To the Editor
Traffic-related air pollutants, such as diesel exhaust particles (DEP), significantly contribute to the pathogenesis of wheezing and asthma in early childhood.1 These illnesses are characterized by chronic airway inflammation caused by a dysregulated immune system.2 Attention has recently been directed towards regulatory T cells (Tregs) because they are important in suppressing immune responses against non-specific stimuli3, such as DEP. The suppressive phenotype of Tregs is conferred by stable expression of the forkhead box protein 3 (FOXP3).3 Transcriptional silencing of FOXP3, via hypermethylation of CpG islands in the promoter and intronic regions, has been identified as a hallmark of committed Tregs and human diseases including asthma.3,4 As such, Nadeau et al.4 reported increased FOXP3 hypermethylation in blood DNA to be associated with diminished Treg function and increased asthma severity in children exposed to polycyclic aromatic hydrocarbons, a component of DEP. In this study, we test the novel hypothesis that early (birth) and consistent exposure to high levels of traffic pollution alters FOXP3 methylation status, in saliva DNA, in a manner that correlates with DEP exposure and/or predicts wheezing/asthma in later-life. The oral cavity provides an important first line of defense against DEP exposure for children, as mouth breathing is a common path of exposure.5 Further, other aerodigestive track tissues, such as buccal cells, have been successful in characterizing DNA methylation with respect to air pollutants and airway inflammation.6,7 The ancillary goal is to establish a non-invasive, high throughput and quantitative assay for measuring risk of developing asthma linked to traffic-related air pollution.
A subset of children (n=92) were selected from the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) 8 using stringent inclusion criteria as described in the online repository. Inclusion required children to have a consistent DEP exposure throughout childhood and no exposure to environmental tobacco smoke. DEP exposure was calculated by estimating each child’s exposure to elemental carbon attributable to traffic (ECAT), a proxy for DEP, using a land-use regression model. 9 The model captures all subsequent changes in exposure throughout childhood including exposures outside of the home.
At age seven, an asthma diagnosis was based on symptom history, spirometry, exhaled nitric oxide, and airway reversibility.10 Wheezing phenotypes were defined as follows: 1) never wheeze - No parental report of wheeze in the previous 12 months at ages one through four and at age seven, 2) early transient wheeze - parental report of wheeze at ages one or two or three or four but not at age seven, and 3) persistent wheeze - parental report of wheeze at age seven and at least one age at or prior to four. DNA was extracted from saliva and was bisulfite-converted using the EZ Methylation Kit (Zymo Research, Irvine, CA). The methylation status of the only CpG island in the 5′ region of FOXP3 (chromosome X: 49125644-49125671) was measured by quantitative pyrosequencing using a Qiagen PyroMark CpG Assay (PM00032620) in the Q96 MD system (Qiagen). This region encompasses 4 CpG sites in the 3′ end of the island.
Chi-square tests or t-tests were performed to investigate differences in binary/categorical and continuous variables, respectively, between the study cohort and study sample. Univariate analyses were conducted to assess the association between independent variables (DEP exposure or FOXP3 methylation) and potential covariates (race, gender, maternal education, child obesity and parental history of asthma, as defined in the online repository) with each dependent variable (FOXP3 methylation, longitudinal wheezing phenotypes, or asthma). Other covariates not collected, such as dietary intake, could affect the epigenetic state of FOXP3 and this is acknowledged as a limitation.
FOXP3 methylation was not normally distributed; therefore, a negative binomial regression model was applied. The final model included variables and product terms significant at the 15% level. The adjusted β-coefficients and 95% confidence intervals are presented for an interquartile range (IQR) increase (0.14 μg/m3) in DEP. Multinomial logistic regression was used to assess the relationship between FOXP3 methylation and respiratory outcomes. The parameter estimate and standard error for methylation changed less than 15% when adjusted for covariates and interactions; therefore, unadjusted odds ratios are reported. The odds of each outcome represent a one standard deviation increase (17.4%) in methylation. For all analyses a 5% alpha level indicated significance. Analyses were performed using SAS, Version 9.2 (SAS Institute, Cary, NC).
The sample was representative of the cohort population with regard to gender, race, and prevalence of respiratory outcomes (Table E1 in the online repository). The prevalence of persistent and early transient wheezing was 16.3% and 35.8%, respectively, and 16.3% of the children were considered asthmatic. The average daily exposure to DEP during the first 12 months of life was 0.38 μg/m3 (Table E2 in the online repository). FOXP3 methylation ranged from 0–62.4% (SD: 17.4%). A significant two-fold increase in the mean level of FOXP3 methylation was observed among persistent wheezers compared with non-wheezers (p <0.01) (Table 1). Similarly, early transient wheezers and asthmatic children had significantly higher mean levels of FOXP3 methylation compared with non-wheezers (p=0.04) and non-asthmatic children (p=0.02).
Table 1.
Distribution of FOXP3 %methylation in sample population and stratified by respiratory outcomes
| Description | Mean | Min | Max | Std Dev |
|---|---|---|---|---|
| Study Sample | 21.30 | 0.00 | 62.40 | 17.40 |
| Wheezing Phenotype | ||||
| Non-wheezers | 17.00 | 0.00 | 55.10 | 16.20 |
| Persistent wheezers* | 35.84 | 18.10 | 58.35 | 15.20 |
| Early Transient wheezers* | 24.16 | 0.32 | 61.30 | 17.90 |
| Asthma Status | ||||
| Non-asthmatic | 19.50 | 0.00 | 62.40 | 16.90 |
| Asthmatic** | 32.70 | 1.10 | 58.40 | 17.90 |
Significant difference between persistent wheezers (p<0.01) and early transient wheezers (p<0.05) compared with non-wheezers
Significant difference between asthmatic and non-asthmatic children (p<0.05)
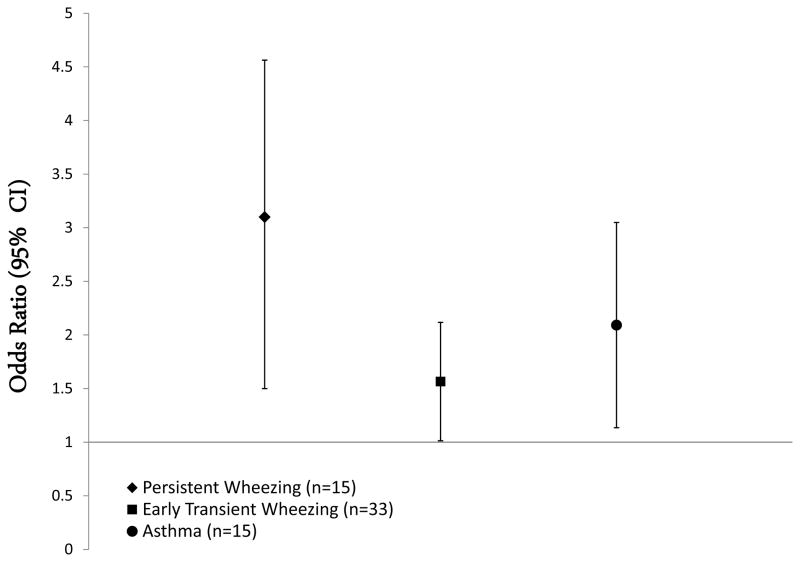
The final negative binomial regression model showed a 4.01% (95% CI: 1.83–6.18) increase in FOXP3 methylation per IQR increase in estimated DEP exposure, while controlling for gender and obesity. Increased FOXP3 methylation was associated with three times the risk of experiencing persistent wheezing during childhood (OR=3.05; 95% CI, 1.54–6.05), compared with children who had never wheezed (Figure 1). Similarly, increased FOXP3 methylation was associated with increased risk for early transient wheezing (OR=1.57; 95% CI, 1.01–2.41). Children with increased FOXP3 methylation were also two times more likely to develop asthma than children with lower FOXP3 methylation (OR=2.12; 95% CI, 1.14–3.85).
Figure 1. Unadjusted Association between FOXP3 %Methylation* and Wheezing Phenotypes and Asthma at Age Seven.
*OR’s calculated for a standard deviation increase in %Methylation
This study provides the first evidence that FOXP3 methylation is associated with chronic DEP exposure during childhood and increased risk for developing persistent wheezing and asthma. Further, we were able to identify epigenetic changes associated with environmental exposure and respiratory disease in saliva. This is an important finding as it represents a less invasive and inexpensive procedure for collecting DNA, which is highly conducive to large epidemiological studies of children. These findings support future strategies targeting Treg expansion through boosting FOXP3 up-regulation for asthma prevention/intervention.
Acknowledgments
This study was supported in part by grants funded by the National Institute of Environmental Health Sciences (T32ES010957, R01ES11170, R01ES015584, U01ES019480, U01ES020988 and P30ES006096, P50ES015905), the Environmental Protection Agency (S.T.A.R. Fellowship FP91736701) and the Veteran Affairs (I01BX000675).
Abbreviations
- DEP
Diesel exhaust particles
- FOXP3
Forkhead box protein 3
- CCAAPS
Cincinnati Childhood Allergy and Air Pollution Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jerrett M, Shankardass K, Berhane K, Gauderman WJ, Künzli N, Avol E, et al. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116:1433–1438. doi: 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 3.Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007;120:227–235. doi: 10.1016/j.jaci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4):845–52. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health A. 2008;71(3):238–43. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- 6.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180(5):462–7. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breton CV, Byun HM, Wang X, Salam MT, Siegmund K, Gilliland FD. DNA methylation in the arginase-nitric oxide synthase pathway is associated with exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med. 2011;15;184(2):191–7. doi: 10.1164/rccm.201012-2029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeMasters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149:505–11. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan PH, LeMasters GK, Levin L, Burkle J, Biswas P, Hu S, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ. 2008;404(1):139–47. doi: 10.1016/j.scitotenv.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 10.Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, et al. High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. Ann Allergy Asthma Immunol. 2011;107(2):120–6. doi: 10.1016/j.anai.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]