Abstract
The sociocommunicative impairments that define autism spectrum disorder (ASD) are not present at birth but emerge gradually over the first two years of life. In typical development, basic attentional processes may provide a critical foundation for sociocommunicative abilities. Therefore early attentional dysfunction in ASD may result in atypical development of social communication. Prior research has demonstrated that persons with ASD exhibit early and lifelong impairments in attention. The primary aim of this paper is to provide a review of the extant research on attention in ASD using a framework of functionally independent attentional networks as conceptualized by Posner and colleagues: the alerting, orienting and executive control networks (Posner and Petersen, 1990; Petersen & Posner, 2012). The neural substrates and typical development of each attentional network is briefly discussed, a review of the ASD attention literature is presented, and a hypothesis is proposed that links aberrant attentional mechanisms, specifically impaired disengagement of attention, with the emergence of core ASD symptoms.
Keywords: autism, attention, development, alerting, arousal, orienting, disengagement, executive control
1. Introduction
From birth, our senses are inundated with information from a diverse and ever-changing environment. Attentional mechanisms are responsible for the selection of a small portion of information from this deluge. What captures our attention automatically and what we choose to attend to influences the way we experience and perceive the world around us and impacts the course of brain and behavioral development. Attention can be broadly defined as information processing mechanisms that mediate perceptual selectivity. This selection is controlled by endogenous, goal-directed processes dependent on the desires, expectations, and/or knowledge of the observer (i.e. top-down control) and exogenous, stimulus-driven (bottom-up) factors may be orthogonal to goal-directed behaviors (Yantis, 1993) and are dependent on the physical characteristics of the stimuli (Wolfe, 1994). Attentional selection rarely consists of exclusively top-down or bottom-up mechanisms; rather, successful and adaptive information processing requires the integration of these two processes (see Pashler, Johnston, & Ruthruff, 2001, for review). Information selection may occur early with irrelevant information filtered from further processing or late with irrelevant information processed to a greater degree (see Pashler, 1999, for review), and is dependent on the perceptual load of the stimuli to be processed (Lavie, 2005).
Posner and Petersen (1990; 2012) conceptualized attention as consisting of three functionally independent attentional networks, which are responsible for a distinct set of cognitive processes: the alerting, orienting, and executive control networks. These three networks have been shown to have some degree of behavioral, neurophysiological, and neuroanatomical independence (Fan et al., 2007; Fan, McCandliss, Sommer, Raz, & Posner, 2002; Fan, McCandliss, Fossella, Flombaum, & Posner, 2005; see Raz & Buhle, 2006, for a recent review). More recently, Posner and Fan (2004) hypothesized that this model may assist in elucidating differences in attentional modulation between typically developing (TD) individuals and individuals with atypical attention. The current review will use this framework of attention to elucidate network-specific attentional abnormalities in autism spectrum disorder (ASD).
Autism spectrum disorder is a behaviorally-defined developmental disorder diagnosed on the basis of impairments and anomalies in the domains of social communication and repetitive and stereotyped behaviors (APA, 2000). While a diagnosis of ASD is based on these features, attentional abnormalities have been associated with the disorder since its first description (Kanner, 1943). Individuals with ASD exhibit early (Baranek, 1999; Elsabbagh et al., 2009; Osterling & Dawson, 1994; Osterling, Dawson, & Munson, 2002; Swettenham et al., 1998; Zwaigenbaum et al., 2005) and pervasive (see Allen & Courchesne, 2001; Burack, Enns, Stauder, Mottron, & Randolph, 1997, for earlier reviews) abnormalities of attention. Importantly, atypical attentional function has been shown in infants at-risk for ASD (at-risk due to having an older sibling diagnosed with ASD) (Elsabbagh et al., 2009; Zwaigenbaum et al., 2005), and may be one of the earliest characteristics that distinguish infants who later receive an ASD diagnosis (Zwaigenbaum et al., 2005). Further, non-social attentional strengths (e.g., visual search) and weaknesses (e.g., novelty detection) in ASD and their neurofunctional correlates have also been associated with increased autism symptomatology (Belmonte, Gomot, & Baron-Cohen, 2010; Gomot, Belmonte, Bullmore, Bernard, & Baron-Cohen, 2008; Joseph, Keehn, Connolly, Wolfe, & Horowitz, 2009; Keehn & Joseph, 2008; Keehn, Lincoln, Muller, & Townsend, 2010). In addition, findings from single gene disorders that have increased rates of ASD (e.g., fragile X syndrome) suggest an association between ASD symptomatology and attentional dysfunction (Cornish et al., 2012; Roberts et al., 2012; Scerif et al., 2011). Together, these findings suggest that attentional processes may impact the development of higher-level sociocommunicative functions. Consequently, understanding the development of attentional mechanisms in children with ASD may help elucidate the abnormal or delayed trajectories of attentional development in ASD, and furthermore, how these attentional abnormalities may contribute to the manifestation of the core ASD impairments.
The primary aim of this paper is to provide a review of attention research in ASD within the framework of neurotypical attentional networks. The first goal is to review the network-specific findings for investigations of attention in ASD after supplying a brief background of the brain regions and neurotypical development associated with each attention network. The second goal of this paper is to put forth a developmental framework regarding how aberrant attentional mechanisms may function as a primary dysfunction with regard to the development of core ASD symptomatology. Specifically, we posit that early deficits in disengaging attention result in cascade of impairments and ultimately contribute to the emergence of the ASD phenotype. Theories of autism have postulated primary impairments in both social and non-social functions (see Happé, 2001, for more in-depth discussion). Although the focus of this section is the role of attention in the development of ASD, this is not meant to imply that attention networks are the only dysfunctional neural networks in ASD. As was aptly put by Goodman, “the very diversity of existing ‘unitary’ psychological and neurological explanations casts doubt on the hypothesis that infantile autism can potentially be explained by a fault in just one psychological or neurological system” (Goodman, 1989, p. 410). Rather, the goals of understanding whether dysfunctional attentional processes are of etiological significance in ASD is two-fold. If early attentional impairments play a causal role in the development of ASD, then 1) attentional deficits may be used as an early neuro-behavioral marker that can be used to identify infants at-risk for ASD and 2) the development of attention-targeted early interventions that may remediate abnormal developmental trajectories and improve outcomes in children with ASD.
2. Attention Networks
2.1 Alerting Network
The alerting network is responsible for achieving and maintaining a state of sensitivity to incoming information. Alertness has been divided into tonic and phasic components (see Sturm & Willmes, 2001, for review). Tonic alertness is a state of general wakefulness or arousal; endogenously-controlled tonic alertness, referred to as vigilance or sustained attention, is the voluntary maintenance of alertness at a certain level. Phasic alertness is a more transient alert state, commonly modified by a behavioral or experimental cue. These components of alertness parallel the tonic and phasic attributes of Sokolov’s orienting response (OR) theory (Sokolov, 1963; note that orienting here does not refer to spatial orienting, as discussed in section 2.2, but rather to a reflexive physiological reaction to a change in the environment). The alerting network is an interacting system of internal state-dependent attentional mechanisms that can increase or decrease information processing capacity (Kahneman, 1973) and influence the breadth of selective attention (Easterbrook, 1959). The efficiency and degree of phasic alerting is modulated by the level of tonic alertness, while goal-directed changes in levels of tonic alertness affect task performance. Thus, multiple factors are involved in typical and pathological alerting responses.
An additional process related to alerting is novelty detection. Novelty detection is a fundamental characteristic of Sokolov’s OR, and is dependent on a mismatch between an individual’s pre-existing representation and a novel stimulus; a phasic OR does not take place without the perception of a novel stimulus. Novelty detection is therefore an important prerequisite for an OR, which subsequently enables an individual to encode and process novel information.
2.1.1 Neuroanatomy of the alerting network
The neuroanatomy of alerting includes a network of subcortical and cortical regions (see Figure 1). The nuclei of the reticular formation in the brain stem, specifically the locus coeruleus-norepinephrine (LC-NE) system, is the core arousal center (see Robbins & Everitt, 1995, for review). The LC projects to the thalamic nuclei and terminates in cerebral cortex (Foote, Bloom, & Aston-Jones, 1983). In general, the LC-NE system supports appropriate levels of alertness in order to maintain efficient information processing. Norepinephrine, the neuromodulator associated with the alerting network, appears to inhibit spontaneous neural activity, permitting increased neural response to sensory stimulation (Foote, Freedman, & Oliver, 1975). The anterior cingulate cortex (ACC) and the right dorsolateral prefrontal frontal cortex (DLPFC) function to maintain endogenous alertness by modulating activity in the LC via the reticular nucleus of the thalamus (Sturm et al., 1999). Lastly, the alerting network is mediated by a right-lateralized ventral frontoparietal network, which is responsible for achieving and maintaining appropriate levels of alertness (Corbetta, Patel, & Shulman, 2008; Posner & Petersen, 1990).
Figure 1.
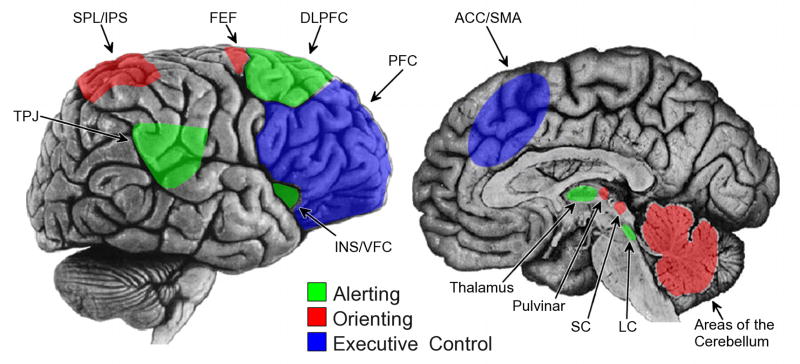
Illustration of cortical and subcortical regions involved in each attentional network. The alerting network (green) is comprised of right lateralized ventral frontoparietal cortical regions, including the temporal-parietal junction (TPJ), dorsolateral prefrontal cortex (DLPFC), and the insula/ventral frontal cortex (INS/VFC), as well as the thalamus and locus coeruleus (LC). The orienting network (red) includes bilateral dorsal frontoparietal areas (superior partial lobe/intraparietal sulci, SPL/IPS; frontal eye fields, FEF) an as well as the superior colliculus (SC), pulvinar, and areas of the cerebellum. The executive control network includes rostral brain locations, including prefrontal cortex (PFC) and anterior cingulate gyrus/supplementary motor area (ACC/SMA).
2.1.2 Development of the alerting network
The development of tonic alertness and the waking state – e.g.,. increased periods with open eyes, toy manipulation, and babbling – in infants changes rapidly between 2 and 24 weeks (Dittrichova & Lapackova, 1964). Early infant attention functions to achieve an optimal state of arousal and is dependent on the amount of external stimulation and on the level of internal arousal (Karmel, Gardner, & Magnano, 1991). The autonomic nervous system and its physiological indices undergo considerable development across the lifespan (see Shields, 1983, for review). Stimulus orienting (i.e., a phasic OR), as measured by a deceleration of heart rate (HR), increases rapidly during the first year of life with no significant developmental changes occurring in infants between 14 and 26 weeks of age (see Reynolds & Richards, 2008, for review). However, sustained attention (i.e., the voluntary maintenance of alertness) undergoes a more protracted developmental time course, with the ability to sustain attention increasing rapidly from 2 to 6 months of age (Richards, 1995).
The development of phasic alertness has also been investigated using event-related potentials (ERP). Two examples include the Nc (negative central; Courchesne, 1977, 1978) and the P3 (see Polich, 2007, for review) components. The Nc component occurs between 350–800ms post-stimulus onset at central electrode sites. This component has been hypothesized to represent an OR that is insensitive to novelty and stimulus probability (Nelson & Collins, 1992; Richards, 2003), although stimulus novelty (Reynolds & Richards, 2005) and/or meaningfulness (de Haan & Nelson, 1999) may play a role in larger Nc responses. The Nc component appears in the first year of life during attentive states, increasing in amplitude while decreasing in latency with development (Richards, 2003). The amplitude of the Nc component decreases significantly from early childhood to early and late adolescence as the electrophysiological response transitions to the more mature P3 waveform (Courchesne, 1978).
The modality-independent P3 response is generally elicited using an odd-ball paradigm (analogous to a continuous performance task; see Figure 2C), which requires a manual response to a prespecified target displayed infrequently amongst standard stimuli (some odd-ball paradigms also include a third novel non-target stimuli). Briefly, the P3 component consists of two subcomponents: 1) a frontocentral P3a (or “novelty P3”; Friedman, Cycowicz, & Gaeta, 2001) component that habituates rapidly and is associated with the redirection of attention monitoring during stimulus discrimination, and 2) a parietal P3b component that reflects a memory comparison and facilitates context maintenance. Previous research has demonstrated that these components reflect activity of separate neural generators and neurotransmitter systems (Polich, 2007) and involve regions such as the temporal-parietal junction, lateral prefrontal cortex, and the locus coeruleus (see Nieuwenhuis, Aston-Jones, & Cohen, 2005, for discussion). Developmental changes of the P3 component from early child- to adulthood include decreased latency (Courchesne, 1978; Cycowicz, Friedman, & Rothstein, 1996; Zenker & Barajas, 1999) and changes in scalp distribution (Courchesne, 1978; Cycowicz et al., 1996). Behaviorally, phasic alerting mechanisms continue to develop until age 8 years, at which point they may have matured to adult levels (Morrison, 1982). These findings are in accord with conclusions from a cross-sectional study of P3a development (Cycowicz et al., 1996), which suggests that despite changes in latency and scalp topography, by age 7–8 years phasic orienting may be functioning at adult levels.
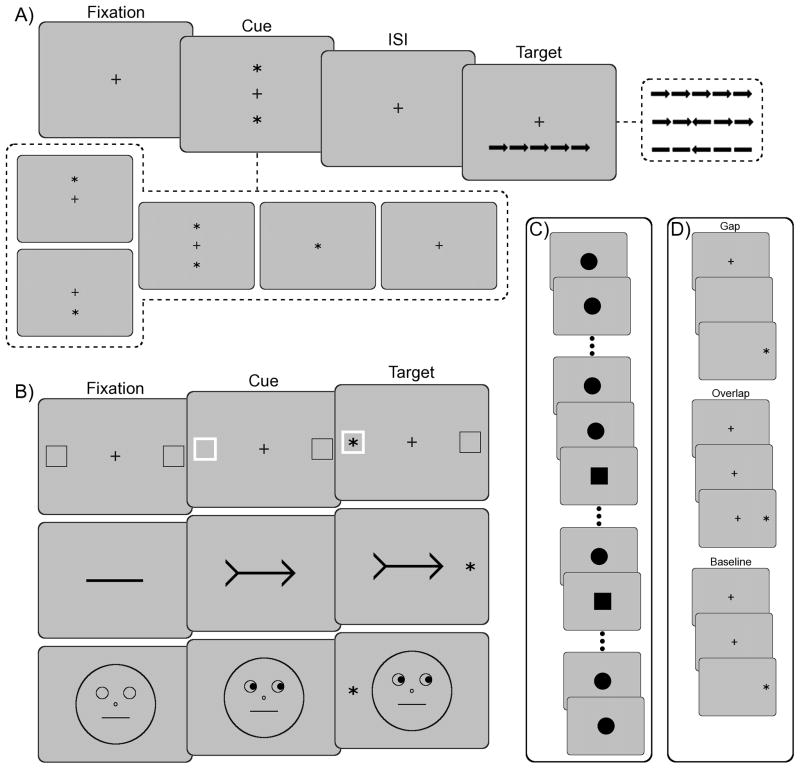
Figure 2.
Paradigms used to study discrete attentional processes: (A) The attention network test (ANT) begins with a fixation cross displayed for a variable duration, then a cue (spatial, double, central, or no cue) is presented, followed by an inter-stimulus interval, and a target (congruent, incongruent, or neutral). The task is to indicate whether the central arrow points left or right. (B) Variations of the Posner cuing paradigm including valid flash (exogenous), valid arrow (endogenous), and invalid gaze (endogenous). (C) A continuous performance test (CPT) or oddball paradigm where a target (square) is presented randomly in a series of standards (circles). (D) A gap-overlap task where a target can occur after fixation offset (gap), with the fixation remaining on screen (overlap), or with the simultaneous offset of the fixation (baseline).
The development of sustained attention across childhood to adolescence has often been measured using Continuous Performance Tests (CPT; see Figure 2C), in which participants are asked to respond to a pre-defined target stimulus presented randomly within a stream of non-target stimuli. Sustained attention abilities, as measured by the CPT, increase significantly from 3 to 6 years of age (Akshoomoff, 2002; Kerns & Rondeau, 1998; Levy, 1980) and continue to develop into late childhood and adolescence, reaching adult-like levels around the age of 12 years (Lin, Hsiao, & Chen, 1999).
The Attention Network Test (ANT), developed by Fan and colleagues (2002), examines the efficiency of each attentional network (see Figure 2A). The ANT consists of both a cued reaction time task (Posner, 1980) and a flanker paradigm (Eriksen & Eriksen, 1974). Alerting, orienting, and executive control scores are calculated by series of cognitive subtractions. The alerting score is calculated by subtracting response times in conditions that maximize and minimize alerting (i.e., double cue vs. no cue). The alerting score which reflects both phasic and tonic components of alertness (Posner, 2008), increases between the ages of four and seven years (Mezzacappa, 2004), but remains similar between the ages of six to ten years (Rueda et al., 2004). However, the process continues to mature as 10-year-old children show significantly higher alerting scores than adults (Rueda et al., 2004). Developmental decreases in alerting scores are likely due to changes in the level of tonic alertness; younger children have difficulty maintaining appropriate levels of tonic alertness. Additionally, the network mediating both phasic and tonic levels of alertness likely continue to develop into adulthood (Konrad et al., 2005).
2.1.3 Alerting network in ASD
Levels of tonic arousal and the modulation of phasic alertness have been areas of intense speculation but modest empirical consensus in ASD-related research (see Bryson, Wainwright-Sharp, & Smith, 1990; Rogers & Ozonoff, 2005, for reviews). Previous authors have argued for hyperarousal (Hutt, Hutt, Lee, & Ounsted, 1964), hypoarousal (Rimland, 1964), and dysfunctional arousal modulation (Ornitz & Ritvo, 1976). Others have proposed that “nonresponsiveness” (i.e. hypoarousal) may develop as a result of sensory overload due to early chronic hyperarousal (van Engeland, 1984). Liss and colleagues (2006) have hypothesized that hyperarousal in ASD may lead to the development of overselective attention, and, similar to van Engeland (1984), that this may result in reduced or absent OR to stimuli may be outside an atypically narrower focus of attention. Alternatively, the varying hypotheses and inconsistent findings of hypo- and hyper-arousal may reflect heterogeneity within the ASD population, with subtypes exhibiting different arousal states (Hirstein, Iversen, & Ramachandran, 2001; Schoen, Miller, Brett-Green, & Hepburn, 2008).
Evidence for hyperarousal in ASD comes from investigations employing multiple modalities. Studies examining skin conductance have demonstrated increased skin conductance levels (SCL) and frequency of skin conductance responses (SCR) (Palkovitz & Wiesenfeld, 1980). Heart rate measures have shown decreased HR deceleration (or a relative acceleration) (Palkovitz & Wiesenfeld, 1980) and increased baseline HR (Ming, Julu, Brimacombe, Connor, & Daniels, 2005) in ASD as compared to TD individuals. More recently, evidence of larger tonic pupil size in children with ASD has also been found (Anderson & Colombo, 2009). Furthermore, children with ASD show atypical HR response between rest and task states (Althaus, Mulder, Mulder, Aarnoudse, & Minderaa, 1999; Toichi & Kamio, 2003), indicative of dysfunctional modulation of arousal.
Contrary to evidence of hyperarousal in ASD, equivalent or reduced levels of arousal have also been shown. Prior studies have reported no difference between low-functioning children and adolescents with ASD and chronological age (CA) and mental age (MA) matched TD individuals in electrodermal response to an auditory habituation paradigm (Stevens & Gruzelier, 1984). Normal SCL, spontaneous SCR, and HR have also been shown in high-functioning adults with ASD during rest (Zahn, Rumsey, & Van Kammen, 1987); however, Zahn and colleagues (1987) also report reduced reactivity for task versus baseline and decreased phasic SCR activity to imperative stimulus in a simple response time (RT) task, which may also be indicative of abnormal task-related modulation of arousal.
A previous study has also demonstrated no difference in spontaneous fluctuations in skin conductance to auditory stimuli between ASD and TD and developmentally delayed (DD) children (van Engeland, 1984). Interestingly, van Engeland (1984) interpreted the findings in a high-functioning subgroup to represent a “paradoxical reaction,” in which participants were atypically open to environmental stimuli, and, thus, potentially overwhelmed with sensory information. The results of the atypically increased openness to sensory information may lead to previously discussed hyperarousal states, and, subsequently, to the development of sensory non-responsiveness to novel stimuli. In support of this hypothesis, van England and colleagues (1991) demonstrated significantly reduced SCR and fixation times to novel visual stimuli in high-functioning children with ASD.
Although behavioral evidence of equivalent phasic alerting in TD and ASD children exists (Raymaekers, van der Meere, & Roeyers, 2006), electrophysiological measures have demonstrated atypical phasic alerting in ASD. The N1c component, which reflects automatic attentional capture resulting from an auditory stimulus, is reduced in amplitude in ASD as compared to TD children (Bruneau, Bonnet-Brilhault, Gomot, Adrien, & Barthelemy, 2003; Orekhova et al., 2009). Reduced (Courchesne et al., 1985) or absent (Ciesielski, Courchesne, & Elmasian, 1990) Nc response has also been shown in adolescents and adults with ASD. More recently, McCleery and colleagues (2009) reported reduced Nc amplitudes in 10-month-old infants at-risk for ASD compared to TD infants during passive viewing of faces and objects. These findings suggest the modulation of phasic alerting mechanisms may be dysfunctional in ASD.
In accord with these findings on phasic alertness, individuals with ASD also display robust insensitivity to novel information. Behaviorally, this has been shown in two separate visual search studies (Greenaway & Plaisted, 2005; Keehn & Joseph, 2008). Multiple studies have shown reduced P3 amplitude in response to novel auditory (Ciesielski et al., 1990; Courchesne, Kilman, Galambos, & Lincoln, 1984; Courchesne, Lincoln, Kilman, & Galambos, 1985; Courchesne, Lincoln, Yeung-Courchesne, Elmasian, & Grillon, 1989; Dawson, Finley, Phillips, Galpert, & Lewy, 1988; Lincoln, Courchesne, Harms, & Allen, 1993; Novick, Kurtzberg, & Vaughn, 1979; Novick, Vaughan, Kurtzberg, & Simson, 1980) and visual targets (Ciesielski et al., 1990; Courchesne et al., 1989; Pritchard, Raz, & August, 1987; Townsend et al., 2001) in ASD. More specifically, Courchesne et al. (1984; 1985) reported decreased P3a amplitudes to novel auditory stimuli, as well as reduced P3b to target auditory compared to standard stimuli in ASD as compared to TD adolescents. Townsend and colleagues (2001) reported abnormalities in both P3a and P3b in adults with ASD to peripheral visual targets.
In fMRI studies, Gomot and colleagues (2008; 2006) examined passive and active detection of auditory oddball stimuli. Passive detection of novel auditory stimuli resulted in greater activation in bilateral temporal parietal junction (TPJ) and right inferior and middle frontal regions in TD compared to ASD participants. In contrast, active novelty detection task (requiring behavioral response) resulted in significantly greater activation in the right prefrontal cortex and left inferior parietal lobule in the ASD compared to the TD group. Thus, atypical response to novel stimuli may be dependent on the context of the experiment. Reduced ASD activation for passive novelty detection may result from a predisposition to ignore novel information. However, the authors suggest that increased ASD activation to active novelty detection may reflect over-focusing in the ASD group, which could result in maladaptive allocation of attention. This is in accord with electrophysiological findings demonstrating that the allocation of attention (in an active task) results in enhanced responses (Kemner et al., 1994) or – in the case where passive tasks have shown reduced amplitudes – equivalent responses (Dunn et al., 2008; Whitehouse and Bishop, 2008) in individuals with ASD.
Keehn and colleagues (2010) recently employed the ANT to investigate attentional networks in ASD. The authors demonstrated similar alerting scores in children and adolescents with ASD and TD children. Interestingly, whereas there were no correlations between network scores for TD children (as reported previously; see Rueda et al., 2004), children with ASD exhibited significant correlations between alerting and executive control networks suggesting that these two networks may not function as independently in children with ASD. Similarly, Raymaeker and colleagues (2004) demonstrated that increased arousal was related to poorer response inhibition performance in ASD but not TD individuals. These findings suggest that alerting and executive networks may interact to a greater degree in individuals with ASD, and as a result difficulty controlling the level of arousal may produce impaired executive control abilities (discussed in greater detail in Section 3.1).
In contrast to studies of phasic alertness and novelty processing, previous studies have demonstrated equivalent sustained attention abilities in ASD and TD individuals (Garretson, Fein, & Waterhouse, 1990; K. A. Johnson et al., 2007; Noterdaeme, Amorosa, Mildenberger, Sitter, & Minow, 2001; Pascualvaca, Fantie, Papageorgiou, & Mirsky, 1998). These findings suggest that endogenous maintenance of tonic alertness may be intact in individuals with ASD.
2.1.4 Summary
The arousal-state and phasic alerting mechanisms develop rapidly during the first year of life. The efficiency and speed of phasic alerting may continue to develop into the early school-age years (Morrison, 1982), while endogenous maintenance of alertness has a more protracted course of development not reaching adult-like levels until early adolescence (Lin et al., 1999).
Inconsistent results have been observed in studies investigating tonic levels and phasic responsiveness of the alerting network in ASD, and limit unequivocal conclusions regarding dysfunction within this attentional network. The alerting network reflects a complicated interaction between the internal state of the individual, their responsiveness to external stimuli, and their task- or goal-related endogenous modulation of alertness. Therefore, elucidating underlying alerting abnormalities in ASD has proven difficult. Further complicating the study of the alerting network is the fact there may be separate subgroups of hyper- and hypo-aroused individuals with ASD.
There is evidence for increased plasma-levels of norepinephrine (Lam, Aman, & Arnold, 2006), increased pupil diameter, aberrant skin conductance responses and levels, atypical heart rate, abnormal electrophysiological alerting response (Nc, N1c) and novelty detection (P3), and atypical neurofunctional activation of the alerting network in persons with ASD. Although some studies report unimpaired phasic and endogenous tonic alertness in ASD, the evidence reviewed here suggests that under some conditions or in some individuals, there are impairments of tonic (arousal) and phasic components of the alerting network in ASD.
Importantly, divergent results on passive and active tasks may reflect atypical control of alertness. Specifically, increased activation in ASD (relative to TD peers) in right frontoparietal regions has been interpreted as over-focusing (Gomot et al., 2008), while atypically decreased activation to novel information in ASD during passive tasks may be indicative of a default processing state that rejects or ignores novelty (Gomot et al., 2006). Likewise, evidence from studies of HR variability suggests that between-group differences arise when examining changes in autonomic state from rest to task conditions (Althaus et al., 1999; Toichi & Kamio, 2003). Thus, alerting abnormalities in ASD cannot be characterized simply by atypical tonic levels of arousal, but may also include the impairments in regulating levels of alertness across different situations and differences in active and passive tasks.
2.2 Orienting Network
The orienting network is responsible for the selection of information from sensory input. Posner and colleagues (1984) have defined visuospatial orienting as disengaging, shifting, and reengaging attention. In contrast to the phasic alerting mechanisms that respond homogeneously across the visual field, orienting visual attention facilitates processing over a localized area (e.g., Mangun & Hillyard, 1991). Although anatomically distinct and functionally independent, alerting and orienting mechanisms do interact (Callejas, Lupianez, Funes, & Tudela, 2005; Callejas, Lupianez, & Tudela, 2004; Fan et al., 2009; Fuentes & Campoy, 2008). For example, non-spatial cues (that result in increased phasic alertness) can facilitate attention orienting (Callejas et al., 2004; 2005), while disengaging and re-orienting attention may serve to attenuate arousal levels (Derryberry & Rothbart, 1988). Thus, alerting (both phasic and tonic components) may be bi-directionally related to orienting abilities.
Orienting visual attention can occur overtly, with concurrent head/eye-movements, or covertly, without corresponding head/eye-movements. Attention may also be directed reflexively (automatically) or voluntarily to a spatial location based on central (endogenous) or peripheral (exogenous) cues. In commonly used spatial attention testing paradigms, reflexive orienting occurs to both peripheral and central (arrows, gaze) non-predictive (i.e., 50% trials validly cued) cues. Predictive (e.g. 80% of trials validly cued) peripheral and central (arrows, gaze) cues engage voluntary as well as reflexive orienting mechanisms, which interact in a super-additive manner (Olk, Cameron, & Kingstone, 2008).
2.2.1 Neuroanatomy of the orienting network
The network of areas responsible for directing attention include superior parietal lobe, intraparietal sulcus, temporal-parietal junction and dorsofrontal (frontal eye fields; FEF) cortices, thalamus, and superior colliculus (Corbetta & Shulman, 2002; Mesulam, 1990; Posner & Petersen, 1990). Furthermore, the cerebellum may also play a role in both covert and overt orienting of attention (Akshoomoff, Courchesne, & Townsend, 1997; Pelisson, Goffart, Guillaume, & Quinet, 2003). Evidence from individuals with cortical and subcortical lesions suggests that reflexive orienting is likely mediated by subcortical and more posterior cortical regions, while a network of frontal-parietal regions underlie voluntary orienting (Rafal, 1998). Acetylcholine (ACh) is the neuromodulator associated with the orienting network, with increased levels of ACh resulting in more rapid attention reorienting (Thiel, Zilles, & Fink, 2005; Witte, Davidson, & Marrocco, 1997). Specifically, ACh may act to expand the attentional spotlight so that attentional misdirection does not elicit a reorienting response (Thiel et al., 2005).
2.2.2 Development of the orienting network
Johnson (1990) proposed a model for the development of overt attention in infants based on the maturation of subcortico-cortical and cortico-cortical pathways as outlined by Schiller (1985, 1998). Briefly, this model proposes that in newborns a subcortical pathway mediates early reflexive shifts of attention; the development of an inhibitory pathway, which is responsible for suppressing visuospatial orienting, leads to “obligatory looking” (difficulty disengaging visual attention) around 1 month. Maturation of cortical layer 4 at two months and subsequently layers 2 and 3 of primary visual cortex at three months allows for development of pathways responsible for smooth pursuit and anticipatory eye-movements, respectively.
Overt orienting to exogenous cues is limited in 2-month-old infants; however, clear orienting including facilitation and inhibition by peripheral cues appears by 4 months of age, with speed of spatial attention shifting increasing from 4 to 7 months (M. H. Johnson & Tucker, 1996). Similarly, the development of covert attention appears to be present by 4 months of age (M. H. Johnson, Posner, & Rothbart, 1994). By the age of 3 months, infants are also sensitive to direction of gaze and direct their attention towards gaze-cued locations (Hood, Willen, & Driver, 1998). However, if the gaze-cue remained onscreen (as opposed to being removed prior to the appearance of the target) 3-month-olds did not orient attention to the gaze-cued location as frequently, perhaps due to difficulties disengaging visual attention (Hood et al., 1998).
Following the period of reduced disengagement efficiency at around 1 month (i.e., obligatory looking), an infant’s ability to disengage attention increases rapidly. Results from investigations employing the gap-overlap eye-movement paradigm have demonstrated that 4-month-old infants exhibit significant improvements in disengaging their attention during an overlap (competing information) condition relative to 3-month-old infants (Frick, Colombo, & Saxon, 1999). In 2-, 4-, and 6-month-old infants, saccadic RT for overlap trials decreased significantly at each age group (6 < 4 < 2 months) (McConnell & Bryson, 2005).
The development of orienting abilities during early childhood has been examined using the ANT. Orienting efficiency increases between the ages of 4 to 7 years (Mezzacappa, 2004); however, no change in orienting score was exhibited from six years of age to adulthood (Rueda et al., 2004). This finding may be task-dependent as the ANT did not include invalid trials, thus reducing the amount of attentional disengagement necessary to complete the task. An fMRI study using a modified ANT (which included invalid trials), demonstrated that children exhibited reduced activity in the right TPJ during attentional reorienting and exhibited increased activation relative to adults in superior frontal gyrus and insula (Konrad et al., 2005)..
During the school-age period and adolescence, the ability to disengage (Wainwright & Bryson, 2002) and shift (Schul, Townsend, & Stiles, 2003) attention to predictive exogenous cues continue to develop, perhaps reaching adult-like levels at approximately 10 years (Wainwright & Bryson, 2002; although see Schul et al., 2003, for evidence of age-related changes after 10 years) as the speed of attentional movements continues to increase during this period (Pearson & Lane, 1990). This is consistent with research using endogenous cuing paradigms, which suggests that the development of orienting may reach adult-like levels by 8 to 9 years of age (Goldberg, Maurer, & Lewis, 2001).
2.2.3 The orienting network in ASD
Deficits in orienting visual attention have been consistently observed in individuals with ASD. Evidence in support of early orienting deficits comes from observational and retrospective video analysis. Swettenham and colleagues (1998) measured spontaneous shifts of attention while 20-month-old ASD, TD, and DD infants participated in a five-minute free play session. In general, infants with ASD showed less attention shifting than did the two comparison groups. Studies using retrospective home video analysis have also reported that ASD infants orient to visual stimuli less (Baranek, 1999), do not orient towards people or human voices as frequently as TD infants (Maestro et al., 2002), and orient to name significantly less as compared to TD and DD infants (Osterling & Dawson, 1994; Osterling et al., 2002).
Dawson and colleagues (1998b) examined orienting to a variety of social and non-social stimuli in 5-year-old children with ASD, children with Down syndrome, and MA-matched TD children. They found that children with ASD failed to orient to both social and non-social sounds as frequently as the comparison groups. Furthermore, those children with ASD who were able to orient attention demonstrated delayed orienting to social stimuli. In a follow-up study, Dawson and colleagues (2004) compared a larger sample of 4-year-old children with ASD to DD and MA-matched TD toddlers. Replicating previous results, children with ASD failed to orient as frequently to social, and to a lesser extent non-social, sounds.
In older children, adolescents, and adults with ASD orienting abilities have been measured using various spatial cuing paradigms (see Figure 2B). Following an attention-directing cue, the response to information at a cued location assesses visuospatial orienting and comparing responses to information at a validly cued versus an invalidly cued location provides a measure of time for attention disengagement and re-orienting. This is typically referred to as a “validity effect” (RT for invalid minus valid cue condition) and represents the behavioral cost of misdirecting attention with an invalid cue. Townsend and colleagues (1996) found slower orienting in adults with ASD compared to TD individuals. A comparison of TD, ASD, and cerebellar lesion participants (Townsend et al., 1999) replicated these findings and suggested that slower orienting was related to decreased size of cerebellar vermis VI-VII. A similar study that included children with ASD reported no difference in RT validity effect; however, similar to adults, children with ASD showed a significant correlation between the orienting speed and vermis size (Harris, Courchesne, Townsend, Carper, & Lord, 1999). A more recent study using the ANT showed that children and adolescents with ASD were slower to shift attention (Keehn et al., 2010).
Ristic et al. (2005) examined predictive and non-predictive orienting to endogenous gaze-cues adolescents with ASD. Individuals with ASD showed similar validity effects for predictive, but not for non-predictive, cues compared to TD individuals. These findings suggest that individuals with ASD do not reflexively orient attention to shifts in gaze, but can use volitional control of attention (but see Wainwright-Sharp & Bryson, 1993, for example of impaired volitional orienting). In agreement, Goldberg et al. (2008) reported that a validity effect to non-predictive endogenous gaze-cues seen in TD children was absent in children with ASD. However, equivalent orienting in ASD and TD children to non-predictive gaze cues has also been reported (Kylliainen & Hietanen, 2004; Senju, Tojo, Dairoku, & Hasegawa, 2004; Swettenham, Condie, Campbell, Milne, & Coleman, 2003), although some evidence suggests that in ASD gaze cues may be processed differently (Vlamings, Stauder, van Son, & Mottron, 2005). In accord with this view, an fMRI study of social and non-social orienting showed that while TD individuals exhibited greater activation to social versus non-social cues, individuals with ASD did not (Greene et al., 2011).
Two studies have investigated exogenous and endogenous cues in the same cohort of individuals. Renner and colleagues (2006) showed intact endogenous, but impaired exogenous orienting in children and adolescents with ASD. These results are in agreement with previous neuroimaging findings that suggest abnormal function of the network underlying reflexive orienting and relatively spared, though still atypical, network underlying more voluntary orienting in ASD (Haist, Adamo, Westerfield, Courchesne, & Townsend, 2005). However, a more recent study by Pruett et al. (2010) demonstrated similar performance on predictive and non-predictive, exogenous and endogenous paradigms in ASD and TD children.
2.2.3.1 Disengagement of attention
Attentional disengagement can also be studied by examining eye-movements. Disengagement efficiency is often determined by examining saccadic RT to targets appearing when the fixation cross remains on the screen (overlap condition) compared to when the cross disappears prior to the target onset (gap condition), i.e., the gap effect (see Figure 2D). The gap effect results from two separate mechanisms: 1) a generalized warning effect (i.e. phasic alerting) as a consequence of fixation offset, and 2) the release of ocular inhibition due to a) the disappearance of a foveal stimulus, and b) the top-down preparation of a saccadic response (Kingstone & Klein, 1993; Taylor, Kingstone, & Klein, 1998).
Landry and Bryson (2004) examined gap-overlap performance in children with ASD, CA-matched children with Downs syndrome, and MA-matched TD children, and demonstrated that the ASD group showed significantly increased latencies to disengage visual attention (on overlap trials), but similar response latencies to shift visual attention on gap trials compared to both comparison groups. Additionally, the authors report that the frequency of fast attentional shifts (i.e., the number of shifts with latency between 100 and 300ms) for the gap condition was significantly reduced in the ASD group, suggesting that in addition to difficulty disengaging attention on overlap trials, children with ASD did not efficiently shift attention to the target even when disengagement mechanisms were not competing with the central stimulus.
Impaired disengagement has also been demonstrated in low-functioning adults with ASD (Kawakubo et al., 2007) and at-risk infants (Elsabbagh et al., 2009; Zwaigenbaum et al., 2005). For at-risk infants, there was no difference between high-risk (i.e., infant with an ASD sibling) and low-risk (i.e., infant with a TD sibling) infants at six months in their ability to shift or disengage visual attention. Importantly, when re-tested at 12 months the high-risk group showed poorer performance in disengaging visual attention compared to performance at 6 months. Specifically, 25% of high-risk infants demonstrated longer latencies to disengage attention. Interestingly, every single child that exhibited increased difficulties disengaging attention between 6 and 12 months received an ASD diagnosis at 24 months (Zwaigenbaum et al., 2005).
While evidence for impaired disengagement of attention in ASD exists across the lifespan, it should be noted that some studies report typical attention disengagement in ASD (Kawakubo, Maekawa, Itoh, Hashimoto, & Iwanami, 2004; Leekam, Lopez, & Moore, 2000; Mosconi et al., 2009). However, conflicting results may in some cases be due to low statistical power and cohort effects in very small sample studies (ASD n = 7 in Kawabuko et al., 2004), or large differences in gap-overlap paradigms (e.g., Leekam et al., 2000). Additionally, although Goldberg and colleagues (2002) found no differences in disengagement or facilitation of visual attention in adolescents with ASD, individuals with ASD made fewer express saccades (short latency saccades occurring between 80 – 140 ms) to the target.
2.2.4 Summary
Subcomponents of the orienting network are established by the middle of the first year of life, but continue to develop at least into the school-age years. Orienting visual attention relies on a distributed network of brain areas, including a dorsal frontal-parietal cortical network as well as subcortical structures (thalamus, superior colliculus, and cerebellum) for shifting and disengaging attention.
In ASD, orienting deficits appear to be present within the first year of life. Retrospective analysis of home videos and prospective analysis of at-risk infant siblings have shown impairments in disengaging and shifting attention to both social and non-social auditory and visual stimuli before the first birthday in infants later diagnosed with ASD. Furthermore, investigations of children, adolescents, and adults with ASD have revealed slower, less efficient visual orienting abilities and fewer express saccades; reflexive orienting may be more impaired than volitional shifts of attention, although there is some conflicting evidence.
2.3 Executive Control Network
The executive control network is a multidimensional attentional system, responsible for inhibition, planning, error monitoring, set shifting, working memory, and cognitive flexibility. Recent studies have shown that executive control is not mediated by a unitary mechanism, but can be dissociated into at least three separate but associated functions – set shifting, working memory, and inhibition, – in TD children and adults (Huizinga, Dolan, & van der Molen, 2006; Miyake et al., 2000). Although typically referred to in the singular, the executive control “network” is undoubtedly comprised of multiple overlapping systems. Set shifting refers not to visuospatial orienting, but rather to shifting between multiple mental sets (also referred to as “task switching”; Monsell, 2003). Working memory corresponds to active monitoring, updating, and maintenance of task-relevant information. Finally, inhibition refers to an individual’s ability to prevent pre-potent or automatic responses. These three domains of executive function will be the focus of the following section; however, it should be noted that separate taxonomies of executive function exist (e.g., Anderson, 2002). Omnibus tests including the Wisconsin Card Sorting Task (WCST) and the Tower of Hanoi or the similar Tower of London have been employed to test executive control in ASD. However, because these tasks tap multiple executive functions, their explanatory significance with regard to unique executive functions is limited. For the purpose of this section, only tasks that attempt to isolate set shifting, working memory, or inhibitory executive control functions will be considered.
Two important considerations should be noted when comparing different populations on executive tasks (Roberts & Pennington, 1996). First, although tasks may be designed to isolate and test a discrete executive function, subtle lapses in a separate function may ultimately result in poorer performance. For example, inhibitory deficits in clinical populations may result from dysfunctional working memory processes (i.e., forgetting task instructions) and not abnormal inhibitory control. Second, the degree of prepotencies may differ between groups resulting in the erroneous appearance of superior/inferior inhibitory abilities (e.g., Adams and Jarrold, 2009).
2.3.1 Neuroanatomy of the executive control network
The neural substrates of executive control include regions within the prefrontal cortex – orbitofrontal, ventrolateral prefrontal, and dorsolateral prefrontal cortex (DLPFC) – as well as medial frontal regions (anterior cingulate cortex; ACC) and subcortical regions such as the basal ganglia and cerebellum (Heyder, Suchan, & Daum, 2004). Additionally, more posterior areas (mainly located in the parietal lobe) may also be important for executive processes (Collette, Hogge, Salmon, & Van der Linden, 2006; Wager, Jonides, & Reading, 2004; Wager & Smith, 2003).
More specifically, the right inferior frontal cortex may mediate inhibitory processes (Aron, Robbins, & Poldrack, 2004), while bilateral dorsal frontal regions (superior frontal sulci) are important for updating working memory and right ventral frontal regions are important for manipulating information in working memory (Wager & Smith, 2003). Finally, shifting may be mediated by bilateral medial frontal cortex (ACC), intraparietal sulci, and to a lesser degree anterior insula and DLPFC (Wager et al., 2004). Dopamine is the primary neuromodulator associated with prefrontal cortex and working memory function; however, norepinephrine also plays an important role in working memory, aside from its potential role in inhibition and set shifting (Robbins, 2000; Robbins & Arnsten, 2009).
2.3.2 Development of the executive control network
Relative to the alerting and orienting networks, the executive control network undergoes the most protracted development (see Diamond, 2002, for review), with differing developmental time courses for each executive component.
2.3.2.1 Set Shifting
The currently limited literature suggests that this executive component continues to develop between 8 and 13 years of age (Lehto, Juujarvi, Kooistra, & Pulkkinen, 2003), and does not reach adult levels until approximately 15 years of age (Huizinga et al., 2006). Surprisingly, performance on the Intradimensional/Extradimensional (ID/ED) subtest of the CANTAB appears to reach adult levels by approximately 8 years of age (De Luca et al., 2003; Luciana & Nelson, 1998). However, this early maturation may be task-dependent. Switching tasks with larger inhibitory and/or working memory demands may result in a slower, more prolonged developmental trajectory.
2.3.2.1 Working memory
The ability to hold information across a delay is present in the first year of life. Working memory functions, such as capacity and the ability to manipulate online information, develop gradually during the preschool period (see Garon, Bryson, & Smith, 2008, for review), and continue to develop between 8 and 13 years of age (Lehto et al., 2003), reaching adult-like levels after age 15 years (Huizinga et al., 2006). The verbal and non-verbal components of working memory appear to follow similar linear performance increases between 4 and 15 years of age (Gathercole, Pickering, Ambridge, & Wearing, 2004).
Results from cross-sectional studies of performance on the Spatial Working Memory CANTAB subtest are consistent with this developmental trajectory. Specifically, adult-like performance is achieved around the age of 15 years (De Luca et al., 2003; Luciana, Conklin, Hooper, & Yarger, 2005). Furthermore, results from cross-sectional studies of memory-guided saccade paradigms have revealed that the latency and accuracy of initial saccades mature by approximately 14–15 years of age (Luna, Velanova, & Geier, 2008).
2.3.2.3 Inhibition
Simple inhibitory processes come online within the first year of life and develop during preschool years, while more complex inhibitory processes appear later in preschool years and continue to develop through school age (Garon et al., 2008). By age 11 years, children perform at adult levels on the Eriksen Flanker task and Stop-Signal task (Huizinga et al., 2006). Inhibitory abilities, as measured by the ANT (identical to the Eriksen flanker task), increase between four and seven years (Mezzacappa, 2004; Rueda et al., 2004), and remain static from the age of seven into adulthood (Rueda et al., 2004). Similarly, there appears to be no relationship between age and inhibition between the ages of 8 and 13 years (Lehto et al., 2003). Yet, performance on the anti-saccade task does not reach adult levels until 14–15 years of age (Luna et al., 2008).
2.3.3 Executive control network in ASD
Executive dysfunction in ASD has been extensively reviewed (Geurts, Corbett, & Solomon, 2009; Hill, 2004a, 2004b; O’Hearn, Asato, Ordaz, & Luna, 2008; Ozonoff, South, & Provencal, 2005; Russo et al., 2007). Ozonoff and colleagues (2005) concluded that individuals with ASD demonstrate relatively intact inhibitory and working memory processes, but impaired cognitive flexibility/set shifting abilities (although see Geurts, Corbett et al., 2009, for discussion of intact cognitive flexibility). Although previously thought to be a primary deficit in ASD, absence of early executive control impairments in 3- to 4-year-old children with ASD (Dawson et al., 2002; Griffith, Pennington, Wehner, & Rogers, 1999; Yerys, Hepburn, Pennington, & Rogers, 2007) suggests that dysfunctional executive control processes may be a secondary to the development of ASD.
Two caveats should be mentioned: First, approximately 30% of children and adolescents with ASD receive a co-morbid diagnosis of ADHD (Leyfer et al., 2006; Simonoff et al., 2008), and profiles of executive impairment may differ between ASD individuals with and without ADHD (Sinzig, Morsch, Bruning, Schmidt, & Lehmkuhl, 2008; Yerys et al., 2009). Thus, differential criteria as to inclusion (or exclusion) of participants with a co-morbid diagnosis of ADHD across studies may contribute to the conflicting findings within the ASD executive control literature. Second, as is likely the case for many of the findings discussed here and above, the intellectual level (high- versus low-functioning) of the sample is an important factor as lower IQ is generally associated with reduced executive control (Keehn et al.,2010; Liss et al., 2001; Lopez, Lincoln, Ozonoff, & Lai, 2005; Steele, Minshew, Luna, & Sweeney, 2007; Williams, Goldstein, Carpenter, & Minshew, 2005).
2.3.3.1 Set Shifting
Initial evidence of impaired set shifting in ASD was established with the robust and well-replicated finding of perseverative deficits on the WCST (see Hill, 2004a; Ozonoff et al., 2005, for review of findings); however, the WCST is not a pure measure of set shifting abilities, and thus impaired performance in ASD may be due to a number of alternative factors (see Geurts, Corbett et al., 2009, for a more detailed discussion).
In addition to the WCST, investigators have begun to use the CANTAB ID/ED subtest as a measure of set shifting in ASD. For example, Hughes and colleagues (1994) found that low-functioning children with ASD performed poorly compared to DD children and MA-matched TD children. Similarly, Ozonoff et al. (2004) observed poorer performance on set shifts in a large ASD sample, ranging in age from 6 to 47 years. However, other studies investigations have failed to find set shift impairments in children and adolescents with ASD (Corbett, Constantine, Hendren, Rocke, & Ozonoff, 2009; Goldberg et al., 2005; Happé, Booth, Charlton, & Hughes, 2006; Ozonoff, South, & Miller, 2000). The absence of set shift impairments on the ID/ED subtest is surprising given the prior findings of impaired shifting on the WCST. However, similar ASD and TD performance may result from ceiling effects, given that TD children reach adult-like performance levels on the ID/ED test around age 8 years (Goldberg et al., 2005). Nevertheless, the absence of set shifting deficits in ASD in these studies suggests that children and adolescents with ASD are capable of shifting mental sets. This appears consistent with a neuroimaging study showing similar behavioral performance and brain activation for set switching in ASD and TD (Shafritz, Dichter, Baranek, & Belger, 2008).
Shifting attention has also been examined with the cross-modal shifting paradigm. Courchesne and colleagues (1994) found impaired attention shifting between auditory and visual modalities in adults with ASD, similar to patients with cerebellar lesions. These deficits reflect slowed rather than absent shifts as both groups were able to shift attention when given more time (>2.5s). Electrophysiological results from the same paradigm suggest poor performance by individuals with ASD may result from aberrant distribution of attentional resources (as indexed by the slow negative wave) (Ciesielski, Knight, Prince, Harris, & Handmaker, 1995).
2.3.3.2 Working Memory
The results of studies examining working memory in ASD are inconsistent, possibly due to the varying degree of capacity, maintenance, and manipulation demands of each task. Prior studies using the A-not-B task have demonstrated intact working memory in 3 year-old children with ASD compared to MA-matched TD and DD children (Dawson et al., 2002; Griffith et al., 1999; Yerys et al., 2007). However, by the age of 5, separate studies have shown that children with ASD perform worse compared to TD comparison children on tasks requiring working memory processes (Dawson, Meltzoff, Osterling, & Rinaldi, 1998a; McEvoy, Rogers, & Pennington, 1993).
Bennetto and colleagues (1996) report impairments on verbal working memory tasks for adolescents with ASD compared to TD individuals. However, poorer performance on these tasks, sentence and counting spans, may be due to the use of a dual-task rather working memory impairment per se. This is supported by Garcia-Villamisar and Della Sala (2002) who found performance to be normal for a single-task working memory condition, but impaired for dual-task working memory in adults with ASD.
Russel and colleagues (1996) reported no difference in working memory between low-functioning children with ASD and DD children. Similarly, Ozonoff and Strayer (2001) tested children on N-back, spatial memory-span, and box search tasks and found no working memory impairment on any task for ASD relative to Tourette syndrome and TD comparison groups.
Williams and colleagues (2005) reported equivalent performance of verbal working memory (N-back, Letter-Number Sequencing), but impaired spatial working memory performance (Spatial Span, Finger-Windows subtests) in children and adults with ASD. More recently, impaired spatial working memory performance in children and adults with ASD has been reported using the CANTAB Spatial Working Memory subtest (Corbett et al., 2009; Goldberg et al., 2005; Happé et al., 2006; Steele et al., 2007). However, Happé et al. (2006) reported impairments in children, but not adults, with ASD compared to TD individuals.
Studies employing memory-guided saccades tasks have reported deficits in saccade accuracy in children and adults with ASD (Luna, Doll, Hegedus, Minshew, & Sweeney, 2007; Minshew, Luna, & Sweeney, 1999) and slower saccade latency but similar accuracy (Goldberg et al., 2002), compared to TD individuals. Additionally, adults with ASD showed reduced activation of DLPFC during a memory-guided saccade task compared to TD adults (Luna et al., 2002).
At first glance, the results of the working memory studies reviewed appear inconsistent. Yet, close inspection suggests that the working memory deficits may be due to load (Garcia-Villamisar & Della Sala, 2002) and/or poor or inefficient use of strategies (Corbett et al., 2009; Steele et al., 2007). Neuroimaging evidence is in accord with these hypotheses, indicating that decreased functional connectivity between regions may, in part, play a role in poorer ASD performance during more complex or dual-task conditions (Kana et al., 2007). In addition, atypical activity during working memory performance suggests that individuals with ASD may rely on less efficient strategies, which may result in impaired performance during more difficult working memory tasks (Koshino et al., 2005).
2.3.3.3 Inhibition
Inhibitory processes seem to function similarly in young children with ASD and in TD children (Dawson et al., 2002; Griffith et al., 1999; Yerys et al., 2007). However, typical developmental improvement in inhibitory abilities may be reduced in ASD (Luna et al., 2007; Ozonoff & McEvoy, 1994; Solomon, Ozonoff, Cummings, & Carter, 2008; although see Happé et al., 2006, for evidence on developmental improvement)
On tasks that tend to isolate inhibitory processing from other executive functions, individuals with ASD have shown typical levels of performance (e.g. Ozonoff & Strayer, 1997); although when measures of inhibition are paired with other executive components (e.g. set switching), deficits in inhibitory abilities may be observed in ASD (e.g. Ozonoff, Strayer, McMahon, & Filloux, 1994). Similarly, individuals with ASD tend to have more difficulty in inhibition tasks that require more cognitive resources (Rinehart, Bradshaw, Tonge, Brereton, & Bellgrove, 2002)
The majority of previous studies have demonstrated intact inhibitory abilities in ASD for Go-NoGo (Geurts, Begeer, & Stockmann, 2009; Happé et al., 2006; Kana, Keller, Minshew, & Just, 2007; Ozonoff et al., 1994; Raymaekers, Antrop, van der Meere, Wiersema, & Roeyers, 2007; Raymaekers et al., 2004, 2006), Eriksen flanker (Henderson et al., 2006; Keehn et al., 2010), Start-Signal (Ozonoff & Strayer, 1997), Negative Priming (Brian, Tipper, Weaver, & Bryson, 2003; Ozonoff & Strayer, 1997), and Stroop (Adams & Jarrold, 2009; Ambery, Russell, Perry, Morris, & Murphy, 2006; Bryson, 1983; Christ, Holt, White, & Green, 2007; Eskes, Bryson, & McCormick, 1990; Goldberg et al., 2005; Ozonoff & Jensen, 1999; Russell, Jarrold, & Hood, 1999) paradigms. However, results from anti-saccade tasks have consistently shown inhibitory impairment in children and adults with ASD (Goldberg et al., 2002; Luna et al., 2007; Minshew et al., 1999; Mosconi et al., 2009; Thakkar et al., 2008); however, performance on the anti-saccade task reaches adult-like levels at much later age compared to other inhibitory tasks. This indicates that the prepotency to saccade towards a peripheral target may be one of the more difficult actions to inhibit, and therefore requires an extended period in order to mature.
2.3.4 Summary
Executive control is not a unitary construct, but instead consists of at least three independent, but associated components: set shifting, working memory, and inhibition. These components are mediated primarily by prefrontal cortex and undergo more protracted development compared to the other attentional networks. Moreover, each executive component follows a distinct developmental trajectory, reaching adults levels at varying times between early and late adolescence.
Although previously thought to be a primary deficit in ASD, the absence of early executive control deficits in preschool-aged children with ASD (Dawson et al., 2002; Griffith et al., 1999; Yerys et al., 2007) suggests that executive control deficits may be secondary to the development of ASD. The degree to which executive control abilities improve with development remains unclear. The trajectory of development appears similar in ASD and TD individuals (Luna et al., 2007); however, there are conflicting reports of age-related improvement (Happé et al., 2006) or decline (Solomon et al., 2008), which may be related to the executive component tested (Ozonoff et al., 2004).
Coordinated communication within the distributed network of brain regions responsible for these processes results in efficient and effective modulation of executive control. Functional neuroimaging studies have reported reduced functional connectivity between regions associated with the executive control network (Just, Cherkassky, Keller, Kana, & Minshew, 2007; Kana et al., 2007; Koshino et al., 2005), although these reductions in functional connectivity may be age-dependent (Lee et al., 2009). Just and colleagues (2007) have hypothesized that executive dysfunction in ASD may result from poor coordination between brain regions necessary to complete complex executive tasks. This hypothesis is supported by behavioral results that demonstrate poorer performance on tasks that necessitate multiple executive components (e.g. WCST; see Hill, 2004a. for review).
3. The Role of Attention in the Development of ASD: A Developmental Framework
Attention has often been considered an associated or secondary deficit within the domain of ASD research; instead, the findings laid out above overwhelmingly indicate early and lifelong impairments and abnormalities in efficiently modulating each attentional network. When viewed from a developmental perspective, the relationship between atypical attentional processes and social and communicative deficits may merge into a coherent model. The following section outlines a developmental framework for understanding the distinct pattern of attentional strengths and weaknesses in ASD, and how these may be related to the emergence of ASD phenotype (Figure 3). Although, much of the following framework is based on inference rather than direct evidence, the model is consistent with the relevant evidence. While ASD is a developmental disorder, very little longitudinal research is available. Therefore many of the links proposed in the current model are suggested, based in part on TD literature, and have yet to be tested in infants and children with ASD.
Figure 3.
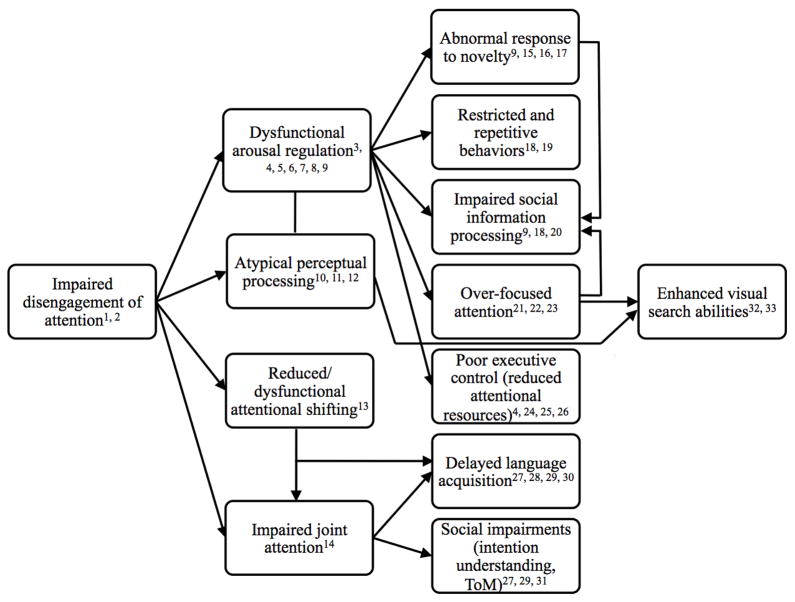
Outline of developmental framework. Bold citations represent longitudinal or correlational findings supporting link or association with ASD symptomatology; Italicized citations represent previous theories that have hypothesized link; Underlined citations represent finding from typically developing literature supporting link. 1Elsabbagh et al., 2009; 2Zwaigenbaum et al., 2005; 3Anderson & Colombo, 2009; 4Keehn et al., 2010; 5Field, 1981; 6Harman et al., 1997; 7Johnson et al., 1991; 8McConnell & Bryson, 2005; 9Dawson & Lewy, 1989a; 10Colombo et al., 1995; 11Gardner et al., 1993; 12Colombo, 1995; 13Courchesne et al., 1994; 14Schietecatte et al., 2011; 15Gomot et al., 2008; 16Keehn & Joseph, 2008; 17Gold & Gold, 1979; 18Garon et al., 2009; 19Hutt et al., 1964; 20Pierce et al., 1997; 21Liss et al., 2006; 22Britton & Delay, 1989; 23Tracy et al., 2000; 24Ciesielski et al., 1995; 25Geurts et al., 2009; 26Raymaekers et al., 2004; 27Charman, 2003; 28Dawson et al., 2004; 29Presmanes et al., 2007; 30Thrum et al., 2006; 31Schietecatte et al., 2011; 32Joseph et al., 2009; 33Keehn et al., in press.
As discussed in the Introduction, lower-level attentional functions may operate as essential elements for the development of higher-level sociocommunicative processes. Such a model has been used by Karmiloff-Smith (2009) to explain delayed language development in Williams syndrome. What follows is a developmental framework that explores the role that atypical attentional processes may have on the emergence of the ASD phenotype. Because impaired disengagement is the earliest attentional deficit reported in infants at-risk for ASD (Elsabbagh et al., 2009; Zwaigenbaum et al., 2005) and is associated with a later autism diagnosis (Zwaigenbaum et al., 2005), the current framework makes the initial assumption that abnormal disengagement of attention represents a primary disturbance in ASD. Efficient disengagement of visual attention has significant influence on two early skills: arousal regulation and joint attention. Additionally, and more speculatively, disengagement of attention may also be associated with the development of perceptual biases (see Colombo, 1995, for discussion). Thus, dysfunctional attentional disengagement may have sequelae that, in combination with other primary disturbances, result in the heterogeneous phenotypic end-state associated with ASD. Secondary impairments and anomalies in arousal modulation, joint attention, and visual-perceptual processes produce more global deficits in a variety of domains. Each will be discussed in turn.
3.1 Atypical arousal regulation
Shifting attention to distracting stimuli temporally suspends distress in infants (Harman, Rothbart, & Posner, 1997). For example, during face-to-face interactions, infants shift attention away from faces in order to regulate arousal levels (Field, 1981). This suggests that early deficits in disengaging attention may result in the development of atypical arousal regulation. The process of arousal modulation in early infancy involves a dynamic interplay between the internal state of the infant and the level of external stimulation, with a purpose of maintaining a homeostatic state (Gardner, Karmel, & Magnano, 1992).
As reviewed above, individuals with ASD may demonstrate both hyper- and hypo-aroused states, which may be a reflection of either impaired modulation of arousal or separate subgroups of individuals with ASD. While prior studies of older children, adolescents, and adults with ASD have shown atypical arousal regulation, a more recent study by Anderson and Colombo (2009) reported that compared to TD children, 4-year-old children with ASD exhibit increased tonic pupil size, which is indicative of increased arousal. Furthermore, prospective studies investigating temperament in infants at risk for ASD have also demonstrated characteristics of over-reactivity and poor arousal modulation (Bryson et al., 2007; Garon et al., 2009). As hypothesized by previous investigators (Dawson & Lewy, 1989a; Gold & Gold, 1975), abnormal arousal levels would have developmental consequences in a variety of domains including: 1) abnormal perception of novel information, 2) reduced attention to social information, 3) restricted and repetitive behaviors, 4) over-focused attention, and 5) reduced efficiency of executive control abilities.
Arousal levels impact novelty processing (Barry, Clarke, McCarthy, Selikowitz, & Rushby, 2005). In TD infants, decreased efficiency of attentional disengagement is related to greater aversion to novelty (Johnson, Posner, & Rothbart, 1991; McConnell & Bryson, 2005). As a result of poor regulation of arousal (perhaps due in part to impaired disengagement) novel stimuli and environments may be over-arousing, such that new information is perceived as aversive. Multiple studies using a variety of experimental modalities have shown that individuals with ASD have dysfunctional novelty processing (see section 2.1.3). Furthermore, prior studies have demonstrated that an inefficient alerting system (Keehn et al., 2010) and decreased sensitivity to new information (Keehn & Joseph, 2008) are both related to increased sociocommunicative deficits in ASD.
A largely consistent finding across multiple studies has been reduced amplitude of the frontal (novelty) P3 or P3a component in ASD (Jeste and Nelson, 2009). The P3a, may reflect inhibition of ongoing processing in order to attend and evaluate novel stimuli (Polich, 2007). One model of ASD has hypothesized that the disorder may reflect an abnormally increased ratio of excitation to inhibition (Hussman, 2001; Rubenstein & Merzenich, 2003). Thus, decreased response to novelty in ASD (as reflected by reduced P3a amplitudes) may reflect a primary disturbance in the balance of excitation and inhibition, which is necessary to stop ongoing activity.
Alternatively, the locus coeruleus-P3 hypothesis suggests that the cortical sources of the P3 component (lateral prefrontal cortex and temporal-parietal junction) are modulated by the LC-NE system (Nieuwenhuis, Aston-Jones, & Cohen, 2005). Reduced amplitude of the P3 component in ASD could also reflect atypical subcortical modulation of the LC-NE system, which as discussed in section 2.1.1, is important for modulation of tonic and phasic alertness. The idea that atypical subcortical-cortical anatomical connectivity could result in reduced P3a amplitudes in ASD has been previously hypothesized (Dawson & Lewy, 1989b). More recently, increased tonic pupil size in young children with ASD has been observed (Anderson & Colombo, 2009). Pupil diameter has been shown to be correlated with LC tonic activity (Aston-Jones & Cohen, 2005), thus increased pupil diameter in ASD may indirectly reflect increased tonic activity of the LC-NE system, which could result in reduced phasic responsiveness to novel stimuli.
Due to an unstable arousal system, the novel, dynamic, and complex features of social stimuli and unpredictable nature of social interactions may be overarousing to individuals with ASD (Dawson & Lewy, 1989a). Because infants use attentional shifts to regulate arousal during early face-to-face interactions (Field, 1981), impaired disengagement during these interactions could lead to hyperarousal in infants and toddlers with ASD. Following repeated instances of overarousal during early social interactions, social information may become aversive and individuals with ASD may not perceive social interactions as intrinsically rewarding. This would be in agreement with a previous theory of ASD that suggests that social dysfunction may result from an abnormal reward system associated with social stimuli (Dawson et al., 2002). This appears consistent with the surprising finding that attention to faces in 6 month old at-risk infants is not associated with later ASD diagnosis (Young, Merin, Rogers, & Ozonoff, 2009), which may in turn suggest that inattention to faces and atypical face processing are secondary to impaired attention disengagement and develop later due to earlier overarousal during face-to-face exchanges.
In accord with this idea, Dalton et al. (2005) showed that increased attention to eyes was associated with hyperactivation of the amygdala in ASD, indicative of overarousal resulting from direct gaze. These authors hypothesized that the avoidance of eye contact may be a strategy used by individuals with ASD in order to reduce arousal, and may result in dysfunctional face and gaze processing strategies. These results were replicated by Kliemann et al. (2012), who also report increased amygdala activity in ASD in response to attention to eyes, and, moreover, subsequent gaze avoidance as measured by the frequency of shifting attention away from the eye region. Likewise, previous studies have also shown that children with ASD exhibit increased arousal (as measured by SCR) to direct gaze compared to TD children (Joseph, Ehrman, McNally, & Keehn, 2008; Kylliainen & Hietanen, 2006), and that increased arousal to direct gaze is related to poorer face recognition skills in ASD (Joseph et al., 2008).
Also in agreement with the hypothesis that the complex nature of social stimuli may be overarousing, Pierce and colleagues (1997) found that the social perception abilities of children with ASD was more impaired with an increasing number of social cues. As argued by the authors, if dysfunctional social cognition was due to impaired perception of social information in ASD, trials with redundant social information (i.e. multiple cues) should improve task performance; however, if ASD social deficits are related to an attentional impairment, then task performance should improve in conditions with reduced attentional requirements. In accord with the latter hypothesis, the authors reported similar performance between ASD and comparison groups for the single cue condition, but impaired performance for the ASD group for the multiple cue conditions, and suggest that dysfunctional arousal modulation may influence attentional capacity for social information, resulting in poorer social perception in situations with redundant social information.
In addition to deficits in communication and reciprocal social interaction, ASD is defined by the presence of restricted and repetitive behaviors (see Turner, 1999, for review). Hutt and colleagues (1964) hypothesized that chronically hyperaroused individuals with ASD may engage in repetitive behaviors (e.g., hand flapping) in order to reduce levels of arousal. Furthermore, they hypothesized that aversive response to novel objects or events may be exacerbated by increased arousal levels, resulting in rigid patterns of behavior and insistence on sameness. A more recent study showing an association between restricted and repetitive behaviors and atypical sensory responsiveness (Gabriels et al., 2008) is in agreement with findings relating arousal level to increased repetitive behaviors (Colman, Frankel, Ritvo, & Freeman, 1976). Interestingly, Garon and colleagues (2009) recently demonstrated that an observational measure of restricted and repetitive behaviors was related to measures of temperament, including attentional shifting and activity level. Because impaired disengagement of attention may result in overarousal, the persistent use of repetitive movements may reflect an alternative means of “self-soothing” (Liss et al., 2006, p. 167) for individuals with ASD. These findings suggest that atypical disengagement and hyper-arousal may play a part in the presence of these behaviors.
Liss and colleagues (2006) have hypothesized that over-focused attentional style in ASD may be the result of hyperarousal. Additionally, the authors hypothesize that over-focused attention in these individuals would result in an amplification of sensory information at the locus of attention (and reduced processing of information outside the atypically narrow attentional focus). This is in agreement with Townsend and Courchesne (1994) who showed enhanced sensory processing within a narrower attentional spotlight specifically in adult ASD participants with parietal lobe abnormalities. Prior studies with TD adults suggest that increased arousal may result in a narrowed attentional focus and increased suppression of peripheral information (Britton & Delay, 1989; Tracy et al., 2000). Over-focused attention may help explain the superior performance of individuals with ASD (as compared to their TD peers) on a variety of visuospatial tasks (see Dakin & Frith, 2005, for review). Eye-tracking studies have shown reduced fixation durations in children and adolescents with ASD (Joseph et al., 2009; Keehn et al., 2009), indicative of enhanced perceptual discrimination at the locus of attention. Lastly, Liss and colleagues (2006) found that a subgroup of individuals with ASD who exhibited this over-focused profile had greater social impairment, which is congruent with the findings by Joseph et al. (2009) of enhanced visual search ability being associated with increased sociocommunicative impairments in children with ASD. This is consistent with the hypothesis of early increases in arousal resulting in a narrowed visual field, i.e., enhanced processing within and reduced orienting to stimuli outside a restricted attentional spotlight.
Abnormal modulation of arousal could also have important consequences for the development of efficient executive control abilities. For example, Raymaekers et al. (2004) and Geurts et al. (2009) found that stimulus presentation rate (which modulates arousal levels) differentially affects inhibitory control abilities in individuals with ASD. In addition, children and adolescents with ASD show atypical interdependence between alerting and executive control networks (Keehn et al., 2010). Although speculative, dysmodulation of arousal in ASD may result in differential task-related increases in arousal and subsequent decreases in performance according to the Yerkes-Dodson law (Yerkes & Dodson, 1908). Previous studies have shown that executive tasks result in changes in arousal levels (Hoshikawa & Yamamoto, 1997). The dysmodulation of arousal observed in ASD may be associated with an abnormal task-related autonomic response, which may in turn result in poorer executive performance (because in ASD regulation of autonomic response taxes executive resources). This drain on attentional resources may be especially challenging as children with ASD may have generally reduced attentional capacity (Ming et al., 2005; Vaughan Van Hecke et al., 2009).
Previous research has demonstrated that early measures of executive function are related to novelty processing (Sheese, Rothbart, Posner, White, & Fraundorf, 2008) and self-regulation (Gerardi-Caulton, 2000). Due to early impairments in arousal regulation in ASD, individuals with ASD may recruit or rely on effortful control or self-regulation in order to mediate the states of both hypo- and hyperarousal. Alternatively, Posner et al. (2011) have proposed that efficient executive control abilities may arise from earlier reliance on orienting abilities. They suggest that orienting to novel information may activate executive network functions necessary for self-regulation and facilitating further development of that network. Therefore, early impairments in the orienting network (i.e. impaired disengagement) may have implications for the development of efficient executive control processes.
As a consequence of early-onset abnormalities in the orienting network and dysfunctional response to novelty, networks associated with arousal and executive control would be expected to show atypical interdependence in ASD. This hypothesis is supported by findings of executive deficits being related to arousal modulation (Geurts, Begeer et al., 2009; Keehn et al., 2010; Raymaekers et al., 2004). High levels of arousal (or stress) result in increased release of norepinephrine and dopamine in prefrontal cortex, and have been found to produce impairments in working memory (Robbins & Arnsten, 2009). Children and adolescents with ASD show increased variability of cortisol levels and elevated cortisol after exposure to novel, non-social stimuli (Corbett, Mendoza, Abdullah, Wegelin, & Levine, 2006). Therefore increased reactivity and variability of stress response may contribute to poorer performance in difficult executive tasks (Geurts, Corbett et al., 2009).
Although potential links between impaired disengagement and aberrant arousal regulation in ASD have not been systematically investigated, some studies have shown the presence of both types of deficit in the same individuals (Bryson et al., 2007; Watson et al., 2007). Disengagement of attention has an important regulatory function early in development (e.g., Harman, Rothbart, & Posner, 1997). If this function is impaired, atypical arousal and responsivity to environmental stimuli are a possible developmental consequence. As outlined above, deficits within these functions could have implications for a broad array of domains associated with ASD, including novelty processing, social attention, over-focused attention, restricted and repetitive behaviors, and executive function.
3.2 Impaired joint attention
Joint attention is the coordinated attention between an individual and his/her social partner. In the most common scenario, it simply implies “looking where someone else is looking” (Butterworth & Jarrett, 1991, p. 223, as cited in Moore & Corkum, 1994). Prior research has shown impaired joint attention in children with ASD (see Bruinsma, Koegel, & Koegel, 2004, for review). Early joint attention may depend on the attention-capturing characteristics of an environmental stimulus and on changes in head/gaze direction of the caregiver (Butterworth & Grover, 1990). These early joint attention abilities rely on more basic attentional mechanisms (Frischen, Bayliss, & Tipper, 2007) and begin to develop between 6 and 12 months, continuing to mature into the second year of life.
One such mechanism necessary for successful joint attention is the ability to efficiently disengage attention from the current focus in order to shift and engage the object or event at the locus of the caregiver’s attention (Hood, Willen, & Driver, 1998). Early difficulties disengaging attention could have important implications for successfully responding and initiating joint attention in infants with ASD. In accord with this idea, Charman referred to joint attention abilities as “not a starting point but merely a staging post in early sociocommunicative development” (Charman, 2003, p. 321). Perhaps early disengagement difficulties reflect a possible origin for joint attention difficulties and its developmental consequences. Impaired disengagement of attention in at-risk infants has been observed within the second half of the first year of life (Elsabbagh et al., 2009; Zwaigenbaum et al., 2005), a period in which joint attention abilities begin to appear. Recently, Schietecatte and colleagues (2011) reported a relationship between attention disengagement and joint attention abilities in ASD, i.e., faster disengagement was associated with more joint attention initiations.
Importantly, joint attention abilities in TD infants and toddlers have been linked to both language development and understanding the psychological states of others (Carpenter, Nagell, & Tomasello, 1998). Multiple studies have also linked early joint attention skills to later language (Charman, 2003; Dawson et al., 2004; Presmanes, Walden, Stone, & Yoder, 2007; Thurm, Lord, Lee, & Newschaffer, 2007) and social functioning (Charman, 2003; Presmanes et al., 2007; Schietecatte et al., 2011) in children with ASD. Thus, deficits in disengaging attention may lead to failure to initiate and response to joint attention, which in turn, could result in delayed or abnormal language acquisition and the impaired mental state attribution skills.
3.3 Atypical perceptual processes
The role of disengagement on the development of perceptual processing biases is currently the most speculative consequence of impaired disengagement (Elsabbagh et al., 2009), and evidence in support of this hypothesis is limited. Individuals with ASD have been shown to excel at a variety of visuospatial tasks relative to their TD peers (see Dakin & Frith, 2005, for review). Two theoretical models, Weak Central Coherence (Happé & Frith, 2006) and Enhanced Perceptual Functioning (Mottron, Dawson, Soulieres, Hubert, & Burack, 2006), have been proposed to explain these “islets of ability.” Both theories posit that enhanced ASD performance is due, at least in part, to a local processing bias. That is, individuals with ASD are biased towards local or featural information rather than global properties of a stimulus. Previous research investigating the mechanisms underlying fixation durations in TD infants has demonstrated that “long lookers” tend to show a local processing bias (Colombo, Freeseman, Coldren, & Frick, 1995). Colombo (1995) hypothesized that one possible explanation for increased fixation durations (i.e., “long lookers”) may be impaired disengagement of attention associated with immature development of the brain regions associated with the orienting network. These infants may find it difficult to shift attention away from salient local features of stimuli. However, at this point, whether impaired disengagement leads to the development of local processing bias and enhanced abilities in ASD remains conjecture. Future research investigating the relationship between disengagement abilities and local-global processing biases in both TD and ASD populations is necessary in order to establish this link.
In addition to disengagement abilities, Gardner and Karmel (1995) have shown that level of arousal also interacts with infants’ preference for certain stimulus characteristics; higher arousal results in increased looking to less intense stimuli, lower arousal in increased looking to more intense stimuli. Therefore, level of arousal (which, again, is influenced by the efficiency of attentional disengagement) may have important implications for the development of visual-perceptual preferences or sensitivities, and may contribute to the development of atypical perceptual processes in ASD.
3.2 Future Research
Typical development of attentional systems undergoes rapid change during the first years of life. The maturation of alerting, orienting, and executive control functions continues into the school-age and adolescence. Fifty years of attention research on ASD has largely given us static pictures of a developmental disorder after these attentional mechanisms have generally reached adult-like levels in TD individuals. While these studies have provided important information regarding the attentional strengths and weaknesses in ASD, the question whether these impairments are a cause or a consequence of ASD remains to be resolved. More recently, prospective studies of infants at-risk for ASD have provided researchers with a glimpse of early attentional function and its relationship to the development of the disorder. These studies have provided a nascent understanding of the emergence of the autistic phenotype. However, important questions remain; these include, but are not limited to:
How does attention set (i.e., top-down modulation of attention) affect the processing of new information in ASD? Results from neurofunctional assays of novelty processing show intact or enhanced processing of novel information when individuals with ASD are given an active task, but deficient response to new information during passive tasks. Future research investigating novelty processing in ASD should include conditions with and without a top-down attentional set to examine how task-relevant and task-irrelevant novel information captures attention in ASD.
-
Why do individuals with ASD take longer to disengage attention?
Although many studies using the gap-overlap task have reported impaired disengagement, specifically slower saccadic RT to the overlap condition, these studies have yet to determine whether these effects are due to 1) differences in tonic alertness (i.e. increased saccadic RT when target onset is not preceded by fixation offset [phasic warning]), and 2) abnormal or inefficient release of ocular inhibition. Future studies should attempt to elucidate which of these mechanisms results in impaired disengagement in ASD.
Do early arousal levels influence sensitivity to new information and does this impact sociocommunicative development? A consistent finding across multiple experimental methodologies indicates that individuals with ASD demonstrate dysfunctional novelty detection. However, the question that remains to be fully answered is why individuals with ASD do not respond in a typical fashion to the onset of novel information and how this aberrant response develops. Prior studies have shown that inefficient alerting and insensitivity to new non-social information are both related to greater sociocommunicative dysfunction. Future prospective studies of at-risk infants should investigate how early arousal levels influence sensitivity to new information later in life to determine if atypical arousal regulation results in abnormal novelty processing and deficits in sociocommunicative functioning
How might visuospatial processing strengths in autism develop, do these superior abilities arise from early impairments in disengaging attention or dysregulation of arousal resulting in over-focused attention, and how are they related to sociocommunicative dysfunction? Future studies should examine the relationship between early arousal regulation and attentional disengagement and the development of visuospatial processing biases, and how over-focused attention and/or a local processing bias may impact the development of social information processing skills in ASD.
Are social and communicative impairments in autism developmental sequelae of abnormal disengagement of attention? Resolving this question has important implications for both the early identification of infants at-risk for ASD and for developing targeted early interventions. Evidence reviewed above suggests that attentional abnormalities are present and that they are related to impairments in higher-level sociocommunicative processes. However, longitudinal behavioral and physiological research with young children at risk for ASD will help to resolve the question of causality. Such research can tell us whether impairments in attentional disengagement play a role in the development of sociocommunicative deficits associated with ASD.
Lastly, and more generally, future research endeavors would also benefit from a cross-syndrome perspective (see Cornish, Scerif, & Karmiloff-Smith, 2007, for example). Tracking the developmental trajectories of attentional functions in variety of developmental disorders may help to understand how attention impairments may ultimately result in unique phenotypic outcomes. Importantly, understanding the maturation of attentional function in single gene disorders with a high prevalence of ASD (e.g., fragile X, Tuberous Sclerosis) may provide unique insights into the role of attentional dysfunction in those with and without ASD.
3.3 Clinical Implications
If early attentional impairments play a causal role in the development of ASD, then 1) attentional deficits may be used as an early neuro-behavioral marker to identify infants at-risk for ASD and 2) the development of attention-targeted early interventions may remediate abnormal developmental trajectories and improve outcomes in children with ASD.
Early identification of infants at risk for ASD is of paramount importance for successful early intervention. Thus far, prospective research investigating socioemotional function in infants at-risk for ASD has not revealed significant patterns of early social dysfunction (see Rogers, 2009, for review). Thus, current diagnostic tools may rely on abnormal behaviors that appear on a more consistent basis later in development. If attentional abnormalities are one of the first characteristics that distinguish infants who are later diagnosed with ASD, diagnostic tools should employ an approach that emphasizes these attentional deficits. These tools may result in earlier diagnosis, which has important implications for successful early intervention that may help reduce the atypical development trajectories in ASD.
Furthermore, if early attentional dysfunction is a primary impairment in ASD, the development of early attention-targeted interventions may help ameliorate the development of higher-level sociocommunicative deficits. Posner and Rothbart (2005) have suggested that early attentional interventions may be a useful tool to promote cognitive and social development. Because children with ASD evidence early attentional impairments, interventions targeted at atypical attentional networks may produce generalized improvement across multiple domains. For example, previous research has demonstrated that early interventions have been successful in improving joint attention abilities in children with ASD (Kasari, Freeman, & Paparella, 2006; Whalen & Schreibman, 2003). Perhaps training the constituent functions of joint attention earlier in development (disengaging, shifting) may have important implications for both the development of joint attention and arousal regulation abilities. A recent study indicates that these abilities (e.g. disengagement) can be trained in TD infants, and that training may make significant impact on attentional function (e.g. more shifts of attention between individuals and objects during free play) (Wass, Porayska-Pomsta, & Johnson, 2011). Furthermore, the distribution of infant attention seems to be malleable (Jankowski, Rose, & Feldman, 2001) and subtle changes may have a large impact on information processing strategies. Finally, regardless of whether atypical attention plays a causal role in the development of sociocommunicative deficits, integrating knowledge associated with attentional strengths and weakness may improve current intervention strategies (see Koegel, Shirotova, & Koegel, 2009, for example).
3.4 Conclusion
This paper reviews attentional function in ASD within the framework of three attentional networks. Autism spectrum disorder is characterized by attentional dysfunction of the alerting, orienting, and executive control networks. The current body of research has only begun to describe the onset and development of attentional impairments in ASD. Nevertheless, ASD may be characterized by dysmodulation of arousal (perhaps with subgroups of hyper- and hypoarousal) and impaired novelty processing, slowed attentional disengagement and shifting, and poorer performance on complex executive control tasks (i.e. requiring more than one executive function).
As was said by Gold and Gold, “using attentional mechanisms as our fulcrum, we may be able to understand the global nature of autism and appreciate the clinical manifestations of this disease” (Gold & Gold, 1975, p. 76). The proposed developmental framework attempts to explain the diverse and heterogeneous nature of ASD by exploring the hypothesis that atypical attentional disengagement may be one of many primary impairments associated with the disorder. This deficit in attentional disengagement results in a cascade of impairment, including poor joint attention abilities that lead to delayed language acquisition and atypical arousal modulation that influences novelty and social-information processing. Ultimately, one goal of future research should be to describe the development of attentional networks in ASD, which may be used to understand the emergence of the ASD phenotype and to develop efficacious early interventions that will aid children with ASD and their families.
Highlights.
Attention in ASD is reviewed in comparison with neurotypical attentional networks
ASD is characterized by deficits in alerting, orienting, and executive networks
A model proposes that deficits in disengagement contribute to emergence of ASD
Atypical attention may be a useful behavioral marker for early ASD identification
Efficacious early interventions may benefit from knowledge of attentional function
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams NC, Jarrold C. Inhibition and the validity of the Stroop task for children with autism. J Autism Dev Disord. 2009;39:1112–1121. doi: 10.1007/s10803-009-0721-8. [DOI] [PubMed] [Google Scholar]
- Akshoomoff N. Selective attention and active engagement in young children. Dev Neuropsychol. 2002;22:625–642. doi: 10.1207/S15326942DN2203_4. [DOI] [PubMed] [Google Scholar]
- Akshoomoff N, Courchesne E, Townsend J. Attention coordination and anticipatory control. Int Rev Neurobiol. 1997;41:575–598. doi: 10.1016/s0074-7742(08)60371-2. [DOI] [PubMed] [Google Scholar]
- Allen G, Courchesne E. Attention function and dysfunction in autism. Front Biosci. 2001;6:D105–119. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- Althaus M, Mulder LJ, Mulder G, Aarnoudse CC, Minderaa RB. Cardiac adaptivity to attention-demanding tasks in children with a pervasive developmental disorder not otherwise specified (PDD-NOS) Biol Psychiatry. 1999;46:799–809. doi: 10.1016/s0006-3223(98)00374-6. [DOI] [PubMed] [Google Scholar]
- Ambery FZ, Russell AJ, Perry K, Morris R, Murphy DG. Neuropsychological functioning in adults with Asperger syndrome. Autism. 2006;10:551–564. doi: 10.1177/1362361306068507. [DOI] [PubMed] [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Anderson CJ, Colombo J. Larger tonic pupil size in young children with autism spectrum disorder. Dev Psychobio. 2009;51:207–211. doi: 10.1002/dev.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. American Psychological Association; Washington D.C: 2000. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Dev Disord. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Rushby JA. Arousal and activation in a continuous performance task. Journal of Psychophysiology. 2005;19:91–99. [Google Scholar]
- Belmonte MK, Gomot M, Baron-Cohen S. Visual attention in autism families: ‘unaffected’ sibs share atypical frontal activation. J Child Psychol Psychiatry. 2010;51:259–276. doi: 10.1111/j.1469-7610.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Dev. 1996;67:1816–1835. [PubMed] [Google Scholar]
- Brian JA, Tipper SP, Weaver B, Bryson SE. Inhibitory mechanisms in autism spectrum disorders: typical selective inhibition of location versus facilitated perceptual processing. J Child Psychol Psychiatry. 2003;44:552–560. doi: 10.1111/1469-7610.00144. [DOI] [PubMed] [Google Scholar]
- Britton LA, Delay ER. Effects of noise on a simple visual attentional task. Percept Mot Skills. 1989;68:875–878. doi: 10.2466/pms.1989.68.3.875. [DOI] [PubMed] [Google Scholar]
- Bruinsma Y, Koegel RL, Koegel LK. Joint attention and children with autism: A review of the literature. Ment Retard Dev Disabil Res Rev. 2004;10:169–175. doi: 10.1002/mrdd.20036. [DOI] [PubMed] [Google Scholar]
- Bruneau N, Bonnet-Brilhault F, Gomot M, Adrien JL, Barthelemy C. Cortical auditory processing and communication in children with autism: Electrophysiological/behavioral relations. Int J Psychophysiol. 2003;51:17–25. doi: 10.1016/s0167-8760(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Bryson SE. Interference effects in autistic children: Evidence for the comprehension of single stimuli. J Abnorm Psychol. 1983;92:250–254. doi: 10.1037//0021-843x.92.2.250. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Wainwright-Sharp JA, Smith IM. Autism: A developmental spatial neglect syndrome? In: Enns JT, editor. The development of attention: Research and theory. Elsevier; North-Holland: 1990. pp. 405–427. [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, McDermott C. A prospective case series of high-risk infants who developed autism. J Autism and Dev Disord. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Burack JA, Enns JT, Stauder JEA, Mottron L, Randolph B. Attention and autism: Behavioral and electrophysiological evidence. In: Cohen DJ, Volkmar FR, editors. Handbook of autism and pervasive developmental disorders. 2. John Wiley and Sons; New York: 1997. pp. 226–247. [Google Scholar]
- Butterworth G, Grover L. Joint visual attention, manual pointing, and preverbal communication in human infancy. In: Jeannerod M, editor. Attention and performance XIII. Erlbaum; Hillside, NJ: 1990. [Google Scholar]
- Butterworth G, Jarrett N. What minds have in common is space: Spatial mechanisms serving joint attention in infancy. British Journal of Developmental Psychology. 1991;9:55–72. [Google Scholar]
- Callejas A, Lupianez J, Funes MJ, Tudela P. Modulations among the alerting, orienting and executive control networks. Exp Brain Res. 2005;167:27–37. doi: 10.1007/s00221-005-2365-z. [DOI] [PubMed] [Google Scholar]
- Callejas A, Lupianez J, Tudela P. The three attentional networks: On their independence and interactions. Brain Cogn. 2004;54:225–227. doi: 10.1016/j.bandc.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs for the Society for Research on Child Development. 1998;63:i–vi. 1–143. [PubMed] [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism? Philos Trans R Soc Lond B Biol Sci. 2003;358:315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SE, Holt DD, White DA, Green L. Inhibitory control in children with autism spectrum disorder. J Autism Dev Disord. 2007;37:1155–1165. doi: 10.1007/s10803-006-0259-y. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Courchesne E, Elmasian R. Effects of focused selective attention tasks on event-related potentials in autistic and normal individuals. Clin Neurophysiol. 1990;75:207–220. doi: 10.1016/0013-4694(90)90174-i. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Knight JE, Prince RJ, Harris RJ, Handmaker SD. Event-related potentials in cross-modal divided attention in autism. Neuropsychologia. 1995;33:225–246. doi: 10.1016/0028-3932(94)00094-6. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Colman RS, Frankel F, Ritvo E, Freeman BJ. The effects of fluorescent and incandescent illumination upon repetitive behaviors in autistic children. J Autism Child Schizophr. 1976;6:157–162. doi: 10.1007/BF01538059. [DOI] [PubMed] [Google Scholar]
- Colombo J. On the neural mechanisms underlying developmental and individual differences in visual fixation: Two hypotheses. Dev Rev. 1995;15:97–135. [Google Scholar]
- Colombo J, Freeseman LJ, Coldren JT, Frick JE. Individual differences in infant fixation duration: Dominance of global versus local stimulus properties. Cog Dev. 1995;10:271–285. [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166:210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31:59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cornish K, Cole V, Longhi E, Karmiloff-Smith A, Scerif G. Does attention constrain developmental trajectories in fragile x syndrome? A 3-year prospective longitudinal study. Am J Intellect Dev Disabil. 2012;117:103–120. doi: 10.1352/1944-7558-117.2.103. [DOI] [PubMed] [Google Scholar]
- Cornish K, Scerif G, Karmiloff-Smith A. Tracing syndrome-specific trajectories of attention across the lifespan. Cortex. 2007;43:672–685. doi: 10.1016/s0010-9452(08)70497-0. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Event-related brain potentials: Comparison between children and adults. Science. 1977;197:589–592. doi: 10.1126/science.877575. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: changes in long-latency event-related potentials from childhood to adulthood. Clin Neurophysiol. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Kilman BA, Galambos R, Lincoln AJ. Autism: Processing of novel auditory information assessed by event-related brain potentials. Electroencephalography and Clinical Neurophysiology. 1984;59:238–248. doi: 10.1016/0168-5597(84)90063-7. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Lincoln AJ, Kilman BA, Galambos R. Event-related brain potential correlates of the processing of novel visual and auditory information in autism. J Autism and Dev Disord. 1985;15:55–76. doi: 10.1007/BF01837899. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Lincoln AJ, Yeung-Courchesne R, Elmasian R, Grillon C. Pathophysiologic findings in nonretarded autism and receptive developmental language disorder. J Autism Dev Disord. 1989;19:1–17. doi: 10.1007/BF02212714. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, James HE, Haas RH, Schreibman L, Lau L. Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Rothstein M. An ERP developmental study of repetition priming by auditory novel stimuli. Psychophysiology. 1996;33:680–690. doi: 10.1111/j.1469-8986.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nature Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Finley C, Phillips S, Galpert L, Lewy A. Reduced P3 amplitude of the event-related brain potential: Its relationship to language ability in autism. J Autism Dev Disord. 1988;18:493–504. doi: 10.1007/BF02211869. [DOI] [PubMed] [Google Scholar]
- Dawson G, Lewy A. Arousal, attention, and the socioemotional impairments of individuals with autism. In: Dawson G, editor. Autism: Nature, diagnosis, and treatment. Guilford Press; New York: 1989a. pp. 49–74. [Google Scholar]
- Dawson G, Lewy A. Reciprocal subcortical-cortical influences in autism. In: Dawson G, editor. Autism: Nature, diagnosis, and treatment. Guilford Press; New York: 1989b. pp. 144–173. [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J. Neuropsychological correlates of early symptoms of autism. Child Dev. 1998a;69:1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998b;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Munson J, Estes A, Osterling J, McPartland J, Toth K, Carver L, Abbott R. Neurocognitive function and joint attention ability in young children with autism spectrum disorder versus developmental delay. Child Dev. 2002;73:345–358. doi: 10.1111/1467-8624.00411. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Dev Psychol. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C. Normative data from the CANTAB. I: Development of executive function over the lifespan. J Clin Exp Neuropsychol. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Arousal, affect, and attention as components of temperament. J Pers Soc Psychol. 1988;55:958–966. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Struss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; Oxford: 2002. [Google Scholar]
- Dittrichova J, Lapackova V. Development Of The Waking State In Young Infants. Child Dev. 1964;35:365–370. doi: 10.1111/j.1467-8624.1964.tb05945.x. [DOI] [PubMed] [Google Scholar]
- Dunn MA, Gomes H, Gravel J. Mismatch negativity in children with autism and typical development. J Autism and Dev Disord. 2008;38:52–71. doi: 10.1007/s10803-007-0359-3. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychol Rev. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, Bolton P, Charman T, Baird G, Johnson MH. Visual orienting in the early broader autism phenotype: Disengagement and facilitation. J Child Psychol Psychiatry. 2009 doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns JT. The development of attention: Reseach and theory. Elsevier; North-Holland, NY: 1990. [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letter upon the identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Eskes GA, Bryson SE, McCormick TA. Comprehension of concrete and abstract words in autistic children. J Autism Dev Disord. 1990;20:61–73. doi: 10.1007/BF02206857. [DOI] [PubMed] [Google Scholar]
- Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI. The relation of brain oscillations to attentional networks. J Neurosci. 2007;27:6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Gu X, Guise KG, Liu X, Fossella J, Wang H, Posner MI. Testing the behavioral interaction and integration of attentional networks. Brain Cogn. 2009;70:209–220. doi: 10.1016/j.bandc.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Posner MI. Relating the mechanisms of orienting and alerting. Neuropsychologia. 1997;35:477–486. doi: 10.1016/s0028-3932(96)00103-0. [DOI] [PubMed] [Google Scholar]
- Field T. Infant gaze aversion and heart rate during face-to-face interactions. Infant Behavior & Development. 1981:4. [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: New evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Foote SL, Freedman R, Oliver AP. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975;86:229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- Frick JE, Colombo J, Saxon TF. Individual and developmental differences in disengagement of fixation in early infancy. Child Dev. 1999;70:537–548. doi: 10.1111/1467-8624.00039. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuro Biobehav Rev. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychol Bul. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes LJ, Campoy G. The time course of alerting effect over orienting in the attention network test. Exp Brain Res. 2008;185:667–672. doi: 10.1007/s00221-007-1193-8. [DOI] [PubMed] [Google Scholar]
- Gabriels RL, Agnew JA, Miller LJ, Gralla J, Pan Z, Goldson E, Ledbetter JC, Dinkins JP, Hooks E. Is there a relationship between restricted, repetitive, stereoyped behaviors and interests and abnormal sensory response in children with autism spectrum disorders? Res Autism Spectrum Disord. 2008:660–670. [Google Scholar]
- Garcia-Villamisar D, Della Sala S. Dual-task performance in adults with autism. Cogn Neuropsychiatry. 2002;7:63–74. doi: 10.1080/13546800143000140. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Karmel BZ, Magnano CL. Arousal/visual preference interactions in high-risk neonates. Dev Psychol. 1992;28:821–830. [Google Scholar]
- Gardner JM, Karmel BZ. Development of arousal-modulated visual preferences in early infancy. Dev Psychol. 1995;31:473–482. [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive function in preschoolers: A review using an integrative framework. Psychol Bull. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, Szatmari P. Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. Journal of Abnormal Child Psychol. 2009;37:59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- Garretson HB, Fein D, Waterhouse L. Sustained attention in children with autism. J Autism Dev Disord. 1990;20:101–114. doi: 10.1007/BF02206860. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Dev Psychol. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Gerardi-Caulton G. Sensitivity to spatial conflict and the development of self-regulation in children 24–26 months of age. Dev Sci. 2000;3:397–404. [Google Scholar]
- Geurts HM, Begeer S, Stockmann L. Brief report: Inhibitory control of socially relevant stimuli in children with high functioning autism. J Autism Dev Disord. 2009a;39:1603–1607. doi: 10.1007/s10803-009-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends Cogn Sci. 2009b;13:74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? J Child Psychol Psychiatry. 2004;45:836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Gold MS, Gold JR. Autism and attention: Theoretical considerations and a pilot study using set reaction time. Child Psychiatry and Human Dev. 1975;6:68–80. doi: 10.1007/BF01438301. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40:2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Maurer D, Lewis TL. Developmental changes in attention: The effects of endogenous cueing and distractors. Dev Sci. 2001;4:209–219. [Google Scholar]
- Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, Denckla MB, Landa RJ. Subtle executive impairment in children with autism and children with ADHD. J Autism Dev Disord. 2005;35:279–293. doi: 10.1007/s10803-005-3291-4. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostow AJ, Vecera SP, Larson JC, Mostofsky SH, Mahone EM, Denckla MB. Evidence for impairments in using static line drawings of eye gaze cues to orient visual-spatial attention in children with high functioning autism. J Autism Dev Disord. 2008;38:1405–1413. doi: 10.1007/s10803-007-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131:2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, Baron-Cohen S. Change detection in children with autism: An auditory event-related fMRI study. Neuroimage. 2006;29:475–484. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Goodman R. Infantile autism: a syndrome of multiple primary deficits? J Autism Dev Disord. 1989;19:409–424. doi: 10.1007/BF02212939. [DOI] [PubMed] [Google Scholar]
- Greenaway R, Plaisted K. Top-down attentional modulation in autistic spectrum disorders is stimulus-specific. Psychol Sci. 2005;16:987–994. doi: 10.1111/j.1467-9280.2005.01648.x. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Colich N, Iacoboni M, Zaidel E, Bookheimer SY, Dapretto M. Atypical neural networks for social orienting in autism spectrum disorders. Neuroimage. 2011;56:354–362. doi: 10.1016/j.neuroimage.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith EM, Pennington BF, Wehner EA, Rogers SJ. Executive functions in young children with autism. Child Dev. 1999;70:817–832. doi: 10.1111/1467-8624.00059. [DOI] [PubMed] [Google Scholar]
- Haist F, Adamo M, Westerfield M, Courchesne E, Townsend J. The functional neuroanatomy of spatial attention in autism spectrum disorder. Dev Neuropsychol. 2005;27:425–458. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- Happé F. Social and nonsocial development in autism: Where are the links? In: Burack JA, Charman T, Yirmiya N, Zelazo PD, editors. The Development of Autism: Perspectives from Theory and Research. Lawrence Erlbaum Associates, Inc; Mahwah, NJ: 2001. pp. 215–230. [Google Scholar]
- Happé F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: Examining profiles across domains and ages. Brain Cogn. 2006;61:25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Harman C, Rothbart MK, Posner MI. Distress and attention interactions in early infancy. Motivation and Emotion. 1997;21:27–43. [Google Scholar]
- Harris NS, Courchesne E, Townsend J, Carper RA, Lord C. Neuroanatomic contributions to slowed orienting of attention in children with autism. Brain Res. 1999;8:61–71. doi: 10.1016/s0926-6410(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Henderson H, Schwartz C, Mundy P, Burnette C, Sutton S, Zahka N, Pradella A. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain Cogn. 2006;61:96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychol (Amst) 2004;115:271–289. doi: 10.1016/j.actpsy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Hill EL. Evaluating the theory of executive dysfunction in autism. Dev Rev. 2004a;24:189–233. [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004b;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Philos Trans R Soc Lond B Biol Sci. 2001;268:1883–1888. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood BM, Willen D, Driver J. Adult’s eyes trigger shifts of visual attention in human infants. Psychol Sci. 1998;9:131–134. [Google Scholar]
- Hoshikawa Y, Yamamoto Y. Effects of Stroop color-word conflict test on the autonomic nervous system responses. Am J Physiol. 1997;272:H1113–1121. doi: 10.1152/ajpheart.1997.272.3.H1113. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Hussman JP. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord. 2001;31:247–248. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- Hutt C, Hutt SJ, Lee D, Ounsted C. Arousal and Childhood Autism. Nature. 1964;204:908–909. doi: 10.1038/204908a0. [DOI] [PubMed] [Google Scholar]
- Jankowski JJ, Rose SA, Feldman JF. Modifying the distribution of attention in infants. Child Dev. 2001;72:339–351. doi: 10.1111/1467-8624.00282. [DOI] [PubMed] [Google Scholar]
- Jeste SS, Nelson CA., 3rd Event related potentials in the understanding of autism spectrum disorders: an analytical review. J Autism and Dev Disord. 2009;39:495–510. doi: 10.1007/s10803-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Daibhis A, Watchorn A, Keavey M, Fitzgerald M, Gallagher L, Gill M, Bellgrove MA. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45:2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Cortical maturation and the development of visual attenion in early infancy. J Cogn Neurosci. 1990;2:81–95. doi: 10.1162/jocn.1990.2.2.81. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Posner MI, Rothbart MK. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. J Cogn Neurosci. 1991;3:334–344. doi: 10.1162/jocn.1991.3.4.335. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Posner MI, Rothbart MK. Facilitation of saccades toward a covertly attended location in early infancy. Psychol Sci. 1994;5:90–93. [Google Scholar]
- Johnson MH, Tucker LA. The development and temporal dynamics of spatial orienting in infants. J Exp Child Psychol. 1996;63:171–188. doi: 10.1006/jecp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Ehrman K, McNally R, Keehn B. Affective response to eye contact and face recognition ability in children with ASD. J Int Neuropsychol Soc. 2008;14:947–955. doi: 10.1017/S1355617708081344. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, Horowitz TS. Why is visual search superior in autism spectrum disorder? Dev Sci. 2009;12:1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Prentice Hall; Englewood-Cliffs: 1973. [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: Decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Karmel BZ, Gardner JM, Magnano CL. Attention and arousal in early infancy. In: Weiss MJS, Zelazo PR, editors. Newborn attention: Biological constraints and the influence of experience. Ablex Publishing Corporation; Norwood, NJ: 1991. [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends Cogn Sci. 1998;2:389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Kasari C, Freeman S, Paparella T. Joint attention and symbolic play in young children with autism: a randomized controlled intervention study. J Child Psychol Psychiatry. 2006;47:611–620. doi: 10.1111/j.1469-7610.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, Okazaki S, Hosokawa-Kakurai M, Watanabe K, Kuwabara H, Ishijima M, Yamasue H, Iwanami A, Kato N, Maekawa H. Electrophysiological abnormalities of spatial attention in adults with autism during the gap overlap task. Clin Neurophysiol. 2007;118:1464–1471. doi: 10.1016/j.clinph.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Maekawa H, Itoh K, Hashimoto O, Iwanami A. Spatial attention in individuals with pervasive developmental disorders using the gap overlap task. Psychiatry Res. 2004;125:269–275. doi: 10.1016/j.psychres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Keehn B, Brenner LA, Ramos AI, Lincoln AJ, Marshall SP, Muller RA. Brief report: Eye-movement patterns during an embedded figures test in children with ASD. J Autism Dev Disord. 2009;39:383–387. doi: 10.1007/s10803-008-0608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Joseph RM. Impaired prioritization of novel onset stimuli in autism spectrum disorder. J Child Psychol and Psychiatry. 2008;49:1296–1303. doi: 10.1111/j.1469-7610.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- Keehn B, Lincoln AJ, Muller RA, Townsend J. Attentional networks in children and adolescents with autism spectrum disorder. J Child Psychol Psychiatry. 2010;51:1251–1259. doi: 10.1111/j.1469-7610.2010.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Cuperus JM, Camfferman G, Van Engeland H. Visual and somatosensory event-related brain potentials in autistic children and three different control groups. Electroencephalogr Clin Neurophysiol. 1994;92:225–237. doi: 10.1016/0168-5597(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Rondeau LA. Development of a continuous performance test for preschool children. J Atten Disord. 1998;2:229–238. [Google Scholar]
- Kingstone A, Klein RM. Visual offsets facilitate saccadic latency: Does predisengagement of visuospatial attention mediate this gap effect? J Exp Psychol Hum Percept Perform. 1993;19:1251–1265. doi: 10.1037//0096-1523.19.6.1251. [DOI] [PubMed] [Google Scholar]
- Kliemann D, Dziobek I, Hatri A, Baudewig J, Heekeren HR. The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. J Neurosci. 2012;32:9469–9476. doi: 10.1523/JNEUROSCI.5294-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Shirotova L, Koegel LK. Brief report: Using individualized orienting cues to facilitate first-word acquisition in non-responders with autism. J Autism Dev Disord. 2009;39:1587–1592. doi: 10.1007/s10803-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz-Dahlmann B, Fink GR. Development of attentional networks: An fMRI study with children and adults. Neuroimage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen JK. Attention orienting by another’s gaze direction in children with autism. J Child Psychol Psychiatry. 2004;45:435–444. doi: 10.1111/j.1469-7610.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen JK. Skin conductance responses to another person’s gaze in children with autism. J Autism Dev Disord. 2006;36:517–525. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: A review of the literature. Res Dev Disabil. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. J Child Psychol Psychiatry. 2004;45:1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: Selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD, VanMeter J, Vaidya CJ, Gaillard WD, Kenworthy LE. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: A fcMRI study of response inhibition. Cereb Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C. Attention and joint attention in preschool children with autism. Dev Psychol. 2000;36:261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Lehto JE, Juujarvi P, Kooistra L, Pulkkinen L. Dimensions of executive functioning: Evidence from children. Br J Dev Psychol. 2003;21:59–80. [Google Scholar]
- Levy F. The development of sustained attention (vigilance) and inhibition in children: Some normative data. J Child Psychol Psychiatry. 1980;21:77–84. doi: 10.1111/j.1469-7610.1980.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lin CC, Hsiao CK, Chen WJ. Development of sustained attention assessed using the continuous performance test among children 6–15 years of age. J Abnorm Child Psychol. 1999;27:403–412. doi: 10.1023/a:1021932119311. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Courchesne E, Harms L, Allen M. Contextual probability evaluation in autistic, receptive developmental language disorder, and control children: Event-related brain potential evidence. J Autism Dev Disord. 1993;23:37–58. doi: 10.1007/BF01066417. [DOI] [PubMed] [Google Scholar]
- Liss M, Fein D, Allen D, Dunn M, Feinstein C, Morris R, Waterhouse L, Rapin I. Executive functioning in high-functioning children with autism. J Child Psychol Psychiatry. 2001;42:261–270. [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10:155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Dev. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biol Psychiatry. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, Sweeney JA. Neocortical system abnormalities in autism: An fMRI study of spatial working memory. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- Luna B, Velanova K, Geier CF. Development of eye-movement control. Brain Cogn. 2008;68:293–308. doi: 10.1016/j.bandc.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D, Golse B, Palacio-Espasa F. Attentional skills during the first 6 months of age in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2002;41:1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol Hum Percept Perform. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biol Psychiatry. 2009;66:950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BA, Bryson S. Visual attention and temperament: Developmental data from the first 6 months of life. Infant Behav Dev. 2005;28:537–544. [Google Scholar]
- McEvoy RE, Rogers SJ, Pennington BF. Executive function and social communication deficits in young autistic children. J Child Psychol Psychiatry. 1993;34:563–578. doi: 10.1111/j.1469-7610.1993.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: Developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Dev. 2004;75:1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain Dev. 2005;27:509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52:917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Morrison FJ. The development of alertness. J Exp Child Psychol. 1982;34:187–199. doi: 10.1016/0022-0965(82)90041-8. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz AM, Seidenfeld A, Guter S, Stanford LD, Sweeney JA. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychol Med. 2009;39:1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain Cognition. 1992;19:105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Noterdaeme M, Amorosa H, Mildenberger K, Sitter S, Minow F. Evaluation of attention problems in children with autism and children with a specific language disorder. Eur Child Adolesc Psychiatry. 2001;10:58–66. doi: 10.1007/s007870170048. [DOI] [PubMed] [Google Scholar]
- Novick B, Kurtzberg D, Vaughn HG., Jr An electrophysiologic indication of defective information storage in childhood autism. Psychiatry Res. 1979;1:101–108. doi: 10.1016/0165-1781(79)90034-9. [DOI] [PubMed] [Google Scholar]
- Novick B, Vaughan HG, Jr, Kurtzberg D, Simson R. An electrophysiologic indication of auditory processing defects in autism. Psychiatry Res. 1980;3:107–114. doi: 10.1016/0165-1781(80)90052-9. [DOI] [PubMed] [Google Scholar]
- O’Hearn K, Asato M, Ordaz S, Luna B. Neurodevelopment and executive function in autism. Dev Psychopathol. 2008;20:1103–1132. doi: 10.1017/S0954579408000527. [DOI] [PubMed] [Google Scholar]
- Olk B, Cameron B, Kingstone A. Enhanced orienting effects: Evidence for an interaction principle. Visual Cognition. 2008;16:979–1000. [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofiev AO, Nygren G, Gillberg C, Elam M. The right hemisphere fails to respond to temporal novelty in autism: evidence from an ERP study. Clin Neurophysiol. 2009;120:520–529. doi: 10.1016/j.clinph.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Ritvo ER. The syndrome of autism: a critical review. Am J Psychiatry. 1976;133:609–621. doi: 10.1176/ajp.133.6.609. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: A study of first birthday home videotapes. J Autism Dev Disord. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, McMahon WM, Minshew N, Munson JA, Pennington BF, Rogers SJ, Spence MA, Tager-Flusberg H, Volkmar FR, Wrathall D. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism network. J Autism Dev Disord. 2004;34:139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Brief report: specific executive function profiles in three neurodevelopmental disorders. J Autism Dev Disord. 1999;29:171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, McEvoy RE. A longitudinal study of executive function and theory of mind development in autism. Dev Psychopathol. 1994;6:415–431. [Google Scholar]
- Ozonoff S, South M, Miller JN. DSM-IV-defined Asperger syndrome: Cognitive, behavioral, and early history differentiation from high-functioning autism. Autism. 2000;4:29–46. [Google Scholar]
- Ozonoff S, South M, Provencal S. Executive Functions. In: Volkmar FR, Paul R, Klin A, Cohen DJ, editors. Handbook of autism and pervasive developmental disorders. 3. John Wiley and Sons; New York: 2005. pp. 606–627. [Google Scholar]
- Ozonoff S, Strayer DL. Inhibitory function in nonretarded children with autism. J Autism Dev Disord. 1997;27:59–77. doi: 10.1023/a:1025821222046. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Further evidence of intact working memory in autism. J Autism Dev Disord. 2001;31:257–263. doi: 10.1023/a:1010794902139. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL, McMahon WM, Filloux F. Executive function abilities in autism and Tourette syndrome: an information processing approach. J Child Psychol Psychiatry. 1994;35:1015–1032. doi: 10.1111/j.1469-7610.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Palkovitz RJ, Wiesenfeld AR. Differential autonomic responses of autistic and normal children. J Autism Dev Disord. 1980;10:347–360. doi: 10.1007/BF02408294. [DOI] [PubMed] [Google Scholar]
- Pascualvaca DM, Fantie BD, Papageorgiou M, Mirsky AF. Attentional capacities in children with autism: is there a general deficit in shifting focus? J Autism Dev Disord. 1998;28:467–478. doi: 10.1023/a:1026091809650. [DOI] [PubMed] [Google Scholar]
- Pashler H. The psychology of attention. MIT Press; Cambridge: 1999. [Google Scholar]
- Pashler H, Johnston JC, Ruthruff E. Attention and performance. Annu Rev Psychol. 2001;52:629–651. doi: 10.1146/annurev.psych.52.1.629. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Lane DM. Visual attention movements: a developmental study. Child Dev. 1990;61:1779–1795. [PubMed] [Google Scholar]
- Pierce K, Glad KS, Schreibman L. Social perception in children with autism: An attentional deficit? J Autism and Dev Disord. 1997;27:265–282. doi: 10.1023/a:1025898314332. [DOI] [PubMed] [Google Scholar]
- Pelisson D, Goffart L, Guillaume A, Quinet J. Visuo-motor deficits induced by fastigial nucleus inactivation. Cerebellum. 2003;2:71–76. doi: 10.1080/14734220310015629. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Cognitive neuroscience of attention. Guilford Press; New York, NY: 2004. [Google Scholar]
- Posner MI. Measuring alertness. Ann N Y Acad Sci. 2008;1129:193–199. doi: 10.1196/annals.1417.011. [DOI] [PubMed] [Google Scholar]
- Posner MI, Fan J. Attention as an organ system. In: Pomerantz JR, Crair MC, editors. Topics in integrative neuroscience: From cells to cognition. Cambridge University Press; Cambridge: 2004. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Influencing brain networks: Implications for education. Trends Cog Sci. 2005;9:99–103. doi: 10.1016/j.tics.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Voelker P. Control networks and neuromodulators of early development. Dev Psychol. 2011 doi: 10.1037/a0025530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presmanes AG, Walden TA, Stone WL, Yoder PJ. Effects of different attentional cues on responding to joint attention in younger siblings of children with autism spectrum disorders. J Autism Dev Disord. 2007;37:133–144. doi: 10.1007/s10803-006-0338-0. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Raz N, August GJ. Visual augmenting/reducing and P300 in autistic children. J Autism Dev Disord. 1987;17:231–242. doi: 10.1007/BF01495058. [DOI] [PubMed] [Google Scholar]
- Pruett JR, Jr, LaMacchia A, Hoertel S, Squire E, McVey K, Todd RD, Constantino JN, Petersen SE. Social and non-social cueing of visuospatial attention in autism and typical development. J Autism Dev Disorders. 2010;41:715–731. doi: 10.1007/s10803-010-1090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal R. The neurology of visual orienting: A pathological disintergration of development. In: Richards JE, editor. Cognitive neuroscience of attention: A developmental perspective. Lawrence Erlbaum Associates, Inc; Mahwah, NJ: 1998. [Google Scholar]
- Raymaekers R, Antrop I, van der Meere JJ, Wiersema JR, Roeyers H. HFA and ADHD: a direct comparison on state regulation and response inhibition. J Clin Exp Neuropsychol. 2007;29:418–427. doi: 10.1080/13803390600737990. [DOI] [PubMed] [Google Scholar]
- Raymaekers R, van der Meere J, Roeyers H. Event-rate manipulation and its effect on arousal modulation and response inhibition in adults with high functioning autism. J Clin Exp Neuropsychol. 2004;26:74–82. doi: 10.1076/jcen.26.1.74.23927. [DOI] [PubMed] [Google Scholar]
- Raymaekers R, van der Meere J, Roeyers H. Response inhibition and immediate arousal in children with high-functioning autism. Child Neuropsychol. 2006;12:349–359. doi: 10.1080/09297040600760457. [DOI] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nat Rev Neurosci. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Renner P, Grofer Klinger L, Klinger MR. Exogenous and endogenous attention orienting in autism spectrum disorders. Child Neuropsychol. 2006;12:361–382. doi: 10.1080/09297040600770753. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: an event-related potential and cortical source localization study. Dev Psychol. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Infant heart rate: A developmental psychophysiological perspective. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and methods. Cambridge University Press; New York: 2008. [Google Scholar]
- Richards JE. Infant cognitive psychophysiology: Normal development and implications for abnormal developmental outcomes. In: Ollendick TH, Prinz RJ, editors. Advances in Clinical Child Psychology. Plenum Press; New York: 1995. pp. 77–107. [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Dev Sci. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimland B. Infantile autism: The syndrome and its implication for natural theory and behavior. Meredith Publishing Company; New York: 1964. [Google Scholar]
- Rinehart NJ, Bradshaw JL, Tonge BJ, Brereton AV, Bellgrove MA. A neurobehavioral examination of individuals with high-functioning autism and Asperger’s disorder using a fronto-striatal model of dysfunction. Behavior and Cognitive Neuroscience Review. 2002;1:164–177. doi: 10.1177/1534582302001002004. [DOI] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychon Bull Rev. 2002;9:507–513. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special but not for everyone: The case of autism. Brain Res. 2005;24:715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzangia MS, editor. The cognitive neurosciences. MIT Press; Cambridge: 1995. pp. 703–720. [Google Scholar]
- Roberts JE, Hatton DD, Long AC, Anello V, Colombo J. Visual attention and autistic behavior in infants with fragile X syndrome. J Autism Dev Disord. 2012;42:937–946. doi: 10.1007/s10803-011-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RJ, Pennington BF. An interactive framework for examining prefrontal cognitive processes. Dev Neuropsychol. 1996;12:105–126. [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, Posner MI. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Rothbart MK. Attention in early development. Oxford University Press; New York: 1996. [Google Scholar]
- Russell J, Jarrold C, Henry L. Working memory in children with autism and with moderate learning difficulties. J Child Psychol Psychiatry. 1996;37:673–686. doi: 10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Russell J, Jarrold C, Hood B. Two intact executive capacities in children with autism: implications for the core executive dysfunctions in the disorder. J Autism Dev Disord. 1999;29:103–112. doi: 10.1023/a:1023084425406. [DOI] [PubMed] [Google Scholar]
- Russo N, Flanagan T, Iarocci G, Berringer D, Zelazo PD, Burack JA. Deconstructing executive deficits among persons with autism: implications for cognitive neuroscience. Brain Cogn. 2007;65:77–86. doi: 10.1016/j.bandc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Scerif G, Longhi E, Cole V, Karmiloff-Smith A, Cornish K. Attention across modalities as a longitudinal predictor of early outcomes: the case of fragile X syndrome. J Child Psychol Psychiatry. 2011;53:641–650. doi: 10.1111/j.1469-7610.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- Schietecatte I, Roeyers H, Warreyn P. Exploring the Nature of Joint Attention Impairments in Young Children with Autism Spectrum Disorder: Associated Social and Cognitive Skills. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1209-x. [DOI] [PubMed] [Google Scholar]
- Schiller PH. A model for the generation of visually guided saccadic eye movements. In: Rose D, Dobson VG, editors. Models of the Visual Cortex. Wiley; Chichester: 1985. [Google Scholar]
- Schiller PH. The neural control of visually guided eye movements. In: Richards JE, editor. Cognitive Neuroscience of Attention: A Developmental Perspective. Lawrence Erlbaum Assoicates, Inc; Mahwah, NJ: 1998. [Google Scholar]
- Schoen SA, Miller LJ, Brett-Green B, Hepburn SL. Psychophysiology of children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2008;2:417–429. [Google Scholar]
- Schul R, Townsend J, Stiles J. The development of attentional orienting during school-age years. Dev Sci. 2003;6:262–272. [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. J Child Psychol Psychiatry. 2004;45:445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 2008;63:974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheese BE, Rothbart MK, Posner MI, White LK, Fraundorf SH. Executive attention and self-regulation in infancy. Infant Behav Dev. 2008;31:501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Shields SA. Development of autonomic nervous system responsitivity in children: A review of the literature. Int J Beh Dev. 1983;6:291–319. [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Morsch D, Bruning N, Schmidt MH, Lehmkuhl G. Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child and Adolescent Psychiatry and Mental Health. 2008;2:4. doi: 10.1186/1753-2000-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions; the orienting reflex. Annu Rev Physiol. 1963;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. Int J Dev Neurosci. 2008;26:239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele SD, Minshew NJ, Luna B, Sweeney JA. Spatial working memory deficits in autism. J Autism Dev Disord. 2007;37:605–612. doi: 10.1007/s10803-006-0202-2. [DOI] [PubMed] [Google Scholar]
- Stevens S, Gruzelier J. Electrodermal activity to auditory stimuli in autistic, retarded, and normal children. J Autism Dev Disord. 1984;14:245–260. doi: 10.1007/BF02409577. [DOI] [PubMed] [Google Scholar]
- Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, Herzog H, Tellmann L, Muller-Gartner HW, Willmes K. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14:S76–84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, Rees L, Wheelwright S. The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. J Child Psychol Psychiatry. 1998;39:747–753. [PubMed] [Google Scholar]
- Swettenham J, Condie S, Campbell R, Milne E, Coleman M. Does the perception of moving eyes trigger reflexive visual orienting in autism? Philos Trans R Soc Lond B Biol Sci. 2003;358:325–334. doi: 10.1098/rstb.2002.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TL, Kingstone A, Klein RM. The disapperance of foveal and nonfoveal stimuli: Decomposing the gap effect. Can J Exp Psychol. 1998;52:192–199. [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kamio Y. Paradoxical autonomic response to mental tasks in autism. J Autism Dev Disord. 2003;33:417–426. doi: 10.1023/a:1025062812374. [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E. Parietal damage and narrow “spotlight” spatial attention. J Cogn Neurosci. 1994;6:220–232. doi: 10.1162/jocn.1994.6.3.220. [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, Lowry TP, Press GA. Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J Neurosci. 1999;19:5632–5643. doi: 10.1523/JNEUROSCI.19-13-05632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Harris NS, Courchesne E. Visual attention abnormalities in autism: delayed orienting to location. J Int Neuropsychol Soc. 1996;2:541–550. doi: 10.1017/s1355617700001715. [DOI] [PubMed] [Google Scholar]
- Townsend J, Westerfield M, Leaver E, Makeig S, Jung T, Pierce K, Courchesne E. Event-related brain response abnormalities in autism: evidence for impaired cerebello-frontal spatial attention networks. Cog Brain Res. 2001;11:127–145. doi: 10.1016/s0926-6410(00)00072-0. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Mohamed F, Faro S, Tiver R, Pinus A, Bloomer C, Pyrros A, Harvan J. The effect of autonomic arousal on attentional focus. Neuroreport. 2000;11:4037–4042. doi: 10.1097/00001756-200012180-00027. [DOI] [PubMed] [Google Scholar]
- Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40:839–849. [PubMed] [Google Scholar]
- van Engeland H. The electrodermal orienting response to auditive stimuli in autistic children, normal children, mentally retarded children, and child psychiatric patients. J Autism Dev Disord. 1984;14:261–279. doi: 10.1007/BF02409578. [DOI] [PubMed] [Google Scholar]
- van Engeland H, Roelofs JW, Verbaten MN, Slangen JL. Abnormal electrodermal reactivity to novel visual stimuli in autistic children. Psychiatry Res. 1991;38:27–38. doi: 10.1016/0165-1781(91)90050-y. [DOI] [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, Denver J, Bazhenova O, Porges SW. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Dev. 2009;80:1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Vlamings PH, Stauder JE, van Son IA, Mottron L. Atypical visual orienting to gaze- and arrow-cues in adults with high functioning autism. J Autism Dev Disord. 2005;35:267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective, and Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wainwright A, Bryson SE. The development of exogenous orienting: mechanisms of control. J Exp Child Psychol. 2002;82:141–155. doi: 10.1016/s0022-0965(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Wainwright-Sharp JA, Bryson SE. Visual orienting deficits in high-functioning people with autism. J Autism Dev Disord. 1993;23:1–13. doi: 10.1007/BF01066415. [DOI] [PubMed] [Google Scholar]
- Wass S, Porayska-Pomsta K, Johnson MH. Training attentional control in infancy. Current Biology. 2011;21:1543–1547. doi: 10.1016/j.cub.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen C, Schreibman L. Joint attention training for children with autism using behavior modification procedures. J Child Psychol Psychiatry. 2003;44:456–468. doi: 10.1111/1469-7610.00135. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV. Do children with autism ‘switch off’ to speech sounds? An investigation using event-related potentials. Dev Sci. 2008;11:516–524. doi: 10.1111/j.1467-7687.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. J Autism Dev Disord. 2005;35:747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Witte EA, Davidson MC, Marrocco RT. Effects of altering brain cholinergic activity on covert orienting of attention: comparison of monkey and human performance. Psychopharmacology. 1997;132:324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 2.0. A revised model of visual search. Psychon Bull Rev. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Yantis S. Stimulus-driven attentional capture and attentional control settings. J Exp Psychol Hum Percept Perform. 1993;19:676–681. doi: 10.1037//0096-1523.19.3.676. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol. 1908;18:459–482. [Google Scholar]
- Yerys BE, Hepburn SL, Pennington BF, Rogers SJ. Executive function in preschoolers with autism: evidence consistent with a secondary deficit. J Autism Dev Disord. 2007;37:1068–1079. doi: 10.1007/s10803-006-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2009 doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Dev Sci. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn TP, Rumsey JM, Van Kammen DP. Autonomic nervous system activity in autistic, schizophrenic, and normal men: effects of stimulus significance. J Abnorm Psychol. 1987;96:135–144. doi: 10.1037//0021-843x.96.2.135. [DOI] [PubMed] [Google Scholar]
- Zenker F, Barajas JJ. Auditory P300 development from an active, passive and single-tone paradigms. Int J Psychophysiol. 1999;33:99–111. doi: 10.1016/s0167-8760(99)00033-1. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]