; Ibrahim et al., 2008). To construct a more complete picture of respiratory variability, we employed a set of analytic techniques that we developed recently (Dhingra et al., 2011; Jacono et al., 2010) to separate linear and nonlinear components of variability and to quantify deterministic variability in the ventilatory pattern. The following tools were used: coefficient of variation, a test which captures distributional variance in the pattern; mutual information, which quantifies statistical dependence; sample entropy, a measure of self-similarity in a time series; and surrogate data sets, which were constructed to reflect the linear but destroy the nonlinear sources of variability. These techniques were used in a complementary fashion, as the control mechanism for respiratory pattern generation is complex and incompletely described by frequency and coefficient of variation as applied to respiratory cycle length.
2. Materials and Methods
2.1 Animals
Experiments were performed on 12 adult male Sprague-Dawley rats (Harlan, Indianapolis, PA) weighing 100-150g. The experimental protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
2.2 Plethysmography recordings
Respiratory pattern was measured using whole-body flow-through plethysmography chambers (Buxco Research Systems, Wilmington, NC), which were cleaned prior to each experiment to reduce sniffing behavior. A visual barrier was placed around the chambers once the rats were in place to minimize environmental stimuli and movement artifacts during the plethysmography recording. The chambers (volume 3.9 L, diameter 8”) were connected to a high-gain differential pressure transducer (model MP45, Validyne, Northridge, CA), whose signal was amplified (model BMA 830, CWE, Ardmore, PA) and subsequently processed and stored by Spike 2 Software (Cambridge Electronic Design, Cambridge, England). The animals were video-recorded using software integrated into Spike 2, and the resulting video was used to verify that movement-free epochs were selected for analysis.
With the plethysmography tracing recording continuously, the rats remained in the chambers for 1h of baseline recordings with room air flowing into the chambers. Tracings from the first 40 min were not used because the rats need to acclimatize to the chamber. In all the protocols, anesthesia was induced with 3% isoflurane in room air. For the isoflurane experiments (n=7), the concentration of isoflurane was reduced to 1.5% in room air, and the rats remained at this setting for 1h. Then they recovered in the chambers for 1h with the chambers being flushed with room air. For the ketamine experiments (n=5), a weight-based (100mg/kg) intraperitoneal dose of ketamine (Borchard, et al., 1991) was administered once the animals were initially sedated with isoflurane. These rats were returned to the chambers flushed with room air immediately after the injection with ketamine, and they remained in the chambers until 2h after exhibiting awake behavior, defined as movements other than breathing.
2.3 Data Analysis
The raw plethysmography data (sampled at 200 Hz) was transferred to a custom software program (Case Western Reserve University). Each state – baseline, anesthesia, recovery – was represented by three 60-s epochs. Baseline epochs (60-s) for data analysis were taken at 43, 45, and 47 min. With the isoflurane anesthesia data were analyzed from 56, 58, and 60 min after the isoflurane was started. The recovery data was taken from 56, 58, and 60 min after the isoflurane was discontinued. For the ketamine animals, the anesthesia period was defined by behavior as well as time. The time point of observable sedation was defined as lying in the chamber without any visible movement except breathing. The time point of observable recovery was visible movement in addition to breathing. The length of this observed anesthesia ranged from 28 to 39 min. For our data analysis, we used the temporal midpoint between observable sedation and recovery to obtain our anesthesia data. For example, if an animal was observed to be anesthetized for 39 min, the tracings from 18, 20, and 22 min were used for analysis of the effects of anesthesia. The recovery data for the ketamine animals was taken from 118, 120, and 122 min after observable recovery began. At this time, the rats were quiescent and this was at least 90 min after non-breathing movements. Video recordings of the experiments were used to verify the animal’s state at each time point.
For each epoch, discrete breaths were identified by the custom software and verified manually. Respiratory rate and coefficient of variation of respiratory cycle length (CV-TTOT) were calculated for each of the three epochs from each state: baseline, anesthesia, and recovery. Reported data represents the average value of those three epochs for every animal. In addition, Spike 2 Software was used to generate cycle-triggered averages to capture average frequency and morphology over the entire 60-s epoch (at least 50 breaths).
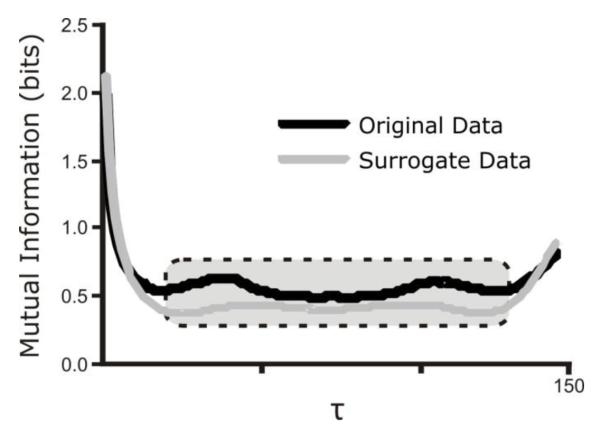
Mutual Information and Sample Entropy of raw and surrogate data were calculated as described previously (Dhingra et al., 2011). Mutual Information (MI) is a measure of statistical dependence in a data set that includes influences of both linear and nonlinear correlations (Fraser and Swinney, 1986; Shannon, 1997). MI quantifies the degree to which the knowledge of a coordinate reduces the amount of uncertainty associated with a time-advanced coordinate x(t+τ), where τ>0 is the time interval between samples as determined by sampling frequency. MI was computed for each time lag (τ) from unity to one cycle length. We report an average value for the middle 65% of MI, which excludes time lags within 17.5% of the minimum and maximum time lags, as these values tended to be high because of the oscillatory nature of the signal (Figure 1).
Figure 1.
Methodology of calculating average value of Mutual Information. The small and large τ values represent periods of high linear correlation and are thus outliers. The inner 65% of data points, represented by the dotted box superimposed on the graph, is averaged to determine the reported value of Mutual Information.
Sample Entropy (SampEn) is a measure of temporal pattern variability reflecting self-similarity in a time-series, with high values denoting less self-similarity, greater complexity and less predictability (Richman and Moorman, 2000). To calculate SampEn, templates consisting of m points and m + 1 points separated by a time interval (τ) was created for every point. Computationally, sample entropy is the negative natural logarithm of the conditional probability that epochs with a certain number of matches for m number of points will also have matches for m + 1 points. Matches are defined as points within a tolerance r. Our analysis was computed using m = 2 and r = 0.2*SD. SampEn was computed over multiple τ’s from unity up to one cycle length. These values were averaged across time lags excluding those for which high linear correlations were present in the data set as defined by the first minimum of the mutual information function.
Surrogate data sets (n=19) were computed using the iterated amplitude adjusted Fourier transform by moving the data into the frequency domain and back into the time domain while ensuring that both the frequency distribution (power spectrum/autocorrelation function) and the amplitude distribution were maintained (Kaffshi et al., 2008; Schreiber and Schmitzm, 2000; Theiler, 1986). Average MI and SampEn calculations were also performed on the surrogate data sets using the techniques described above.
To determine complexity attributable to nonlinear sources, differences were tabulated between SampEn for original and surrogate datasets at each τ. A nonlinear complexity index (NLCI) was computed as the average of the statistically significant differences in SampEn between the surrogate and original data sets. Higher values of NLCI correlate with an increased contribution of nonlinear and/or non-Gaussian sources of variability.
Results are presented as mean ± standard deviation. The data were inspected graphically and were distributed normally. To determine statistical difference between the baseline, anesthesia, and recovery states a repeated measures analysis of variance (rmANOVA) was performed. If statistical significance was found (rmANOVA p<0.05), then adjustments for multiple comparisons to control the type-I error rate were performed using the Fisher-Hayter Procedure. A two-tailed p < 0.05 was considered significant. We performed analyses using STATA 10.0 (STATACORP, College Station, TX).
3. Results
3.1 Effect of anesthesia on ventilatory pattern: rate and shape
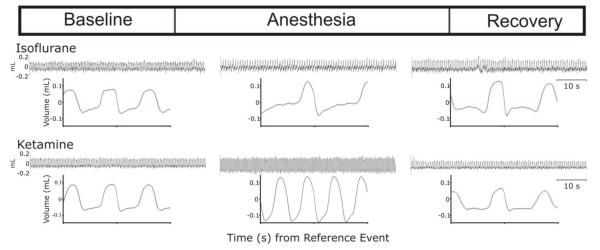
The influences of isoflurane and ketamine on breathing are summarized in Figure 2. Characteristic tracings from each state and for each anesthetic are presented. Changes in frequency and morphology are apparent on qualitative assessment of the cycle-triggered averages that reflect patterning over the entire 60-s epoch. Respiratory rate decreased with isoflurane (baseline vs. anesthesia, p<0.001; anesthesia vs. recovery, p=0.003) but increased with ketamine (baseline vs. anesthesia, p<0.001; anesthesia vs. recovery, p<0.001) (Table 1). With both anesthetics, the coefficient of variation of respiratory cycle length (CV-TTOT) decreased during anesthesia and returned to baseline levels during recovery (Table 1).
Figure 2.
Cycle-triggered histogram representing averaged breaths from each state.
Table 1A.
| baseline | anesthesia | recovery | |
|---|---|---|---|
| RR a (breaths/min) |
104±10 | 77±16 | 92±18 |
| CV- TTOTb | 0.084±0.011 | 0.047±0.016 | 0.076±0.008 |
Data are presented as mean ± standard deviation
Respiratory Rate
Coefficient of variation of respiratory cycle length
3.2 Effects of anesthesia on mutual information
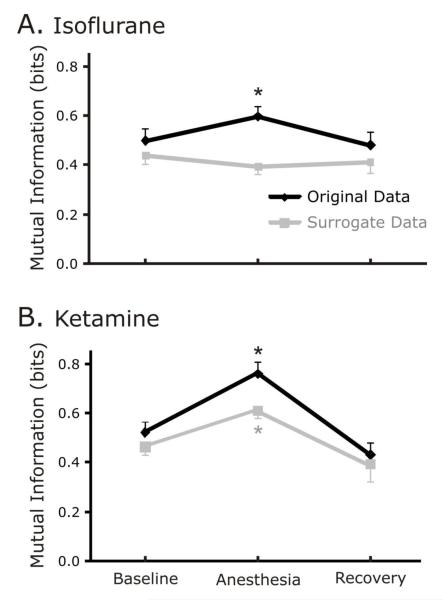
In the rats anesthetized with isoflurane, MI increased from baseline to anesthesia and decreased during recovery (baseline vs. anesthesia, p<0.001; anesthesia vs. recovery, p<0.001) for the original data. However, MI of the surrogate data did not change significantly in the isoflurane group during anesthesia as compared to baseline and recovery (repeated measures rmANOVA p=0.11; Figure 3A.).
Figure 3.
A. Isoflurane increases statistical dependence as measured by Mutual Information for original data but not surrogate. While the Mutual Information increased for the original data set during sedation, the same was not true for the surrogate data set. The asterisk denotes where the value during sedation is significantly different from both the baseline and recovery values. Figure 3B. Ketamine increases statistical dependence as measured by mutual information for both original and surrogate data.
In contrast, in the rats anesthetized with ketamine, both the original and surrogate data sets had significant differences for MI (Figure 3B.). For the original data set, MI increased from baseline to anesthesia and decreased during recovery (baseline vs. anesthesia, p<0.001; anesthesia vs. recovery, p<0.001). For surrogate data sets, MI showed the same pattern; increasing during anesthesia, decreasing during recovery (baseline vs. anesthesia, p=0.009; anesthesia vs. recovery, p<0.001).
3.3 Effects of anesthesia on sample entropy
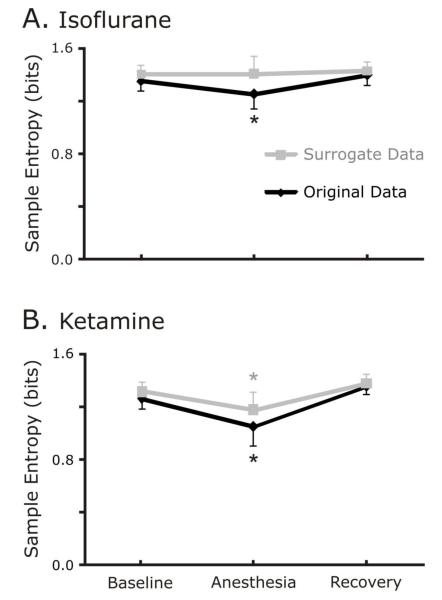
In the animals anesthetized with isoflurane, SampEn decreased significantly for the original but not the surrogate data set (Figure 4A.). For the original data set, SampEn decreased from baseline to anesthesia and increased during recovery (baseline vs. anesthesia, p=0.05; anesthesia vs. recovery, p=0.008). In contrast, SampEn of the surrogate data sets constructed from isoflurane data did not change in the anesthetized segments relative to the unanaesthetized segments (rmANOVA p=0.774).
Figure 4.
A. Isoflurane decreases temporal variability of original data as measured by sample entropy. As we found in the Mutual Information calculations, the isoflurane did not change the Sample Entropy of the surrogate data, but it did change the Sample Entropy of the original data. A decrease in sample entropy indicates less complexity and greater predictability of a data set. The asterik denotes that the values during sedation are significantly different from both baseline and recovery values. Figure 4B. Ketamine decreases temporal variability of original and surrogate data sets. As was the case with the Mutual Information results, both the original and surrogate data sets were influenced by ketamine, despite the result that isoflurane significantly influenced on the original data.
In ketamine-anesthetized animals, SampEn decreased significantly during anesthesia for both the original (baseline vs. anesthesia, p<0.001; anesthesia vs. recovery, p<0.001) and surrogate data sets (baseline vs. anesthesia, p<0.001; anesthesia vs. recovery, p<0.001; Figure 4B.).
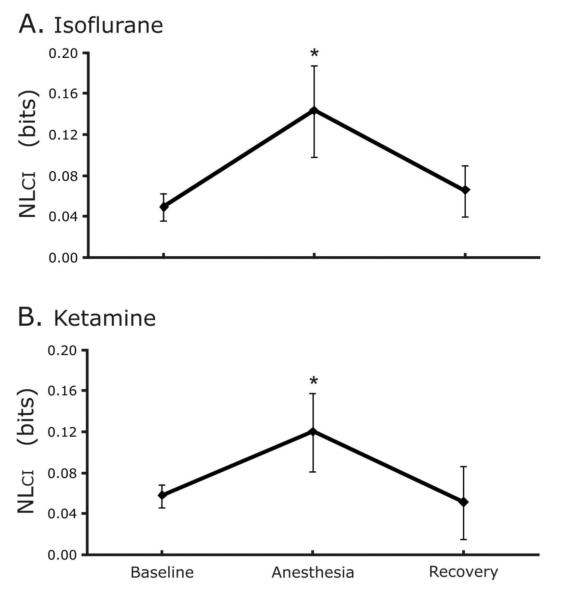
3.4 Effects of anesthesia on nonlinear complexity
Differences between the SampEn of the surrogate and original data sets were used to quantify the NLCI of breathing patterns. NLCI increased during anesthesia with isoflurane from a baseline of 0.049±0.013 to 0.143±0.045 and decreased to 0.065±0.025 during recovery (baseline vs. anesthesia, p<0.001; anesthesia vs. recovery, p<0.001; Figure 5A.).
Figure 5.
A. Sedation with isoflurane increases nonlinear variability. The NLcI quantifies the difference in SampEn between the original and surrogate data, thereby representing a measure of the nonlinear variability contained in the data. The asterisk denotes that the sedation value was significantly different from both the baseline and recovery results. Figure 5B. Sedation with ketamine increases nonlinear variability.
Similarly, NLCI increased during anesthesia with ketamine from baseline (0.058±0.012 to 0.120±0.038) and decreased during recovery (0.051±0.036; baseline vs. anesthesia, p<0.001; anesthesia vs. recovery, p<0.001; Figure 5B.).
4. Discussion
Isoflurane and ketamine have different effects on ventilatory patterns. Isoflurane decreased and ketamine increased respiratory frequency, but both anesthetics decreased the coefficient of variation of respiratory cycle length. Nonlinear determinants of breathing variability as quantified by the NLCI increase with anesthesia with both isoflurane and ketamine; but these anesthetics have differential effects on other measures of VPV, specifically measurements of mutual information and sample entropy as applied to surrogate data sets.
Traditionally, the quantification of VPV has focused on scored interval data primarily analyzed using statistical measures such as mean, variance and CV. This strategy implicitly suggests a paradigm where the mechanics of the respiratory system can be captured solely in timing mechanisms, and it assumes statistical independence between breaths. In contrast, our results demonstrate that specific aspects of variability can be measured and differentiated in both interval and raw waveform data, each contributing to a more complete analysis.
Our analysis of interval data revealed decreases in mean respiratory rate with isoflurane, while ketamine increased mean rate. In contrast, breathing variability as measured by CV-TTOT, decreased decisively during anesthesia with both ketamine and isoflurane. This reflects a more regular breathing pattern during anesthesia, a phenomenon which was reversed during recovery. Taken in isolation, these findings would suggest that both anesthetics have a similar impact on pattern variability. Further, these decreases in variability are not likely to be rate-related as each anesthetic impacted respiratory frequency differently. However, qualitative differences in the shape of breathing patterns appeared in the cycle triggered averages. These observations prompted an extended analysis of respiratory waveform morphology. Analysis of the raw signal confirmed that the overall variability of the waveforms decreased during anesthesia, as represented by increases in statistical dependence between points (quantified by an increase in mutual information). Decreases in sample entropy further highlight the loss of complexity in breathing pattern morphology. An increase in nonlinear relationships between points in the waveform contributed to the observed changes in pattern shape induced by both anesthetics.
Given the similar impact of both sedatives on nonlinear structure in the signal, it is surprising that these anesthetics would affect the linear structure differently. Specifically, the mutual information and sample entropy of the isoflurane surrogates do not change significantly during anesthesia as compared to baseline and recovery; whereas mutual information increases and sample entropy decreases for the ketamine surrogates constructed from anesthetized segments relative to the unanaesthetized segments. Surrogate data sets maintain both the autocorrelation (linear) structure and amplitude distribution of the original time series. As a result, changes in the mutual information or sample entropy of the surrogate data reveal an alteration in the contribution of linear Gaussian stochastic variability to the ventilatory pattern. Thus, ketamine has a stronger influence on linear properties of the signal as compared to isoflurane. Taken together, these findings suggest a complicated control mechanism for pattern generation that is incompletely captured by coefficient of variation as applied to respiratory cycle length. In particular, changes in both linear and nonlinear sources of variability contribute to the observed changes in breathing patterning during anesthesia. Furthermore, the two anesthetics examined had differential impacts on this balance of variability determinants. These observations introduce the possibility that separate mechanisms modulate linear and nonlinear variability.
Many anesthetics depress ventilation (Teppema and Baby, 2011), but increases in respiratory rate with ketamine similar to those observed in the present study have been reported. In particular in humans, Morel and colleagues (1986) reported increases in frequency resulting primarily from decreases in expiratory time. Further, the mechanisms by which ketamine might influence linear aspects of breathing pattern variability that isoflurane does not are not known. Ketamine, in contrast to isoflurane, is a “dissociative” anesthetic occasionally associated with hallucinations and tonic-clonic movements. However, no evidence of these side effects was noted during the anesthetized state in our study. Potential mechanisms postulated by others include direct stimulation of brainstem structures, activation of the pituitary- adrenal axis with adrenal release of catecholamines, increased CO2 production, increases in physiologic dead space due to the bronchodilating properties of ketamine (Morel et al., 1986), and a sparing effect on intercostal muscle activity (Mankikian et al., 1986). Ketamine interacts with N-methyl-D-aspartate (NMDA), opioid, monoaminergic and muscarinic receptors (Hirota and Lambert, 1996). In contrast, isoflurane interacts with γ-aminobutyric acid (GABA) receptors. In particular, isoflurane decreases GABA levels in the pontine reticular formation, and administration of a GABA uptake blocker in this brainstem area abrogates the decreases in respiratory rate during isoflurane anesthesia (Vanini et al., 2008). Interestingly, ketamine and isoflurane differentially alter the synaptic pathways of the trigeminocardiac reflex (Wang et al., 2011). The contribution of these mechanisms to the observed changes in linear and nonlinear aspects of breathing pattern variability with isoflurane and ketamine will be the focus of future studies.
Our strategy for quantifying pattern variability provides a unique insight into characterizing the changes in respiratory waveforms during anesthesia with ketamine and isoflurane. Future directions would include investigating the biological basis for the similarities as well as contrasting ways in which the two anesthetics influence respiration. Our current study describes the effect of these medications on ventilatory pattern variability but does not provide a physiologic explanation for these differences or identify the physiologic consequences of these changes. Another limitation of our study technique is that we did not perform physiologic monitoring such as electroencephalography or electrocardiography, although we did document onset of recovery from anesthesia using clinical indicators. This approach was taken to limit environmental influences on breathing patterns in the baseline and recovery states, but studies that incorporate these measurements may provide further insight into the biology of these sedatives. Another limitation is that depth of anesthesia was not measured, but we used standard dosing of these medications. Such dosing provides a surgical plane of anesthesia. Future studies will include physiologic monitoring such as electroencephalography and electrocardiography, as well as animal models of human disease to make clinical correlations of the impact of these anesthetics on breathing.
In summary, the present study demonstrates that anesthesia with isoflurane and ketamine alters breathing patterns, and that each drug uniquely impacts the balance between the linear and nonlinear components of pattern variability. Anesthetics are routinely utilized in bench research employing animal models, as well as in clinical settings with human patients. Our ultimate aim is to describe not only the changes in ventilatory pattern variability that occur during anesthesia in normal subjects, but also to make correlations between respiratory health or injury with ventilatory pattern variability. We speculate that respiratory variability may eventually be viewed as an “additional vital sign” used to make clinical assessments of patients. Analyses of ventilatory pattern variability may inform the decisions clinicians make for sedated patients in the operating room and intensive care unit.
Highlights.
* Assessed effects of isoflurane and ketamine on ventilatory pattern variability (VPV).
* Isoflurane increased and ketamine decreased respiratory rate.
* Surrogate data sets maintained linear but not nonlinear properties of original data.
* Mutual information & sample entropy of the surrogates increased only after ketamine.
* Thus, linear and nonlinear properties of VPV may be controlled differentially.
Table 1B.
Effect of sedation with ketamine on RRa and CV-TTOTb
| baseline | anesthesia | recovery | |
|---|---|---|---|
| RR a (breaths/min) |
109±9 | 146±11 | 88±12 |
| CV- TTOTb | 0.088±0.016 | 0.055±0.017 | 0.088±0.019 |
Data are presented as mean ± standard deviation
Respiratory Rate
Coefficient of variation of respiratory cycle length
Acknowledgments
We gratefully acknowledge that this work was supported by Award No. I01BX000873 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (to Frank Jacono) and by National Institutes of Health (NIH) HL-080318, NIH HL-087377 (to Thomas Dick).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askanazi J, Silverberg PA, Hyman AI, Rosenbaum SH, Foster R, Kinney JM. Patterns of ventilation in postoperative and acutely ill patients. Crit. Care Med. 1979;7:41–46. doi: 10.1097/00003246-197902000-00002. [DOI] [PubMed] [Google Scholar]
- Bien MY, Hseu SS, Yien HW, Kuo BI, Lin YT, Wang JH, Kou YR. Breathing pattern variability: a weaning predictor in postoperative patients recovering from systemic inflammatory response syndrome. Intensive Care Med. 2004;30:241–247. doi: 10.1007/s00134-003-2073-8. [DOI] [PubMed] [Google Scholar]
- Borchard R, Barnes C, Eltherington L. Drug dosage in laboratory animals. The Telford Press; New Jersey: 1991. [Google Scholar]
- Brack T, Jubran A, Tobin MJ. Dyspnea and decreased variability of breathing in patients with restrictive lung disease. Am. J. Respir. Crit. Care Med. 2002;165:1260–1264. doi: 10.1164/rccm.2201018. [DOI] [PubMed] [Google Scholar]
- Casaseca-de-la-Higuera P, Martin-Fernandez M, Alberola-Lopez C. Weaning from mechanical ventilation: a retrospective analysis leading to a multimodal perspective. IEEE Trans. Biomed. Eng. 2006;53:1330–1345. doi: 10.1109/TBME.2006.873695. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Jacono F, Fishman M, Loparo K, Rybak I, Dick T. Vagal-dependent nonlinear variability in the respiratory pattern of anesthetized, spontaneously breathing rats. J. Appl. Physiol. 2011;111:272–284. doi: 10.1152/japplphysiol.91196.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AM, Swinney HL. Independent coordinates for strange attractors from mutual information. Phys. Rev. A. 1986;33:1134–1140. doi: 10.1103/physreva.33.1134. [DOI] [PubMed] [Google Scholar]
- Giraldo BF, Chaparro J, Ballesteros D, Lopez-Rodriguez L, Geat D, Benito S, Caminal P. Study of the respiratory pattern variability in patients during weaning trials. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004;6:3909–3912. doi: 10.1109/IEMBS.2004.1404093. [DOI] [PubMed] [Google Scholar]
- Hirota K, Lambert DG. Ketamine: its mechanism(s) of action and unusual clinical uses. Br. J. Anaesth. 1996;77:441–444. doi: 10.1093/bja/77.4.441. [DOI] [PubMed] [Google Scholar]
- Ibrahim LH, Patel SR, Modarres M, Johnson NL, Mehra R, Kirchner HL, Redline S. A measure of ventilatory variability at wake-sleep transition predicts sleep apnea severity. Chest. 2008;134:73–78. doi: 10.1378/chest.07-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icaza EE, Huang X, Fu Y, Neubig RR, Baghdoyan HA, Lydic R. Isoflurane-induced changes in righting response and breathing are modulated by RGS proteins. Anesth. Analg. 2009;109:1500–1505. doi: 10.1213/ANE.0b013e3181ba7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacono FJ, De Georgia MA, Wilson CG, Dick TE, Loparo KA. Data acquisition and complex systems analysis in critical care: Developing the intensive care unit of the future. J. Healthc. Eng. 2010;1:337–356. [Google Scholar]
- Jaspar N, Mazzarelli M, Tessier C, Milic-Emili J. Effect of ketamine on control of breathing in cats. J. Appl. Physiol. 1983;55:851–859. doi: 10.1152/jappl.1983.55.3.851. [DOI] [PubMed] [Google Scholar]
- Kaffshi F, Foglyano R, Wilson CG, Loparo KA. The effect of time delay on approximate & sample entropy calculations. Physica D: Nonlinear Phenomena. 2008;237:3069–3074. [Google Scholar]
- Kuratomi Y, Okazaki N, Ishihara T, Arai T, Kira S. Variability of breath-by-breath tidal volume and its characteristics in normal and diseased subjects. Ventilatory monitoring with electrical impedance pneumography. Jpn. J. Med. 1985;24:141–149. doi: 10.2169/internalmedicine1962.24.141. [DOI] [PubMed] [Google Scholar]
- Mankikian B, Cantineau JP, Sartene R, Clergue F, Viars P. Ventilatory pattern and chest wall mechanics during ketamine anesthesia in humans. Anesthesiology. 1986;65:492–499. doi: 10.1097/00000542-198611000-00007. [DOI] [PubMed] [Google Scholar]
- Morel DR, Forster A, Gemperle M. Noninvasive evaluation of breathing pattern and thoraco-abdominal motion following the infusion of ketamine or droperidol in humans. Anesthesiology. 1986;65:392–398. doi: 10.1097/00000542-198610000-00008. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Schmitzm A. Surrogate time series. Physica D. 2000;142:346–382. [Google Scholar]
- Shannon CE. The mathematical theory of communication. MD Comput. 1997;14:306–317. [PubMed] [Google Scholar]
- Teppema LJ, Baby S. Anesthetics and control of breathing. Resp. Physiol. Neurobiol. 2011;177:80–92. doi: 10.1016/j.resp.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Theiler J. Spurious dimension from correlation algorithms applied to limited time-series data. Phys. Rev. A. 1986;34:2427–2432. doi: 10.1103/physreva.34.2427. [DOI] [PubMed] [Google Scholar]
- Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gorini C, Sharp D, Bateman R, Mendelowitz D. Anaesthetics differentially modulate the trigeminocardiac reflex excitatory synaptic pathway in the brainstem. J. Physiol. 2011;589:5431–5442. doi: 10.1113/jphysiol.2011.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki M, Cracco C, Teixeira A, Mercat A, Diehl JL, Lefort Y, Derenne JP, Similowski T. Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation. Crit. Care Med. 2006;34:2076–2083. doi: 10.1097/01.CCM.0000227175.83575.E9. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Radhakrishna RK, Tancer M, Uhde T. Nonlinear measures of respiration: respiratory irregularity and increased chaos of respiration in patients with panic disorder. Neuropsychobiology. 2002;46:111–120. doi: 10.1159/000066388. [DOI] [PubMed] [Google Scholar]