Abstract
Objective
To report a novel cell-surface autoantigen of encephalitis that is a critical regulatory subunit of the Kv4.2 potassium channels.
Methods
Four patients with encephalitis of unclear etiology and antibodies with a similar pattern of neuropil brain immunostaining were selected for autoantigen characterization. Techniques included immunoprecipitation, mass spectrometry, cell-base experiments with Kv4.2 and several dipeptidyl-peptidase-like protein-6 (DPPX) plasmid constructs, and comparative brain immunostaining of wild-type and DPPX-null mice.
Results
Immunoprecipitation studies identified DPPX as the target autoantigen. A cell based assay confirmed that all 4 patients, but not 210 controls, had DPPX antibodies. Symptoms included agitation, confusion, myoclonus, tremor, and seizures (one case with prominent startle response). All patients had pleocytosis, and three had severe prodromal diarrhea of unknown etiology. Given that DPPX “tunes up” the Kv4.2 potassium channels (involved in somatodendritic signal integration and attenuation of dendritic backpropagation of action potentials), we determined the epitope distribution in DPPX, DPP10 (a protein homologous to DPPX) and Kv4.2. Patients’ antibodies were found specific for DPPX, without reacting with DPP10 or Kv4.2. The unexplained diarrhea led to demonstrate a robust expression of DPPX in the myenteric plexus, which strongly reacted with patients’ antibodies. The course of neuropsychiatric symptoms was prolonged and often associated with relapses while decreasing immunotherapy. Long-term follow-up showed substantial improvement in 3 patients (1 is lost to follow-up).
Interpretation
Antibodies to DPPX associate with a protracted encephalitis characterized by CNS hyperexcitability (agitation, myoclonus, tremor, seizures), pleocytosis, and frequent diarrhea at symptom onset. The disorder is potentially treatable with immunotherapy.
Keywords: Antibodies, encephalitis, autoimmune, DPP6, DPPX, potassium channels
Introduction
The discovery that memory, behavior, cognition, and thought processes can be altered by autoantibodies has changed the approach to the diagnosis and treatment of neuropsychiatric disorders previously considered idiopathic. Since 2007, seven such antibodies have been identified (anti-NMDAR, AMPAR, GABA(B), LGI1, Caspr2, GlyR, and mGluR5), all targeting cell surface proteins involved in synaptic transmission, plasticity, or nerve excitability, and associated with syndromes that although severe, often respond to immunotherapy.1 Patients may be comatose for several months, with bizarre behaviors, abnormal movements, or refractory seizures and still recover with immunotherapy and extended care support.2 Considering that until recently these disorders were unknown, the relative high frequency of some has been surprising. For example, in a center focused in the diagnosis and epidemiology of encephalitis (California Encephalitis Project) the frequency of anti-NMDAR encephalitis surpassed that of any individual viral encephalitis.3 For these reasons, similar immune mechanisms are increasingly being considered in patients who develop rapidly progressive neuropsychiatric symptoms in the context of encephalitis of unknown etiology, a situation that occurs frequently. Nowadays about 70% of encephalitis of unclear etiology remain undiagnosed after extensive evaluation for infectious etiologies.4 In this setting, the identification of autoantibodies against neuronal cell surface antigens shifts the management to the use of immunotherapy and may extend the intensive care support in cases that otherwise might be considered futile. We report here the clinical and immunological features of 4 patients with prominent neuropsychiatric symptoms (preceded in 3 by intense diarrhea) and antibodies against a novel cell surface antigen, dipeptidyl-peptidase-like protein-6 (DPP6 or DPPX), a cell surface auxiliary subunit of the Kv4.2 potassium channels. In addition to the known robust expression of DPPX in the hippocampus and cerebellum, we show that DPPX is also expressed in the myenteric plexus.
Patients, Material and Methods
The observation of 4 patients with subacute onset of neuropsychiatric symptoms and serum or CSF antibodies showing a similar pattern of immunostaining of the neuropil of rodent hippocampus and cerebellum, as well as immunolabeling of the cell-surface of dissociated cultured hippocampal neurons led us to immunoprecipitate the target antigen. None of the patients had antibodies to previously known synaptic or cell surface proteins, including among others the antibodies attributed to voltage-gated potassium channels (measured by radioimmunoassay using protein complexes labeled with dendrotoxin), and antibodies against LGI1 or Caspr2. Serum or CSF of 210 subjects including patients with autoimmune inflammatory and non-inflammatory encephalopathies, and normal individuals served as controls (see “serum, CSF samples and controls” in Supplemental material).
Patients
Patients are described in detail below (index case), in supplemental information (cases 2 and 3), and summarized in Table 1. The fourth case was a 76 year-old man who developed prominent diarrhea and weight loss along with rapidly progressive confusion, cognitive decline, seizures, unsteady gait, and evidence of intrathecal IgG production (IgG index 1.49); he is not included in the table due to limited information and lack of follow-up.
Table 1.
Clinical features, treatment, and outcome
| Sex, age | Initial symptoms | Main symptoms | Other | CSF | Brain MRI | EEG | Antibody titer* | Treatment (ordered chronologically) | Outcome (duration follow-up) |
|---|---|---|---|---|---|---|---|---|---|
| M,61 | Abdominal pain, diarrhea, depression, aggression, withdrawal | Paranoid delusions, visual hallucinations, mutism, resting tremor, myoclonus, exaggerated startle response. | Decreased level of consciousness, able to track, but not follow commands, orofacialdyskinesias. Suspected seizures | July 2010: WBC 117, protein 82, no OCB. December 2010: WBC 9, protein 51; IgG index 1.36; May 2011: WBC 5, protein 50, IgG index 0.95; August 2011: WBC 1, protein 34, IgG index 0.92; May 2011: WBC 1, protein 40, IgG index 0.57 | Multiple MRIs: Non-specific patchy periventricular and subcortical white matter T2/FLAIR increased signal. | Video EEG: diffuse slowing, poor organization; no epileptic activity | July 2010: serum 1:6400 CSF: 1:160 September 2011: Serum 1:1280 May 2012: CSF: 1:10 |
IV methylprednisolone, oral steroids: substantial improvement, but relapsed with steroid taper. IVIg: mild improvement. Rituximab: mild improvement. Plasma exchange: substantial improvement. Cyclophosphamide: steady, but incomplete improvement. | Able to return home 15 months after symptom onset. Currently completing the 6th monthly cycle of cyclophosphamide. Oriented to person, place and time, able to follow simple conversations. Occasional episodes of agitation. Persistent deficits in executive functioning, attention, concentration, visual-spatial functioning. (FU=21 months) |
| F, 45 | Diarrhea, 30 kg weight loss, memory deficit, insomnia, anxiety | Agitation, paranoia hallucinations, anxiety, insomnia. Recurrent generalized seizures, episodes of status epilepticus. Myoclonus, coarse resting tremor | Decreased level of consciousness, hyperreflexia, orofacial movements, horizontal nystagmus. ANA >2560 |
WBC 15, normal protein; normal glucose; positive OCB. | Multiple MRIs Normal | Background with intermittent generalized theta, delta. PLEDS. | Serum: 1:1280 CSF: 1:160 |
IV methylprednisolone: no response. IVIg: slow improvement. Rituximab: accelerated improvement (remains on periodic Rituximab). | Good. Mild transient relapse when rituximab was skipped. Living independently, normal cognition. Dependent on Rituximab. (FU=49 months) |
| F, 58 | none | Hallucinations, decreased speech, parasomnias, myoclonus, tremor, unsteady gait. | Psychosis (admitted to psychiatry). Clinically suspected seizures. Congenital nystagmus. Single stranded DNA antibodies; no antibodies to dsDNA. | June 2006: WBC 11, protein 50, increased IgG index, positive OCB; April 2007: WBC 5, protein 52 December 2007: WBC 4, protein 53; April 2008: WBC 62, protein 62; September 2008: WBC 1; protein 81; December 2008: WBC 1; protein 71 | Multiple MRIs: non-specific white matter changes. One showing non-acute but new right frontal infarction (biopsy = resolving infarction without vasculitis) | slow background activity | April 2008: CSF 1: 80 September 2008: Serum 1: 1280 CSF: 1:80 |
Prednisone: improvement (relapses at several tapers). IVIg and rituximab: no clear effect. Cyclophosphamide: 1 cycle (no further cycles due to cryptococcal pneumonia). Plasma exchange: partial improvement. | Alert, attentive, fully oriented, normal short-term memory, knows current events. No tremor, myoclonus, or hallucinations. Walks with a slightly wide base. (FU=68 months) |
OCB: oligoclonal bands; FU: follow-up
Patient 1 (index case)
A 61 year-old man with history of obesity, hypertension, and adult-onset diabetes mellitus was admitted for four weeks of abdominal pain and diarrhea followed by subacute change in mental status, characterized by depression, aggression, withdrawal, visual hallucinations, mutism, myoclonus and an exaggerated startle response. MRI of the abdomen showed a fatty liver but no evidence of tumor, and extensive GI workup was negative for fecal leukocytes, clostridium difficile, parasites and ova. Endoscopic biopsies from stomach, small bowel, and colon showed only chronic gastritis (serum H. Pylori IgG positive without bacterium on histology). The diarrhea persisted for over one month without other symptoms of autonomic dysfunction.
CSF, MRI and EEG studies are described in Table 1. CSF PCR for HSV, VZV, Tropheryma whippelii, and enterovirus were negative. Rheumatologic, paraneoplastic, and neuronal cell surface antibody panels (which also included the glycine receptor) were negative. Whole body CT and PET scans and testicular ultrasound did not reveal a cancer.
The patient was briefly intubated for worsening mental status, and treated with intravenous methylprednisolone (1000 mg/day × 5 days) with notable neurologic improvement. He was then placed on a prolonged oral steroid taper over 4 months and discharged to a skilled nursing facility. Four months later he was readmitted with worsening mental status and a urinary tract infection. Treatment with antibiotics followed by IVIg (2 grams/kg over 5 days) resulted in brief neurologic improvement. He subsequently developed sepsis and was transferred to the ICU requiring a tracheotomy and PEG tube. Repeated IVIg did not improve his mental status. He was then treated with Rituximab (1000 mg iv × 2 doses 15 days apart) and the prednisone was titrated to 5 mg every other day. The clinical course was complicated by urinary tract infections and pneumonia, and his mental status remained poor for 5 more months. At his best he could mouth a few words and follow simple commands. One striking finding on the exam at this stage of disease was an exaggerated startle response to sound and touch. In addition, he exhibited frequent episodic myoclonus, oral dyskinesias and paratonia; the muscle strength was normal.
He was eventually treated with plasma exchange. After the first exchange, he was able to converse readily with his examiners and answer questions, but not enough to participate in formal cognitive testing. Subsequently, he received monthly intravenous pulses of cyclophosphamide with a steady but incomplete improvement in cognition. He was able to return home 15 months after symptom onset. On follow-up 21 months after symptom onset, and after having received 9 monthly doses of intravenous cyclophosphamide, he was still at home with family, but required assistance with many activities of daily living. On cognitive testing, orientation was relatively preserved but there were impairments in attention and concentration, executive functioning, abstraction, visual-spatial functioning and phonemic fluency. Testing of verbal memory revealed successful encoding (cueing required). He scored 9 out of 30 points on the Montreal Cognitive Assessment (MOCA). He has lost 45 kg during the course of the disease.
Techniques used for detection of novel antibodies and precipitation of the autoantigen
The laboratory techniques used for antibody and antigen characterization, including Immunohistochemistry on rodent tissue, immunocytochemistry with neuronal cultures, and immunoprecipitation, mass spectrometry, and immunoblot have been previously reported5,6,7,8 and are shown in detail in supplemental information.
Immunocytochemistry on HEK293 cells
HEK293 cells were transfected with plasmids containing rat DPPX-S (short cytoplasmic domain of 32 amino acids), DPPX-L (long cytoplasmic domain of 88 amino acids), DPPXed-myc (DPPX construct with extracellular domain deleted, and linked to a myc-tag), rat Kv4.2, human DPP10, or plasmid without insert (control).9 In other experiments, cells were co-transfected with DPPX and Kv4.2 in equimolar ratios. The reactivity of patients’ antibodies was then assessed as previously reported7 using a double immunofluorescent assay with patients’ serum or CSF and a commercial antibody against DPPX or Kv4.2 (Supplemental information).
Immunohistochemistry with wild type and DPPX-null mice
Wild-type and DPPX-null mice were generated and genotyped as previously reported.10 The brain and bowel were removed, processed, and examined by standard avidin-biotin-peroxidase immunohistochemistry or immunofluorescence using patients’ serum (1:200) or CSF (1:5) as indicated for brain and small bowel of rat (supplemental information).
Results
All 4 patients (2 male, 2 female; age range 45–76 years) developed a rapidly progressive encephalopathy characterized by agitation, delusions, hallucinations, and myoclonic jerks, which in 3 patients associated with prominent diarrhea of unclear etiology. All had confirmed or clinically suspected seizures and CSF pleocytosis with evidence of intrathecal production of IgG or oligoclonal bands. Detailed information from 3 patients showed that after multiple immunotherapies all had substantial recovery at the last follow-up (18–68 months from symptom onset); minimal follow-up information was available for the fourth patient.
Identification of the target antigen as DPPX
All 4 patients had antibodies in serum or CSF that reacted with the neuropil of brain of rodents (supplementary Figure 1) and the cell surface of live, non-permeabilized cultures of dissociated rat hippocampal neurons (Figure 1A). Immunoprecipitation of the target antigen with serum of one of the patients, followed by electrophoretic protein separation and EZBlue gel staining showed a distinct band of approximately 100 kDa that was not present in the immunoprecipitate using a control serum (Figure 1B). Excision of the band from the gel and analysis by mass spectrometry demonstrated that it contained sequences derived from DPPX (scores 6441, 5945, and 383; cutoff score for a confident protein identification ≥ 70). This finding was confirmed by immunoblotting of the precipitate with an antibody specific for DPPX (Figure 1C).
Figure 1. Immunoprecipitation of DPPX.
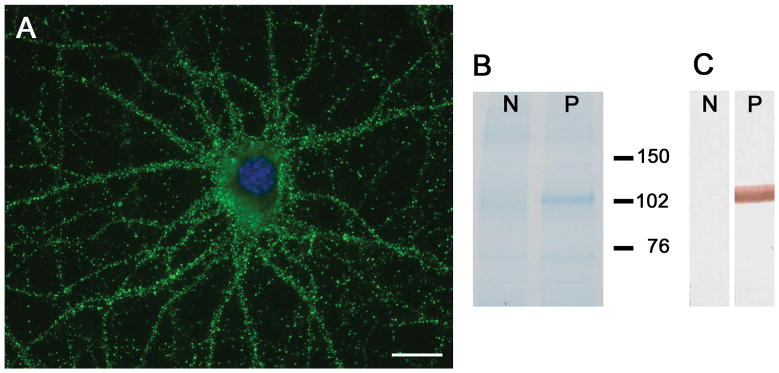
In cultures of dissociated rat hippocampal neurons, patients’ antibodies showed intense reactivity with the neuronal cell surface (A), bar = 10 μm. Immunoprecipitation of the antigen with serum of the index case is shown in B, where the precipitated proteins were run in a gel and subsequently stained with EZblue. Note that patient’s antibodies precipitated a protein (band close to 102 kDa in lane P), which was excised from the gel and analyzed by mass spectrometry, demonstrating sequences of DPPX. Lane N is the precipitate obtained from control serum. Immunoblot of these proteins with a rabbit polyclonal antibody against DPPX (1:1000, developed by BR) confirmed that the band corresponded to DPPX (C).
Immunohistochemical analysis of small bowel demonstrated that DPPX was specifically expressed by neurons of the myenteric plexus and that patients’ antibodies also reacted with DPPX expressed in these neurons (Figure 2).
Figure 2. Expression of DPPX in myenteric plexus.
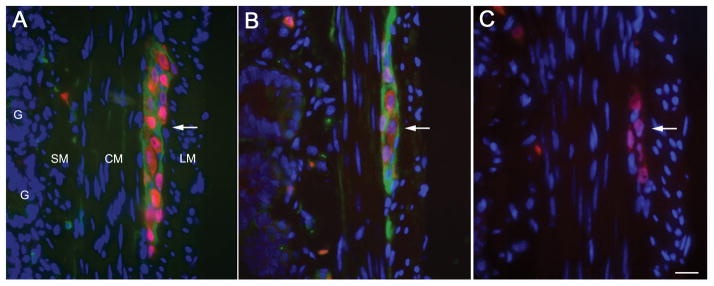
Transverse section of small bowel of rat showing the longitudinal muscular layer (LM), circular muscular layer (CM), submucosal layer (SM), and glans (G). The myenteric plexus (Plex) is revealed as clusters of large neurons between the two muscular layers. In the 3 panels (A–C) the nuclei of the neurons (red) was labeled with anti-Hu (a highly specific neuronal marker). Panel A, shows in green the DPPX immunostaining using a rabbit polyclonal antibody (1:1000, developed by BD); panel B shows the DPPX reactivity of serum from one of the patients with encephalitis, and panel C shows the lack of reactivity of serum from a healthy subject. Note that DPPX is predominantly expressed in the cytoplasm-membrane of the large clustered neurons of the myenteric plexus, and is also detected in a fine longitudinal pattern in CM and SM where the submucosal plexus is located. Bar = 20μm.
Patients’ antibodies recognize epitope regions contained in DPPX, but not Kv4.2
HEK293 cells transfected with DPPX-S or DPPX-L showed similar reactivity with patients’ serum or CSF, consistent with the recognition of an extracellular epitope (Figure 3 shows the reactivity with DPPX-L; similar reactivity was obtained with DPPX-S, not shown). Patients’ antibodies did not react with cells expressing Kv4.2 (supplementary Figure 2), and the reactivity with DPPX was not modified when it was co-expressed with Kv4.2 (data not shown). Further analysis using a DPPX plasmid in which the extracellular domain was deleted (DPPXed-myc)9 showed abrogation of reactivity with serum and CSF of patients 1 and 4, and weak reactivity with serum and CSF of patients 2 and 3, indicating that the latter two patients had antibodies against cell surface and intracellular epitopes (panels D and G of supplementary Figure 3). Although DPPX and DPP10 have 51% amino acid sequence identity,11 patients’ antibodies did not react with DPP10 (data not shown). Overall, these findings demonstrate that patients’ antibodies specifically target DPPX, but not the Kv4.2 channel, and that some patients have antibodies against both, the extracellular and intracellular domains of DPPX.
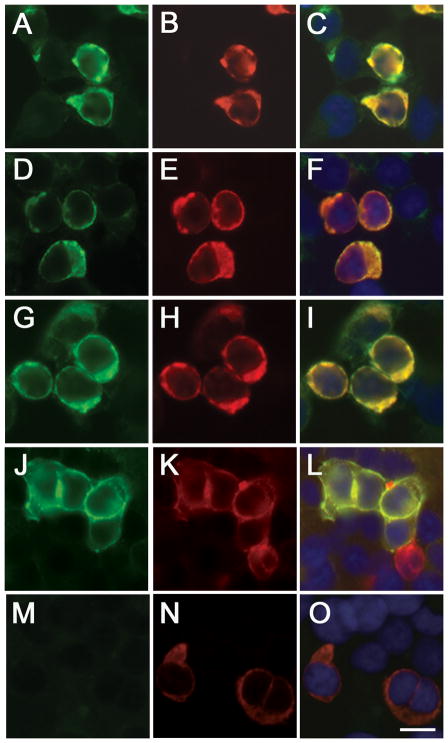
Figure 3. Analysis of DPPX antibodies using a cell-based assay.
HEK 293 cells expressing DPPX-L immunostained with patients’ serum (A, D, G, J) and a mouse monoclonal antibody against DPPX (B, E, H, K). The merged reactivities are shown in the corresponding panels (C, F, I, L). Similar studies comparing the serum of a healthy individual and the DPPX monoclonal antibody are shown in M and N, and the merged reactivities in O. Note that patient’s antibodies immunoreact with cells expressing DPPX. Bar = 10 μm.
We next determined in a cell-based assay co-expressing DPPX and Kv4.2 the reactivity of serum or CSF of the 210 controls. None of these subjects was found to have antibodies reacting against these 2 proteins, suggesting that antibodies against DPPX are specific to a subgroup of patients with autoimmune encephalitis. In contrast, a patient without encephalitis who had a thymoma and seronegative myasthenia gravis (included in the controls) had antibodies to DPP10, but not DPPX (Martinez-Hernandez, data not shown).
Analysis of patients’ antibody reactivity with brain of DPPX-null mice
To further confirm the specificity of patients’ antibodies for DPPX, immunohistochemistry with brain and bowel of wild-type mice was compared with that of DPPX-null mice. These experiments demonstrated abrogation of reactivity of serum or CSF of 3 patients (those shown in Table 1) with brain and bowel of DPPX-null mice indicating that patients’ antibodies were directed only against DPPX (Figure 4 and supplementary Figure 4). One patient had additional antibodies against a protein of unknown identity (Figure 4H).
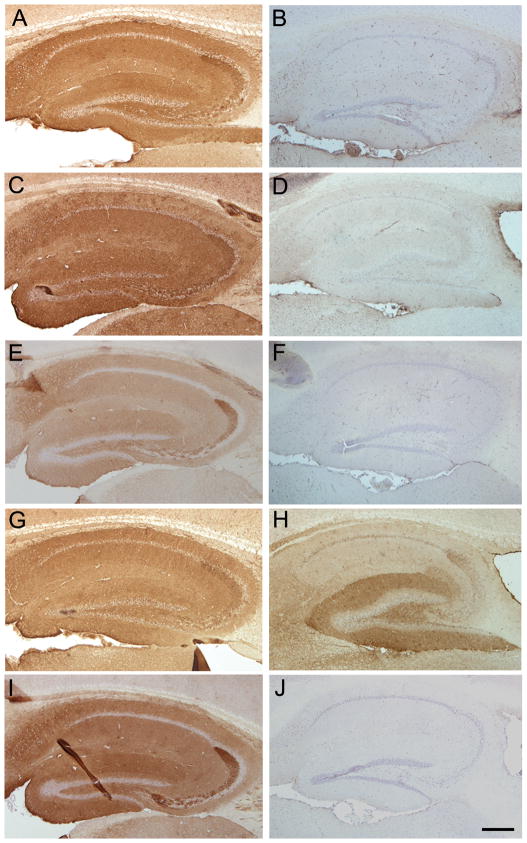
Figure 4. Comparison of patients’ serum reactivity using brain from DPPX-null mutants and wild type mice.
The reactivity of patients’ serum with the hippocampus of wild type mice is shown in A, C, E and G. The reactivity of a rabbit polyclonal DPPX antibody with the hippocampus of wild-type mice is shown in I. Panels on the right side show the results of a similar experiment but using the hippocampus of DPPX-null mice. Note that the reactivities of the sera of the first three patients (cases 1, 2, and 3 of Table 1) and the rabbit polyclonal DPPX antibody are abrogated in the hippocampus of DPPX-null mice (panels B, D, F, J). Patient 4, not included in the table (panels G and H) showed remaining reactivity with the hippocampus of DPPX-null mice indicating that this patient had two antibodies, one against DPPX and the other against an unknown antigen. Bar = 200 μm.
Discussion
We report 4 patients with a novel autoimmune disorder characterized by subacute development of cognitive dysfunction, agitation, hallucinations, confusion, resting tremor and myoclonus in association with antibodies against DPPX, a cell surface auxiliary subunit of the Kv4.2 potassium channel. In three patients, the neurological symptoms were preceded or overlapped with severe diarrhea and weight loss to the point that two patients underwent extensive endoscopic biopsies without a clear diagnosis. Support for an autoimmune etiology of this disorder is provided by the presence of cerebrospinal (CSF) pleocytosis, increased IgG index or oligoclonal bands, and the neurological response to intensive or persistent immunotherapy. Using patients’ antibodies three sets of experiments established DPPX as the main autoantigen: immunoprecipitation of DPPX from cultures of dissociated rat hippocampal neurons; immunostaining of DPPX in a cell-based assay; and comparative brain immunostaining of wild-type and DPPX-null mice, showing abrogation of reactivity of patients’ antibodies with the DPPX-null mice brain, and revealing in one patient additional antibodies to an unknown antigen.
DPPX has a critical role “tuning up” the Kv4.2 channels by remodeling channel gating.12 This type of potassium channel belongs to the mammalian Shal K+ channel family13 which has different properties compared with the Shaker K+ (Kv1) family, previously considered the target of antibody-associated limbic encephalitis, neuromyotonia, or Morvan’s syndrome (the main autoantigens are LGI1 and Caspr2).14,15 The Kv4.2 channels operate in the subthreshold range of membrane potentials.12 This somatodendritic subthreshold A-type K+ current (ISA) is a critical component of the ensemble of voltage-gated ionic currents that determine somatodendritic signal integration.16 In many neurons, action potentials that initiate in the axon hillock propagate down the axon but also backpropagate into the dendrites. In the dendritic tree, these action potentials serve as signals that report the status of the neuron’s output. The transient subthreshold ISA current in dendrites attenuates this backpropagation of action potentials. Under resting conditions ISA shuts the action potential as it tries to spread into the distal regions of the dendritic tree. However, when excitatory synaptic inputs and somatic action potentials are paired within a certain time window, the ensuing subthreshold depolarization in distal dendrites inactivates ISA, and the attenuation of backpropagating the action potential is substantially reduced. 13 It is believed that this interaction provides a coincidence detection mechanism that plays an important role in dendritic Ca++ signaling, signal integration and synaptic plasticity.12,13,16
The function of the Kv4 channels is dependent on two auxiliary subunits, the intracellular Kv-channel-interacting proteins (KChIPs),17 and the extracellular DPPX that is predominantly expressed in hippocampal pyramidal neurons and cerebellum, or DPP10 that has a different brain expression profile and is also present in pancreas.9,11 DPPX is composed of a short cytoplasmic N-terminus, a single trans-membrane domain, and a large extracellular C-terminus. Depending on the length of the cytoplasmic domain, two adult forms, DPPX-S and DPPX-L, have been identified.18,19 Consistent with the presence of antibodies against extracellular epitopes, our 4 patients’ serum and CSF equally recognized DPPX-S and DPPX-L, but two patients had additional antibodies against intracellular epitopes present in a mutant construct in which the extracellular C-terminus was deleted.
The extensive evaluation and prolonged follow-up of three patients indicate that this disorder is severe, resulting in lengthy hospitalizations or multiple relapses that usually occurred while the immunotherapy was decreased. Patient # 1 was able to return home 15 months after symptom onset, and had a clinical relapse while the prednisone was tapered. Patient #2 spent 10 months in the hospital and currently continues to receive rituximab treatments when the CD19 count increases to 1%. On one occasion delay of treatment resulted in symptom recurrence. Patient #3 had 7 relapses in 5 years, most related with attempts to decrease the dose of steroids.
The main symptoms of this disorder including, agitation, myoclonus, tremor, and seizures, although not characteristic of a specific syndrome, are compatible with neuronal hyperexcitability, and consistent with the increased excitability noted in electrophysiological studies of DPPX-knockouts.20 Interestingly, a truncation mutation of Kv4.2 identified in a patient with temporal lobe epilepsy resulted in aberrant excitability of cells expressing the mutant channel.21 Altogether, these findings suggest that genetic or immunological alteration of the DPPX-Kv4.2 complex leads to neuronal hyperexcitability. In clinical practice, the combination of the neurological symptoms indicated above with severe diarrhea and non-organ specific antibodies (e.g. ANA) may lead to a wide differential diagnosis including among other Whipple’s disease or lupus erythematosus, as occurred in our patients.
At this time the significance of the diarrhea is unclear, but this symptom is notable because it was severe, lasted for several weeks, and only occurred at the initial episode of encephalitis. Moreover, review of our experience with encephalitis suspected to be autoimmune suggests a link between diarrhea and DPPX antibody-associated encephalitis. Indeed, among 1429 cases of encephalitis of unclear etiology examined by us in the last 4 years, only 11 had severe diarrhea at symptom onset. Three of these 11 patients correspond to the cases reported here, and the other 8 did not have DPPX antibodies; none of the 1418 cases without diarrhea had serum or CSF brain reactivity as that shown by DPPX antibody positive samples (see supplementary Figure 1A). A plausible explanation for the association of diarrhea and DPPX antibody-associated encephalitis is that in some patients the immune response may result from molecular mimicry between DPPX and a yet unknown infectious agent. This paradigm would be similar to the mechanism that triggers GM1 autoantibodies in patients with Guillain-Barré syndrome and Campylobacter jejuni infection. Moreover, the robust expression of DPPX by neurons of the myenteric plexus support the possibility that patients’ antibodies may alter the function of the plexus resulting in gastrointestinal hyperactivity, similar to the CNS hyperexcitability that occur when DPPX is ablated in brain.20
A practical implication of this study is that detection of antibodies to DPPX in patients with encephalitis of unclear etiology should prompt the use of immunotherapy. Although the frequency of this disorder is unknown, the triad of diarrhea, encephalitis with signs of hyperexcitability and CSF pleocytosis will likely lead to the recognition of new cases.
Supplementary Material
Sagittal sections of rat brain immunostained with CSF of a patient (A) and a healthy individual (B). Note that the patient’s CSF shows intense reactivity with the neuropil of brain, predominantly the hippocampus and cerebellum, while control CSF produces no reactivity. Bar = 500 μm. A higher magnification of selected areas of brain immunostained with patient’s serum is shown in panels C–F, including fascia dentata and hilus (C), cerebellum (D), and cornu ammonis of hippocampus at the level of CA2 (E and F). Note the intense immunolabeling of the neuropil sparing the body of the neurons. Bars in C–E = 20 μm; bar in F = 10 μm.
HEK 293 cells expressing Kv4.2 immunostained with patients’ serum (A, D, G, J) and a rabbit polyclonal antibody (Alomone labs, #APC-023) against Kv4.2 (B, E, H, K). The merged reactivities are shown in the corresponding panels (C, F, I, L). Similar studies comparing the serum of a healthy individual and the rabbit polyclonal antibody are shown in M and N, and the merged images in O. Note that patient’s antibodies do not recognize Kv4.2. Bar = 10 μm.
HEK 293 cells expressing the mutated DPPXed-myc construct immunostained with patients’ serum (A, D, G, J) and a mouse monoclonal Myc-tag antibody diluted 1:500 (B, E, H, K). The merged reactivities are shown in the corresponding panels (C, F, I, L). Similar studies using the serum of a healthy individual and the anti-Myc-tag antibody are shown in M and N, and the merged images in O. Note that two patients (panels A and J) had antibodies that did not react with this construct indicating that the target epitopes were present only in the extracellular domain (Figure 3); in contrast, two patients had antibodies that reacted with this construct indicating that they recognized intracellular epitopes (D and G) in addition to extracellular epitopes present in the DPPX full construct (Figure 3) and in cultures of live neurons. Bar = 10 μm.
Immunostaining of myenteric plexus of wild type mice (A, B) and DPPX-null mice (C, D) with serum of a patient with anti-DPPX antibodies (A, C) and a rabbit polyclonal antibody against DPPX (B, D). The reactivity of patient’s and rabbit antibodies against DPPX is shown in green, and the reactivity of Hu (a marker of neurons) is shown in red. Note that panels C and D show lack of DPPX reactivity. Bar = 20 μm.
Acknowledgments
We thank Dr. Carol Glaser (California Encephalitis Project) for providing serum and CSF samples of 10 patients with encephalitis of unclear etiology. This work is supported in part by grants from the National Institutes of Health RO1NS077851 (JD), RO1MH094741 (RB-G and JD), the National Cancer Institute RO1CA089054 (JD), Fundació la Marató TV3 (JD), Fondo de Investigaciones Sanitarias (FIS, PI11/01780 to JD, FI08/00285 to EMH, and PS09/0193 to FG and AB), and a McKnight Neuroscience of Brain Disorders award (RB-G and JD). Dr. Dalmau has received a research grant from Euroimmun, and receives royalties from patents for the use of Ma2 and NMDAR as autoantibody test.
References
- 1.Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179–189. doi: 10.1212/WNL.0b013e318224afde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frechette ES, Zhou L, Galetta SL, Chen L, Dalmau J. Prolonged follow-up and CSF antibody titers in a patient with anti-NMDA receptor encephalitis. Neurology. 2011;76:S64–S66. doi: 10.1212/WNL.0b013e31820c34de. [DOI] [PubMed] [Google Scholar]
- 3.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The Frequency of Autoimmune N-Methyl-D-Aspartate Receptor Encephalitis Surpasses That of Individual Viral Etiologies in Young Individuals Enrolled in the California Encephalitis Project. Clin Infect Dis. 2012 doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gable MS, Gavali S, Radner A, Tilley DH, Lee B, Dyner L, et al. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis. 2009;28:1421–1429. doi: 10.1007/s10096-009-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchhalter JR, Dichter MA. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991;26:333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 7.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zagha E, Ozaita A, Chang SY, Nadal MS, Lin U, Saganich MJ, et al. DPP10 modulates Kv4-mediated A-type potassium channels. J Biol Chem. 2005;280:18853–18861. doi: 10.1074/jbc.M410613200. [DOI] [PubMed] [Google Scholar]
- 10.Clark BD, Kwon E, Maffie J, Jeong HY, Nadal M, Strop P, Rudy B. DPP6 Localization in Brain Supports Function as a Kv4 Channel Associated Protein. Front Mol Neurosci. 2008;1:8. doi: 10.3389/neuro.02.008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi SY, Riviere PJ, Trojnar J, Junien JL, Akinsanya KO. Cloning and characterization of dipeptidyl peptidase 10, a new member of an emerging subgroup of serine proteases. Biochem J. 2003;373:179–189. doi: 10.1042/BJ20021914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de ME, Ma Y, Mo W, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- 13.Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, et al. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2008;100:1835–1847. doi: 10.1152/jn.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadal MS, Amarillo Y, Vega-Saenz de ME, Rudy B. Evidence for the presence of a novel Kv4-mediated A-type K(+) channel-modifying factor. J Physiol. 2001;537:801–809. doi: 10.1111/j.1469-7793.2001.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada K, Yokotani N, Hunter C, Doi K, Wenthold RJ, Shimasaki S. Differential expression of two distinct forms of mRNA encoding members of a dipeptidyl aminopeptidase family. Proc Natl Acad Sci U S A. 1992;89:197–201. doi: 10.1073/pnas.89.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadal MS, Amarillo Y, Vega-Saenz de ME, Rudy B. Differential characterization of three alternative spliced isoforms of DPPX. Brain Res. 2006;1094:1–12. doi: 10.1016/j.brainres.2006.03.106. [DOI] [PubMed] [Google Scholar]
- 20.Kaulin YA, De Santiago-Castillo JA, Rocha CA, Nadal MS, Rudy B, Covarrubias M. The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4. 2 channels. J Neurosci. 2009;29:3242–3251. doi: 10.1523/JNEUROSCI.4767-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh B, Ogiwara I, Kaneda M, Tokonami N, Mazaki E, Baba K, et al. A Kv4. 2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol Dis. 2006;24:245–253. doi: 10.1016/j.nbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sagittal sections of rat brain immunostained with CSF of a patient (A) and a healthy individual (B). Note that the patient’s CSF shows intense reactivity with the neuropil of brain, predominantly the hippocampus and cerebellum, while control CSF produces no reactivity. Bar = 500 μm. A higher magnification of selected areas of brain immunostained with patient’s serum is shown in panels C–F, including fascia dentata and hilus (C), cerebellum (D), and cornu ammonis of hippocampus at the level of CA2 (E and F). Note the intense immunolabeling of the neuropil sparing the body of the neurons. Bars in C–E = 20 μm; bar in F = 10 μm.
HEK 293 cells expressing Kv4.2 immunostained with patients’ serum (A, D, G, J) and a rabbit polyclonal antibody (Alomone labs, #APC-023) against Kv4.2 (B, E, H, K). The merged reactivities are shown in the corresponding panels (C, F, I, L). Similar studies comparing the serum of a healthy individual and the rabbit polyclonal antibody are shown in M and N, and the merged images in O. Note that patient’s antibodies do not recognize Kv4.2. Bar = 10 μm.
HEK 293 cells expressing the mutated DPPXed-myc construct immunostained with patients’ serum (A, D, G, J) and a mouse monoclonal Myc-tag antibody diluted 1:500 (B, E, H, K). The merged reactivities are shown in the corresponding panels (C, F, I, L). Similar studies using the serum of a healthy individual and the anti-Myc-tag antibody are shown in M and N, and the merged images in O. Note that two patients (panels A and J) had antibodies that did not react with this construct indicating that the target epitopes were present only in the extracellular domain (Figure 3); in contrast, two patients had antibodies that reacted with this construct indicating that they recognized intracellular epitopes (D and G) in addition to extracellular epitopes present in the DPPX full construct (Figure 3) and in cultures of live neurons. Bar = 10 μm.
Immunostaining of myenteric plexus of wild type mice (A, B) and DPPX-null mice (C, D) with serum of a patient with anti-DPPX antibodies (A, C) and a rabbit polyclonal antibody against DPPX (B, D). The reactivity of patient’s and rabbit antibodies against DPPX is shown in green, and the reactivity of Hu (a marker of neurons) is shown in red. Note that panels C and D show lack of DPPX reactivity. Bar = 20 μm.