Abstract
Objective
Interleukin-33 (IL-33) is the newest member of the IL-1 cytokine family, a group of key regulators of inflammation. The purpose of this study was to determine whether IL-33 is expressed in the human placenta and to investigate its expression in the context of acute and chronic chorioamnionitis.
Methods
Placental tissues were obtained from five groups of patients: (1) normal pregnancy at term without labor (n=10); (2) normal pregnancy at term in labor (n=10); (3) preterm labor without inflammation (n=10); (4) preterm labor with acute chorioamnionitis (n=10); and (5) preterm labor with chronic chorioamnionitis (n=10). Immunostaining was performed to determine IL-33 protein expression patterns in the placental disk, chorioamniotic membranes, and umbilical cord. mRNA expression of IL-33 and its receptor IL1RL1 (ST2) was measured in primary amnion epithelial and mesenchymal cells (AECs and AMCs, n=4) and human umbilical vein endothelial cells (HUVECs, n=4) treated with IL-1β (1ng/ml and 10ng/ml) and CXCL10 (0.5ng/ml and 1ng/ml or 5ng/ml).
Results
1) Nuclear IL-33 expression was found in endothelial and smooth muscle cells in the placenta, chorioamniotic membranes, and umbilical cord; 2) IL-33 was detected in the nucleus of CD14+ macrophages in the chorioamniotic membranes, chorionic plate, and umbilical cord, and in the cytoplasm of myofibroblasts in the Wharton’s jelly; 3) acute (but not chronic) chorioamnionitis was associated with the presence of IL-33+ macrophages in the chorioamniotic membranes and umbilical cord; 4) expression of IL-33 or IL1RL1 (ST2) mRNA in AECs was undetectable; 5) IL-33 mRNA expression increased in AMCs and HUVECs after IL-1β treatment but did not change with CXCL10 treatment; and 6) IL1RL1 (ST2) expression decreased in AMCs and increased in HUVECs after IL-1β but not CXCL10 treatment.
Conclusions
IL-33 is expressed in the nucleus of placental endothelial cells, CD14+ macrophages, and myofibroblasts in the Wharton’s jelly. IL-1β can induce the expression of IL-33 and its receptor. Protein expression of IL-33 is detectable in macrophages of the chorioamniotic membranes in acute (but not chronic) chorioamnionitis.
Keywords: alarmin, chorioamnionitis, interleukin-1 family, pregnancy, preterm birth, preterm labor
Introduction
Inflammation is a key feature of “the great obstetrical syndromes”, which are associated with increased maternal and perinatal mortality and morbidity [1,2]. Systemic inflammation in the mother has been observed in preeclampsia [3,4], preterm labor [5,6], preterm prelabor rupture of the membranes [7], fetal death [8–10], pyelonephritis [11], and small-for-gestational-age (SGA) pregnancies [12]. Systemic inflammation and multi-organ involvement of the fetus have been described in preterm labor with intact membranes, preterm prelabor rupture of the membranes, and fetal viral infections, and have been termed as the fetal inflammatory response syndrome (FIRS) [13–16]. Localized inflammation at the maternal-fetal interface has also been observed in various obstetrical syndromes, including preeclampsia [17–23], spontaneous preterm birth [5,24–33] and unexplained fetal death [34].
The most common inflammatory lesion in the placenta in spontaneous preterm birth is acute chorioamnionitis (ACA), while chronic chorioamnionitis (CCA) is a major placental inflammatory lesion in cases of late spontaneous preterm birth [5,26,35]. ACA often results from microbial invasion of the amniotic cavity (MIAC) [27,36–48], and is characterized by amniotropic infiltration of maternal neutrophils into the chorioamniotic membranes [49,50]. Acute chorioamnionitis and intra-amniotic infection are associated with adverse pregnancy outcomes, including short-term neonatal complications [28,30,51–63], as well as neurologic disorders (e.g., cerebral palsy [16,29,64–72]) and chronic lung disease [73–77]). Chronic chorioamnionitis is defined as amniotropic infiltration of maternal T cells into the chorioamniotic membranes [24,25]. Chronic chorioamnionitis is associated with preterm labor and preterm prelabor rupture of membranes, and higher amniotic fluid concentrations of the chemokine CXCL10, as well as increased mRNA expression of T-cell chemokines CXCL9, CXCL10, and CXCL11 in the chorioamniotic membranes [26]. CXCL9, CXCL10, and CXCL11 are functionally involved in allograft rejection [78–81] and exert their effects by binding to CXCR3, present in T cells and natural killer cells [82,83]. CCA shares a common pathogenesis with villitis of unknown etiology (VUE). CCA and VUE have been reported in patients with fetal HLA-specific maternal HLA alloantibodies [32,33]. Therefore, CCA and VUE are considered as maternal anti-fetal rejection involving chorioamniotic membranes and the villous placenta, respectively [26,32,33,84]. Additionally, systemic T-cell-mediated cytotoxicity has been shown to increase in pregnant women with CCA as a manifestation of maternal anti-fetal rejection [85].
Cytokines of the interleukin-1 (IL-1) family play important roles in immune regulation and inflammation for several types of tissues, and have been implicated in several disease states [86–88]. Members of this family include IL-1α, IL-1β, the natural IL-1 receptor antagonist (IL-1RA), IL-18, the IL-18 binding protein (IL-18BP), and a newly described molecule, interleukin-33 (IL-33). IL-1 family members bind to specific IL-1 receptors, including IL-1 receptor types I and II (IL-1RI and IL-1RII) for IL-1, and the IL-18 receptor (IL-18R) for IL-18. Additional binding of their respective associated proteins, the IL-1R accessory protein (IL-1RAcP) and IL-18 receptor accessory protein (IL-18RAcP), regulate cytokine activity [89–91].
IL-1 was the first cytokine to be implicated in the mechanisms of preterm parturition associated with infection/acute inflammation and spontaneous parturition at term [92]. Members of the pro-inflammatory cytokine IL-1 family are important mediators in ACA [28,51,55,56,59,60,62,92,93], as IL-1 expression increases in response to microorganisms and bacterial products (e.g., endotoxin) [30,54]. IL-1β can induce both prostaglandin production in the amnion, chorion, and decidual and myometrial cells [58,60,94,95], and in the onset of labor when administered to pregnant mice. Moreover, amniotic fluid IL-1α and IL-1β bioactivity [59] and concentrations [55,62] are elevated in women with preterm labor and MIAC, and IL-1 administration to pregnant animals results in preterm labor [52,61,63]. In addition, the robust up-regulation of amniotic fluid IL-1 is a feature of spontaneous term parturition [92,96,97]. IL-1-induced preterm delivery is mediated by the IL-1 receptor [98]. The natural IL-1 receptor antagonist, present in fetal, maternal, and amniotic fluid compartments during pregnancy, has been shown to reduce IL-1β-induced prostaglandin production in the amnion and chorion [98–100]. Similarly, the natural IL-1 receptor antagonist has been shown to prevent IL-1-induced preterm delivery in mice [101], but this was not true of endotoxin-induced preterm labor and delivery in mice [102]. Lastly, IL-18, a member of the IL-1 cytokine family, is also detectable in fetal, maternal, and amniotic fluid compartments, increases with MIAC [103], and may play a role in host defense against infection [103,104].
In 2005, Schmitz et al.[91] identified IL-33 as a new member of the IL-1 family, based upon its structural similarities to IL-18 and IL-1β. IL-33 has been shown to lead to the production of pro-inflammatory and TH2-associated cytokines, and to increase serum immunoglobulin concentrations by signaling through IL-1R family member IL1RL1 (also known as ST2 [91]), which belongs to the Toll-like receptor (TLR)/IL-1R (TIR) superfamily. Moreover, Carrière et al.[105] showed that IL-33 is a nuclear factor which possesses strong transcriptional repressor properties. Thus, IL-33, like IL-1α, may function as both a pro-inflammatory cytokine and a transcription factor [106–108].
Like IL-1β and IL-18, IL-33 was originally thought to be activated by caspase-1 [91,106,107]. It has been suggested that infection may induce caspase-1 production and activation of the inflammasome, leading to IL-1β processing and secretion, and activation of the common pathway of parturition [109–111]. However, while caspase-1 cleaves IL-33 from pro-IL-33 in vitro [91], the effect is lost in the absence of all other proteases [112], suggesting that activation of other proteases by caspase-1 [113] may be responsible for the previously observed effect. IL-33 is also cleaved by caspase-3 [114,115] and caspase-7 [114], and it is thought that IL-33 cleavage may be mediated by calpain [116].
Pro-IL-33 has a different caspase cleavage site from those described for pro-IL-1β and pro-IL-18. Protease-induced cleavage of IL-33 results in a molecule that does not have cytokine-like activity and is not actively secreted. This is a major difference between IL-33 and IL-1β, and IL-33 and IL-18. Indeed, IL-33 is sequestered in apoptotic cells [112,114,115] which do not generally induce inflammation; thus, it has been suggested that the inactivation of IL-33 may be a mechanism to limit the release of bioactive, full-length IL-33 from apoptotic cells [107].
Similar to full-length pro-IL-1α [117,118], pro-IL-33 is sequestered in the nucleus [105–108] and passively secreted from necrotic cells, thereby becoming biologically active and initiating inflammation [105–108,114,115]. High motility group box 1 (HMGB1) is another protein with properties similar to IL-33 in that it is also localized in the nucleus [119,120], released by necrotic cells [121], and inactivated during apoptosis [122]. Both HMGB1 and IL-1α are classified as “damage associated molecular patterns” (DAMPS), also known as alarmins, which are endogenous danger signals released by necrotic cells in injured tissues during trauma or infection and elicit an immune response [123,124]. Thus, given its similarities to IL-1α and HMGB1, IL-33 has been considered to act as a DAMP/alarmin [125–127]. Previously, we have shown that HMGB1 released from stressed or injured cells into amniotic fluid may be responsible, in part, for intra-amniotic inflammation due to non-microbial insults [128]. Since IL-33 has been implicated in the development of a wide variety of human inflammatory diseases including allergy, asthma, arthritis, and ulcerative colitis [106–108], it is possible that IL-33 may also play a role in pathological inflammation at the maternal-fetal interface. This study was conducted to investigate the expression patterns of IL-33 in the human placenta and changes associated with acute chorioamnionitis and chronic chorioamnionitis.
Materials and Methods
Study Design
A cross-sectional study was designed to evaluate IL-33 expression patterns in the human placenta, chorioamniotic membranes, and umbilical cord of normal and pathologic pregnancies. Tissue samples were retrieved from the Bank of Biological Materials of the Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, and the Wayne State University.
Placenta, chorioamniotic membranes and umbilical cord samples were collected from five study groups: (1) normal pregnancy at term not in labor (TNL; n=10); (2) normal pregnancy at term in labor (TIL; n=10); (3) preterm labor without inflammation (PTL; n=10); (4) preterm labor with acute chorioamnionitis and funisitis (PTL-ACA; n=10); and (5) preterm labor with chronic chorioamnionitis (PTL-CCA; n=10). Preterm labor was defined as the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical changes that led to delivery before 37 weeks of gestation [129]. Placentas from patients with preterm labor were derived from gestational-age-matched groups. All patients had singleton gestations with intact membranes at the time of onset of preterm labor. Patients with medical complications or fetal congenital or chromosomal abnormalities were excluded. Criteria for the diagnosis of acute chorioamnionitis, funisitis [130], and chronic chorioamnionitis [26] have been previously described. All patients provided written informed consent prior to the collection of clinical data and tissue samples. Collection and utilization of samples for research purposes were approved by the Institutional Review Boards of the NICHD and Wayne State University.
Immunohistochemistry
Immunohistochemistry (IHC) was performed to determine IL-33 protein expression and localization of expression in the placenta, chorioamniotic membranes and umbilical cord. Five-micrometer-thick sections of formalin-fixed, paraffin-embedded tissues were placed on silanized slides and stained using a Ventana Discovery automatic staining system (Ventana Medical Systems, Tucson, AZ, USA). Immunostaining was performed using a mouse monoclonal anti-IL-33 antibody (1:200, Alexis Biochemicals, Lausen, Switzerland) and a Discovery™ Universal Secondary Antibody (Ventana Medical Systems). The Discovery® DAB Map™ Kit (Ventana Medical Systems) was used to detect the chromogenic reaction of horseradish peroxidase (HRP).
Immunofluorescence staining
Immunofluorescence (IF) staining was conducted on frozen sections of placentas, chorioamniotic membranes and umbilical cord specimens to identify IL-33-positive cells. Five-micrometer-thick frozen sections were fixed with 4% (w/v) paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 30 min at room temperature, permeabilized with 0.25% Triton X-100 (Promega, Fitchburg, WI, USA) for 5 min at room temperature, and incubated with 5% (w/v) BSA (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) in PBS (Invitrogen, Carlsbad, CA, USA) for 30 min at room temperature. Immunofluorescence staining was performed using a rabbit polyclonal anti-IL-33 antibody (1:200, Alexis Biochemicals), mouse monoclonal anti-CD31 antibody (1:1000, Dako, Glostrup, Denmark), mouse monoclonal anti-CD14 antibody (1:1000, Abcam, Cambridge, MA, USA), mouse monoclonal anti-cytokeratin 7 antibody (1:1000, Dako), and mouse monoclonal anti-type 1 procollagen antibody (1:50, Developmental Studies Hybridoma Bank at The University of Iowa, Iowa City, IA, USA). Sections were incubated with primary antibodies in 1% (w/v) BSA in PBS for 1 h, followed by incubation with Alexa Fluor 488 goat anti-mouse IgG (Invitrogen) or Alexa Fluor 594 donkey anti-rabbit IgG (Invitrogen) in 1% (w/v) BSA for 30 min. Sections were mounted in ProLong® Gold antifade reagent with DAPI (Invitrogen). Stained sections were examined using a Leica TCS SP5 MP spectral confocal system (Leica Microsystems, Wetzlar, Germany).
Primary amnion epithelial and mesenchymal cell cultures
The method for isolation and primary culture of amnion epithelial cells (AECs) and amnion mesenchymal cells (AMCs) used in our laboratory has been previously described in detail [131]. Briefly, reflected amnion (n=4) was separated from the chorion and washed with PBS (Invitrogen) before and after being cut into 2 cm × 2 cm pieces. These amnion fragments were transferred into two tubes containing 25 ml of collagenase A (1 mg/ml; Roche, Basel, Switzerland) and incubated at 37°C with gentle shaking for 3 h. Amnion digests were then filtered through a 100-micron nylon mesh and centrifuged at 2,500xg for 10 min for the collection of AMCs. These AMCs were washed and suspended in DMEM (Mediatech, Herndon, VA, USA), then supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 UI/ml penicillin and 100 μg/ml streptomycin (Invitrogen) for culture. Remaining amnion fragments were then placed in 10 ml of 0.05% (w/v) trypsin/EDTA (Invitrogen) and gently shaken for 30 sec. Fragments were transferred into two new tubes for isolation of AECs, and 15 ml of trypsin/EDTA were added, followed by incubation at 37°C with gentle shaking for 10 min. This trypsin digestion supernatant was discarded. Amnion fragments were transferred into a new tube containing 25 ml of fresh trypsin/EDTA solution and incubated for an additional 40 min at 37°C. After digestion, the supernatant was mixed with an equal volume of DMEM and centrifuged for 10 min at 200xg, 4°C. The pellet was resuspended with 10 ml of DMEM. Remaining amnion trypsin digests were treated again with 25 ml of trypsin/EDTA at 37°C for 40 min. AECs were collected and pooled with the previous cell suspension. AECs and AMCs were cultured in DMEM at 37°C in a humidified incubator containing 5% CO2. All experiments were performed with the cells at passages 2 and 4.
Primary human umbilical vein endothelial cell (HUVEC) culture
Samples of human umbilical cord (n=4) were obtained from patients immediately after delivery, and umbilical vein endothelial cells were isolated as previously described [132,133] Briefly, sections of umbilical cord were trimmed at both ends with a scalpel. A cannula was introduced at one end of the vein, which was then perfused with PBS (Invitrogen) using a 20 ml syringe to wash the vein of red blood cells. A second cannula attached to a syringe was introduced at the other end of the vein. Collagenase A (0.2% w/v in Hanks Balanced Salt Solution, Invitrogen) was then injected into one end of the vein using a 30 ml syringe (~10–12ml/cord). Clean aluminum foil was used to maintain syringes at either end of the cord, which was then placed into a 400 ml beaker containing 200 ml of PBS with 1x Antibiotic-Antimyotic Buffer (Invitrogen) and pre-incubated in a 37°C water bath for 10 min. After incubation, the umbilical cord was gently squeezed to facilitate endothelial cell detachment. Collagenase solution containing endothelial cells was then flushed from the cord by perfusion with 40 ml of PBS. Effluent was collected in a sterile 50 ml tube containing 10 ml of F-12K culture medium (Invitrogen) supplemented with 20% FBS (Invitrogen) and 100 UI/ml penicillin and 100 μg/ml streptomycin (Invitrogen). The cells were centrifuged for 5 min at 1,500xg, after which the medium was discarded and endothelial cells were resuspended in 5 ml of fresh culture medium. Cells were then incubated in a T25 cell culture flask (BD Biosciences, Franklin Lakes, NJ, USA) at 37°C in a humidified atmosphere containing 5% CO2. Culture medium was replaced at 24 h to remove non-adherent cells, and then every two days until the cells achieved approximately 70% confluence. Cells were then transferred into a T75 flask (BD Biosciences) and grown to confluence. Experiments were performed with the cells at passages 3 and 4.
Cytokine and chemokine treatment of AECs, AMCs, and HUVECs
Cells were plated in 6-well cell culture plates (BD Biosciences) at a density of 1 × 106 cells per well. AECs and AMCs were plated in RPMI 1640 media (Invitrogen) supplemented with 10 % FBS and 100 UI/ml penicillin and 100 μg/ml streptomycin. AECs and AMCs were treated with recombinant IL-1β (R&D Systems, Minneapolis, MN, USA) at a concentration of 1 ng/ml or 10 ng/ml for 18 hours. The concentration of recombinant CXCL10 (R&D Systems) was 0.5 ng/ml or 1 ng/ml. HUVECs were plated in F-12K (Invitrogen) media supplemented with 20% FBS, 0.1 mg/ml heparin (Sigma), 60 μg/ml Endothelial Cell Growth Supplement (ECGS, Sigma), and 100 UI/ml penicillin and 100 μg/ml streptomycin (Invitrogen). HUVECs were treated for 18 hours with IL-1β (R&D Systems) at 1 ng/ml or 10 ng/ml, or CXCL10 (R&D Systems) at 0.5 ng/ml or 5 ng/ml. All cells were then collected for isolation of RNA for qRT-PCR.
RNA isolation, cDNA generation and quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNAs was isolated from AECs, AMCs, and HUVECs by using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Isolated RNA was reverse transcribed using the ImpromII Reverse Transcription System (Promega). All qRT-PCR analyses were carried out using TaqMan assays (Applied Biosystems, Foster City, CA, USA) using 50 ng of cDNA. Human RPLP0 (large ribosomal protein) Endogenous Control (4326314E, Applied Biosystems) was used for the normalization of IL-33 mRNA expression (Hs00369211_m1, Applied Biosystems) and IL1RL1 (ST2) mRNA expression (IL1RL1, Hs00545033_m1, Applied Biosystems). qRT-PCR reactions were performed using the 7500 Fast Real-Time PCR System (Applied Biosystems).
Statistical analysis
Clinical and demographic characteristics were compared among the groups using a Kruskal–Wallis test, followed by the Mann–Whitney U-test for continuous variables, as well as the chi-square or Fisher’s exact test to compare proportions. The effect of a given treatment (e.g. IL-1β, CXCL10) on gene expression levels was tested using Linear Mixed-Effects (LME) models implemented in the R package nlme [134] In these models, the dependent variable was the gene expression level (−DCt values), and the fixed effects included the differences between treatment groups (dose 1 vs. control and dose 2 vs. control), while random effects were assigned to each of the four individuals from which the samples were collected. A p value of <0.05 was considered statistically significant for all comparisons.
Results
I. IL-33 protein expression in normal term pregnancies
IL-33 protein was primarily localized in the nuclei of endothelial and smooth muscle cells of the blood vessels in the chorionic plate, chorionic villi, and basal plate (Fig. 1A and 1B). Villous and extravillous trophoblasts and Hofbauer cells (placental macrophages) were negative for IL-33, which was verified by double-staining with IL-33 and cytokeratin or CD14 (data not shown).
Figure 1. IL-33 protein expression in the placenta and chorioamniotic membranes of normal term pregnancies.
(A) IL-33 is expressed in the nuclei of endothelial (arrowhead) and smooth muscle cells (arrow) of blood vessels in the chorionic plate and chorionic villi. CP = chorionic plate; CV = chorionic villi. x200 magnification. (B) Immunofluorescence staining of CD31 (red), IL-33 (green) and DAPI (blue) shows nuclear IL-33 staining of vascular endothelial cells in chorionic villi. x900 magnification. (C) IL-33 is expressed in the nuclei of decidual endothelial cells (arrowhead) in blood vessels in chorioamniotic membranes. AE = amnion epithelium; AM = amnion mesoderm; CM = chorionic mesoderm; CT = chorionic trophoblast layer; DP = decidua parietalis. x200 magnification. (D) Immunofluorescence staining of CD31 (red), IL-33 (green) and DAPI (blue) show nuclear IL-33 staining in endothelial cells of decidual blood vessels. x400 magnification. (E) Immunofluorescence staining of CD14 (red), IL-33 (green) and DAPI (blue) shows nuclear IL-33 staining in macrophages in the mesodermal layer. x1500 magnification.
Immunoreactivity for IL-33 was readily detectable in the endothelial cell nuclei of decidual blood vessels found in the chorioamniotic membranes. AECs, trophoblasts in the chorion laeve, and decidual cells were negative for IL-33 expression (Fig. 1C and 1D). IL-33 nuclear immunoreactivity was also detected in the nuclei of chorioamniotic mesenchymal cells in 60% of the placentas from normal pregnant women who delivered at term. To characterize the IL-33-positive mesenchymal cells, we performed a series of immunofluorescence stainings using antibodies against IL-33, CD14, or procollagen. IL-33-positive mesenchymal cells were positive for CD14 (Fig. 1E), but not for procollagen, indicating that these cells were macrophages and not myofibroblasts.
IL-33 protein expression was localized in the nuclei of endothelial and smooth muscle cells in the umbilical veins and arteries (Fig. 2A and 2B). The nuclei of stromal cells in the Wharton’s jelly were positive for IL-33, which was observed in 15% of the umbilical cords from normal pregnant women who delivered at term (Fig. 2C). Immunofluorescence staining revealed cytoplasmic IL-33 expression in several myofibroblasts stained with procollagen (Fig. 2D) and CD14 positive macrophages of the Wharton’s jelly (Fig. 2E). Nuclei of these cells were not positive for IL-33.
Figure 2. IL-33 protein expression in the umbilical cord of normal term pregnancies.

(A) IL-33 is expressed in the umbilical artery (B) and Wharton’s jelly (C). x100 magnification. (B) IL-33 is expressed in the nuclei of endothelial (arrowhead) and smooth muscle cells (arrow) in the umbilical vessels. x200 magnification. (C) IL-33 is expressed in the nuclei of stromal cells in the Wharton’s jelly (inset). x100 magnification. (D) Immunofluorescence staining of procollagen (red), IL-33 (green) and DAPI (blue) shows cytoplasmic IL-33 staining in myofibroblasts in the Wharton’s jelly. x630 magnification. (E) Immunofluorescence staining of CD14 (red), IL-33 (green) and DAPI (blue) shows cytoplasmic IL-33 staining in macrophages in the Wharton’s jelly. x630 magnification. UA = umbilical artery; WJ = Wharton’s jelly.
IL-33 immuno-localization in the placenta, chorioamniotic membranes, and umbilical cord from normal pregnant women who delivered at term did not differ according to the presence or absence of labor. Placentas and chorioamniotic membranes of patients with preterm labor without inflammation had a staining pattern for IL-33 similar to that of normal pregnant women who delivered at term. However, nuclear IL-33 staining of macrophages in the chorioamniotic membranes and stromal cells of the Wharton’s jelly was not observed in cases of preterm labor without evidence of inflammation (data not shown).
II. Placental IL-33 expression in acute and chronic inflammation
In cases of preterm labor and acute chorioamnionitis (PTL-ACA), IL-33 expression was found in the chorioamniotic membranes and umbilical cord, but not in the placental disk. Forty percent of cases with preterm labor and acute chorioamnionitis showed macrophages with IL-33-positive nuclei in the chorioamniotic membranes compared to none of the cases with PTL, indicating that IL-33 expression in macrophages increases with acute chorioamnionitis (Fig. 3A and 3B). Additionally, IL-33 staining in the stromal cells of the Wharton’s jelly was apparent in 40% of cases with preterm labor and acute chorioamnionitis, but this was not observed in any cases with preterm labor without inflammation. There was no difference in IL-33 expression in the placentas of patients with preterm labor and acute chorioamnionitis compared to those with preterm labor without inflammation.
Figure 3. IL-33 expression in pathologic pregnancies.
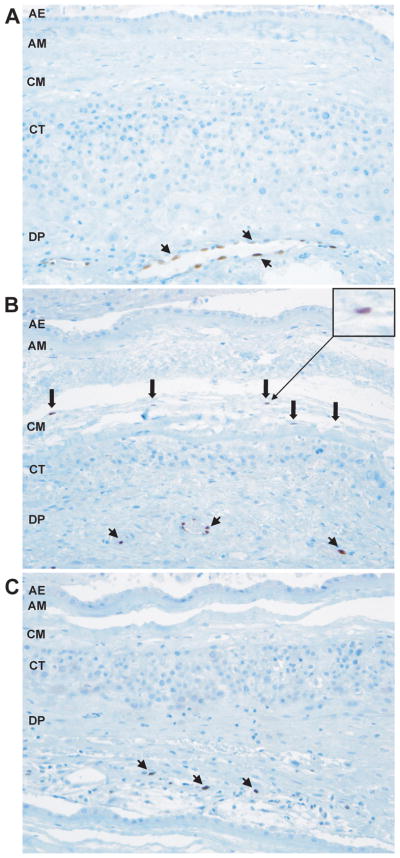
(A) IL-33 is expressed in the nuclei of endothelial cells of decidual blood vessels in patients with preterm labor. IL-33 is not expressed in the mesodermal layer of the amnion. x200 magnification. (B) IL-33 is expressed in the nuclei of macrophages in the chorioamniotic membranes of patients with preterm labor and acute chorioamnionitis. x200 magnification. (C) IL-33 is not expressed in macrophages in the chorioamnionic membranes of patients with preterm labor and chronic chorioamnionitis. x200 magnification. Arrowheads are endothelial cells and thick arrows are macrophages for all. AE = amnion epithelium; AM = amnion mesoderm; CM = chorionic mesoderm; CT = chorionic trophoblast layer; DP = decidua parietalis.
In patients with preterm labor and chronic chorioamnionitis, the placenta, chorioamniotic membranes, and umbilical cord also showed nuclear expression of IL-33 in vascular endothelial and smooth muscle cells. However, contrary to what was observed in preterm labor with acute chorioamnionitis, macrophages and myofibroblasts in these tissues did not express IL-33 (Fig. 3C).
III. In vitro models of acute and chronic inflammation
Amnion epithelial and mesenchymal cells (AECs & AMCs)
We used in vitro models of inflammation to assess the effects of pro-inflammatory cytokines involved in acute and chronic chorioamnionitis on the mRNA expression of IL-33 and its receptor IL1RL1 (ST2). To accomplish this assessment, primary AECs and primary AMCs were treated with IL-1β (1 ng/ml and 10 ng/ml) as a model for acute chorioamnionitis, and with CXCL10 (0.5 ng/ml and 1 ng/ml) for chronic chorioamnionitis. IL-33 and IL1RL1 (ST2) mRNA were not detected in AECs after incubation with IL-1β or CXCL10. IL-33 mRNA was expressed in AMCs, and the level of expression increased after incubation with IL-1β at both concentrations compared to the no-treatment control (p < 0.001 for both; Fig. 4A). IL1RL1 (ST2) mRNA expression decreased in AMCs after IL-1β treatment at both concentrations (1ng/ml and 10 ng/ml; p = 0.007 and p = 0.003, respectively; Fig. 4A), However, IL-33 and IL1RL1 (ST2) mRNA expression in AMCs did not change with CXCL10 treatment at either concentration compared with the control ( p > 0.05 for all; Fig. 4B).
Figure 4. IL-33 and IL1RL1 (ST2) mRNA expression in amnion mesenchymal cells.
(A) Amnion mesenchymal cells (AMCs) treated with IL-1β expressed significantly increased IL-33 mRNA (p<0.001 for both 1 ng/ml and 10 ng/ml) and significantly decreased IL1RL1 (ST2) mRNA (p=0.007 for 1 ng/ml and p=0.003 for 10 ng/ml). (B) IL-33 and IL1RL1 (ST2) mRNA expression did not change in AMCs treated with CXCL10 (p>0.05 for both concentrations).
Human umbilical vein endothelial cells
As seen in Figure 5, IL-33 mRNA expression increased after incubation with IL-1β at concentrations of 1 ng/ml and 10 ng/ml, but only the increase after treatment with 1 ng/ml IL-1β was significant compared to the control (p = 0.02 for 1 ng/ml and p = 0.11 for 10 ng/ml; Fig. 5A), IL1RL1 (ST2) mRNA expression also significantly increased in HUVECs treated with both concentrations of IL-1β (p = 0.001 for 1 ng/ml and p = 0.003 for 10 ng/ml; Fig. 5A). IL-33 and IL1RL1 (ST2) mRNA expression did not change after incubation with CXCL10 (p > 0.05 for 0.5 ng/ml and 5 ng/ml; Fig. 5B).
Figure 5. IL-33 and IL1RL1 (ST2) mRNA expression in HUVECs.
(A) HUVECs treated with IL-1β expressed significantly increased IL-33 mRNA at a concentration of 1 ng/ml (p=0.02) and a slight increase at 10 ng/ml although this difference was not significant (p=0.11). IL1RL1 (ST2) mRNA expression significantly increased with IL-1β treatment (p=0.001 for 1 ng/ml and p=0.003 for 10 ng/ml. (B) IL-33 and IL1RL1 (ST2) mRNA expression did not change in HUVECs treated with CXCL10.
Discussion
Principal findings of the study
1) IL-33 is expressed in the nuclei of endothelial and smooth muscle cells of umbilical and chorionic fetal vessels, endothelial cells of maternal decidual vessels, macrophages in the chorioamniotic membranes and the umbilical cord, as well as in the cytoplasm of myofibroblasts in the Wharton’s jelly; 2) IL-33 staining in the nuclei of macrophages of the chorioamniotic membranes and umbilical cord and cytoplasm of myofibroblasts in the Wharton’s jelly is increased in these tissues in the presence of acute but not chronic chorioamnionitis; and 3) IL-33 and its receptor IL1RL1 (ST2) mRNA expression are differentially regulated by the pro-inflammatory cytokine IL-1β, but not by the chemokine CXCL10 in a cell-type specific manner.
Localization of IL-33 protein in the placenta in term gestations
Using immunohistochemistry, we determined that IL-33 protein is expressed in the nuclei of endothelial cells and vascular smooth muscle cells of the placenta, chorioamniotic membranes and umbilical cord. A recent report described IL-33 protein localization in the villous syncytiotrophoblast of placentas from normal gestations, as well as from patients with preeclampsia [135]. In the present study, however, the syncytiotrophoblast was consistently negative for IL-33 in placentas from normal pregnant women, as well as those with preterm labor and acute or chronic chorioamnionitis. Only the vascular endothelial and smooth muscle cells of the chorionic villi, chorionic plate, and basal plate expressed IL-33 protein. We have not studied placentas from patients with preeclampsia for this purpose.
Our findings support those of other investigators who have shown that endothelial cells constitutively express IL-33 in normal human tissues, including the skin, small intestine, colon, kidney, lung, stomach, liver, skeletal muscle, prostate, fallopian tubes, umbilical vein, mammary gland and placenta [125,135,136]. Smooth muscle cells represent another cell type expressing IL-33 in the placenta. This is also consistent with previous reports that IL-33 is expressed in human vascular [137], visceral [125] and bronchial [138] smooth muscle cells. Therefore, expression of IL-33 in smooth muscle cells of chorionic and umbilical vessels suggests a role for IL-33 in vascular biology during fetal life. It is interesting that AECs were not positive for IL-33 in this study: this finding contrasted with other reports indicating that IL-33 is expressed by epithelial cells exposed to the environment, such as keratinocytes and epithelial cells of the stomach, tonsillar crypts and salivary glands [125]. It is possible that amnion cells do not express IL-33 under physiologic conditions or those associated with preterm labor (with acute or chronic chorioamnionitis).
Additionally, we found nuclear IL-33 expression in a few macrophages within the chorioamniotic membranes and umbilical cord tissues in normal pregnancies, as well as in placentas of patients with acute chorioamnionitis and preterm labor. Of interest, we did not detect IL-33 in placental villous macrophages (Hofbauer cells). Macrophages are an important cell population at the feto-maternal interface.[139–144] Activated macrophages have been shown to express low quantities of IL-33 mRNA [91], and apoptotic monocytes sequester IL-33 in the nuclei after LPS stimulation [145]. However, IL-33 expression in non-activated macrophages has not been reported. Altogether, these findings suggest that IL-33-positive macrophages in the chorioamniotic membranes and umbilical cord at term are activated and/or apoptotic, which may explain why we observed IL-33-positive macrophages in cases of preterm labor and acute chorioamnionitis, but not in those with preterm labor in the absence of inflammation.
IL-33 expression in macrophages in acute chorioamnionitis, but not in chronic chorioamnionitis
IL-33 is expressed in macrophages in the chorioamniotic membranes and umbilical cord of cases with preterm labor and acute chorioamnionitis, but not in cases without inflammation. In contrast, macrophages in the chorioamniotic membranes and umbilical cord in cases with preterm labor with chronic chorioamnionitis did not have detectable expression of IL-33. The results of in vitro experiments reported herein are consistent with the immunohistological findings in that AMCs and HUVECs treated with pro-inflammatory cytokine IL-1β as a model for acute inflammation revealed increased IL-33 mRNA expression compared to no-treatment controls. Notably, IL1RL1 (ST2) mRNA expression decreased in AMCs treated with IL-1β, but increased in HUVECs with IL-1β treatment. This suggests different roles for IL-33 signaling dependent on the cell type and location. When treated with CXCL10 as a model for chronic inflammation, both IL-33 and IL1RL1 (ST2) mRNA expression in AMCs and HUVECs remained unchanged.
Functional properties of IL-33
IL-33 is a dual-function protein initially described as a nuclear factor (NF-HEV, nuclear factor from high endothelial venules) that may act as a transcriptional repressor [106,107,146]. Later, a computational approach searching for protein ligands of the IL1RL1 (ST2) receptor identified this protein as a new member of the IL-1 family, which mediates its biological effects via the ST2 receptor [91,106,107]. Although the exact nuclear functions of IL-33 have not yet been elucidated, many of its extracellular functions have been uncovered [106,107]. Through ST2, IL-33 can drive the production of Th2-associated cytokines from in vitro polarized Th2 cells [106,107]. IL-33 also has prominent pro-inflammatory effects, primarily on mast cells, basophils, eosinophils, monocytes, macrophages, NK cells and activated neutrophils, by inducing the activation of NFkB and MAP kinases, and the production of various cytokines and chemokines [106,107]. The increased production of IL-33 has been shown in several human inflammatory diseases, and IL-33 has been implicated in the development of both Th1- and Th2-mediated diseases, including allergic rhinitis, atopic dermatitis, anaphylaxis, asthma, ulcerative colitis and rheumatoid arthritis [106–108,138,147–150].
Our findings on the nuclear localization of IL-33 in endothelial and smooth muscle cells, as well as macrophages are consistent with previous reports on the nuclear localization and function of this protein [106,107,146]. Additionally, our findings on the increased expression of IL-33 in macrophages in acute chorioamnionitis and its increased expression in AMCs and HUVECs after IL-1β treatment are in accord with previous observations showing that IL-33 expression is induced in resident and infiltrating inflammatory cells of inflamed tissues [106]. Our observations showing no change of IL-33 expression in chronic chorioamnionitis in the placenta and no increase of IL-33 expression in AMCs and HUVECs after CXCL10 treatment support our previous findings on the fundamental differences between the pathophysiological processes between acute and chronic chorioamnionitis [26,32,33,150].
IL-33 as an “alarmin”
It has been shown that IL-33 is constitutively expressed by cells of tissues exposed to the environment, and is released upon cell injury, suggesting that IL-33 acts as an “alarmin” [106–108,125–127]. The term “alarmin” was suggested by Oppenheim and Yang [123] to describe a group of multifunctional molecules that are early endogenous danger signals. Alarmins are rapidly released following a pathogen challenge and/or tissue injury, and signal a warning to the immune system, recruiting and activating immune cells, eliciting effector immune responses and contributing to the resolution of immune responses [123,124,151–153]. The group of alarmins include structurally heterogeneous molecules that can be released by unconventional pathways from the cell, including heat-shock proteins, defensins, S100 proteins, galectins and HMGB1 [129,151–157].
We have recently shown that intra-amniotic infection/inflammation is associated with elevated amniotic fluid HMGB1 concentrations, independent of membrane status [128] Preterm prelabor rupture of membranes was also associated with increased HMGB1 in the amniotic fluid, and immunoreactive HMGB1 was localized to the nuclei and cytoplasm of AECs, myofibroblasts, and infiltrating macrophages of chorioamniotic membranes, as well as stromal cells in the Wharton’s jelly. Given the similarities between the HMGB1 and IL-33 [120,125] (such as their nuclear expression profiles and secretion by necrotic cells), it is possible that IL-33 also plays a DAMP/alarmin-like role in the induction of inflammation [125–127]. In support of this suggestion, alarmins are recognized by trophoblasts through TLR-4 at the feto-maternal interface [17], and both TLR-2 and TLR-4 have been implicated in acute chorioamnionitis [158,159].
Strengths and limitations of this study
A major strength of this study is that we have compared in vivo and in vitro findings in terms of IL-33 regulation by pro-inflammatory signals. The analysis also focused on specific cell types of the placenta using double immunofluorescence staining. A limitation of this study is that changes in IL-33 in the related biological compartments (such as amniotic fluid and maternal/fetal plasma) were not performed. Further studies using immunoassays will be necessary to document both local and systemic changes in IL-33 concentrations during normal pregnancy and pregnancy with complications.
Conclusions
In this study, we show novel aspects of IL-33 expression in the normal placenta, chorioamniotic membranes and umbilical cord. The findings suggest a role for IL-33 in acute inflammation of the human placenta. Further studies are required to investigate the functional role of IL-33 in normal pregnancy, “the great obstetrical syndromes” and the placenta.
Table IA.
Demographic and clinical characteristics of the study population
| Term in labor (n=10) | Term not in labor (n=10) | P value | |
|---|---|---|---|
|
| |||
| Maternal age (years) | 23.9 (6.3) | 30.3 (7.3) | NS |
| Gestational age at delivery (weeks) | 39.1 (1.4) | 38.4 (0.6) | NS |
| Male neonate | 60% | 40% | NS |
| Birth weight (g) | 3299.8 (343.7) | 3307 (480.0) | NS |
| Cesarean section | 0 | 100% | <0.001 |
Values are presented as mean (±S.D.) or percentage.
NS, statistically not significant.
Table IB.
Demographic and clinical characteristics of the study population
| PTL without chorioamnionitis (n=10) | PTL with acute chorioamnionitis (n=10) | PTL with chronic chorioamnionitis (n=10) | P value | |
|---|---|---|---|---|
|
| ||||
| Maternal age (years) | 24.0 (4.7) | 24.2 (6.0) | 25.2 (7.4) | NS |
| Gestational age at delivery (weeks) | 34.2 (1.8) | 34.2 (2.1) | 34.2 (2.7) | NS |
| Male neonate | 70% | 60% | 40% | NS |
| Birth weight (g) | 2462.8 (594.8) | 2264.5 (490.7) | 2430.0 (818.0) | NS |
| Cesarean section | 10% | 0% | 30% | NS |
Values are presented as mean (±S.D.) or percentage.
NS, statistically not significant;
PTL, preterm labor.
Acknowledgments
The authors are grateful to all who made this work possible, including patients, nurses, lab staff, and clinicians. We are grateful to Dr. Yi Xu, Dr. Haidy El-Azzamy, Carla Jenkins, and Gerardo Rodriguez for their technical assistance, as well as to Maureen McGerty, Andrea Bernard and Sara Tipton for their critical readings of the manuscript.
Footnotes
Declaration of Interest
The authors report no declarations of interest. This work was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services.
Reference List
- 1.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 2.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 3.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 4.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes 139. Am J Obstet Gynecol. 2001;185:1124–1129. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 7.Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, Kim JC, Kim YM, Yoshimatsu J, Espinoza J, Romero R. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:171–175. doi: 10.1080/jmf.11.3.171.175. [DOI] [PubMed] [Google Scholar]
- 8.Erez O, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Kim CJ, Chaiworapongsa T, Hoppensteadt D, Fareed J, Than NG, Nhan-Chang CL, Yeo L, Pacora P, Mazor M, Hassan SS, Mittal P, Romero R. Evidence of maternal platelet activation, excessive thrombin generation, and high amniotic fluid tissue factor immunoreactivity and functional activity in patients with fetal death. J Matern Fetal Neonatal Med. 2009;22:672–687. doi: 10.1080/14767050902853117. [DOI] [PubMed] [Google Scholar]
- 9.Richani K, Romero R, Soto E, Espinoza J, Nien JK, Chaiworapongsa T, Refuerzo J, Blackwell S, Edwin SS, Santolaya-Forgas J, Mazor M. Unexplained intrauterine fetal death is accompanied by activation of complement. J Perinat Med. 2005;33:296–305. doi: 10.1515/JPM.2005.052. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell S, Romero R, Chaiworapongsa T, Refuerzo J, Gervasi MT, Yoshimatsu J, Espinoza J, Berman S, Yoon BH. Unexplained fetal death is associated with changes in the adaptive limb of the maternal immune response consistent with prior antigenic exposure. J Matern Fetal Neonatal Med. 2003;14:241–246. doi: 10.1080/jmf.14.4.241.246. [DOI] [PubMed] [Google Scholar]
- 11.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–1123. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 12.Ogge G, Romero R, Chaiworapongsa T, Gervasi MT, Pacora P, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Mittal P, Kim YM, Hassan SS. Leukocytes of pregnant women with small-for-gestational age neonates have a different phenotypic and metabolic activity from those of women with preeclampsia. J Matern Fetal Neonatal Med. 2010;23:476–487. doi: 10.3109/14767050903216033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 14.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, Draghici S. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, Erez O, Vaisbuch E, Gotsch F, Pacora P, Yeo L, Gervasi MT, Lamont RF, Yoon BH, Hassan SS. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009;37:543–552. doi: 10.1515/JPM.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, Nien JK, Gomez R, Mazor M, Saito S, Abrahams VM, Mor G. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005;193:921–927. doi: 10.1016/j.ajog.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 18.Faupel-Badger JM, Fichorova RN, Allred EN, Hecht JL, Dammann O, Leviton A, McElrath TF. Cluster analysis of placental inflammatory proteins can distinguish preeclampsia from preterm labor and premature membrane rupture in singleton deliveries less than 28 weeks of gestation. Am J Reprod Immunol. 2011;66:488–494. doi: 10.1111/j.1600-0897.2011.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centlow M, Wingren C, Borrebaeck C, Brownstein MJ, Hansson SR. Differential gene expression analysis of placentas with increased vascular resistance and pre-eclampsia using whole-genome microarrays. J Pregnancy. 2011;2011:472354. doi: 10.1155/2011/472354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitras V, Paulssen RH, Gronaas H, Leirvik J, Hanssen TA, Vartun A, Acharya G. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30:424–433. doi: 10.1016/j.placenta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Cindrova-Davies T. Gabor Than Award Lecture 2008: pre-eclampsia - from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30(Suppl A):S55–65. doi: 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Varkonyi T, Nagy B, Fule T, Tarca AL, Karaszi K, Schonleber J, Hupuczi P, Mihalik N, Kovalszky I, Rigo J, Jr, Meiri H, Papp Z, Romero R, Than NG. Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta. 2011;32(Suppl):S21–9. doi: 10.1016/j.placenta.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol. 1991;10:217–229. [PubMed] [Google Scholar]
- 25.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:1457–1461. doi: 10.1016/s0046-8177(98)90016-8. [DOI] [PubMed] [Google Scholar]
- 26.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, Kim JS. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet Gynecol. 1989;73:31–34. [PubMed] [Google Scholar]
- 31.Romero R, Chaiworapongsa T, Alpay SZ, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–567. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, Chaiworapongsa T, Hassan SS, Kim CJ. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–526. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, Kusanovic JP, Chaiworapongsa T, Hassan SS, Yoon BH, Kim CJ. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Romero R, Dong Z, Xu Y, Qureshi F, Jacques S, Yoo W, Chaiworapongsa T, Mittal P, Hassan SS, Kim CJ. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59:928–938. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 36.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SK, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Mittal P, Chaiworapongsa T, Pacora P, Ogge G, Gomez R, Yoon BH, Yeo L, Lamont RF, Hassan SS. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD) J Perinat Med. 2010;38:45–53. doi: 10.1515/JPM.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009;89:924–936. doi: 10.1038/labinvest.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seong HS, Lee SE, Kang JH, Romero R, Yoon BH. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol. 2008;199:375. doi: 10.1016/j.ajog.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, Khalek N, Camacho N, Hendler I, Mittal P, Friel LA, Gotsch F, Erez O, Than NG, Mazaki-Tovi S, Schoen ML, Hassan SS. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med. 2006;19:693–697. doi: 10.1080/14767050600927353. [DOI] [PubMed] [Google Scholar]
- 43.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, Sorokin Y. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, Chaiworapongsa T, Espinoza J, Gonzalez R. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–37. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 45.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM, Hassan S, Gomez R, Yoon BH, Chaiworapongsa T, Lee W, Mazor M. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–352. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 46.Blackwell S, Romero R, Chaiworapongsa T, Kim YM, Bujold E, Espinoza J, Camacho N, Hassan S, Yoon BH, Refuerzo JS. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern Fetal Neonatal Med. 2003;14:151–157. doi: 10.1080/jmf.14.3.151.157. [DOI] [PubMed] [Google Scholar]
- 47.Yoon BH, Romero R, Moon J, Chaiworapongsa T, Espinoza J, Kim YM, Edwin S, Kim JC, Camacho N, Bujold E, Gomez R. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J Matern Fetal Neonatal Med. 2003;13:32–38. doi: 10.1080/jmf.13.1.32.38. [DOI] [PubMed] [Google Scholar]
- 48.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, Svinarich D, Cotton DB. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–113. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 49.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73:383–389. [PubMed] [Google Scholar]
- 50.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 51.Baumann P, Romero R. Intra-amniotic infection, cytokines and premature labor. Wien Klin Wochenschr. 1995;107:598–607. [PubMed] [Google Scholar]
- 52.Bry K, Hallman M. Transforming growth factor-beta opposes the stimulatory effects of interleukin-1 and tumor necrosis factor on amnion cell prostaglandin E2 production: implication for preterm labor. Am J Obstet Gynecol. 1992;167:222–226. doi: 10.1016/s0002-9378(11)91662-7. [DOI] [PubMed] [Google Scholar]
- 53.Chen FC, Sarioglu N, Buscher U, Dudenhausen JW. Lipopolysaccharide binding protein in the early diagnosis of intraamniotic infection of pregnant women with premature rupture of the membranes. J Perinat Med. 2009;37:135–139. doi: 10.1515/JPM.2009.004. [DOI] [PubMed] [Google Scholar]
- 54.Fidel PL, Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 55.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–948. [PubMed] [Google Scholar]
- 56.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 57.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, Chaiworapongsa T, Kim JC, Yoon BH, Menon R, Fortunato S, Berry SM. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–316. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 58.Molnar M, Romero R, Hertelendy F. Interleukin-1 and tumor necrosis factor stimulate arachidonic acid release and phospholipid metabolism in human myometrial cells. Am J Obstet Gynecol. 1993;169:825–829. doi: 10.1016/0002-9378(93)90011-7. [DOI] [PubMed] [Google Scholar]
- 59.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 61.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 62.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 63.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 64.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 65.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(Suppl 1):16–8. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 66.Kannan S, Saadani-Makki F, Muzik O, Chakraborty P, Mangner TJ, Janisse J, Romero R, Chugani DC. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med. 2007;48:946–954. doi: 10.2967/jnumed.106.038539. [DOI] [PubMed] [Google Scholar]
- 67.Kannan S, Saadani-Makki F, Balakrishnan B, Chakraborty P, Janisse J, Lu X, Muzik O, Romero R, Chugani DC. Magnitude of [(11)C]PK11195 binding is related to severity of motor deficits in a rabbit model of cerebral palsy induced by intrauterine endotoxin exposure. Dev Neurosci. 2011;33:231–240. doi: 10.1159/000328125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Shea TM, Klinepeter KL, Meis PJ, Dillard RG. Intrauterine infection and the risk of cerebral palsy in very low-birthweight infants. Paediatr Perinat Epidemiol. 1998;12:72–83. [PubMed] [Google Scholar]
- 70.Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, Romero R, Chugani D. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol. 2008;199:651–657. doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, Chi JG. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- 72.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 73.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2:27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol. 2005;25(Suppl 2):S31–5. doi: 10.1038/sj.jp.7211317. [DOI] [PubMed] [Google Scholar]
- 75.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 77.Polglase GR, Dalton RG, Nitsos I, Knox CL, Pillow JJ, Jobe AH, Moss TJ, Newnham JP, Kallapur SG. Pulmonary vascular and alveolar development in preterm lambs chronically colonized with Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol. 2010;299:L232–L241. doi: 10.1152/ajplung.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Panzer U, Reinking RR, Steinmetz OM, Zahner G, Sudbeck U, Fehr S, Pfalzer B, Schneider A, Thaiss F, Mack M, Conrad S, Huland H, Helmchen U, Stahl RA. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341–1350. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 79.Tan J, Zhou G. Chemokine receptors and transplantation. Cell Mol Immunol. 2005;2:343–349. [PubMed] [Google Scholar]
- 80.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piper KP, Horlock C, Curnow SJ, Arrazi J, Nicholls S, Mahendra P, Craddock C, Moss PA. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827–3832. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 82.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–3705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Lopez MA, Sanchez-Madrid F, Rodriguez-Frade JM, Mellado M, Acevedo A, Garcia MI, Albar JP, Martinez C, Marazuela M. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Invest. 2001;81:409–418. doi: 10.1038/labinvest.3780248. [DOI] [PubMed] [Google Scholar]
- 84.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, Lee J, Sundell IB, Mittal P, Kusanovic JP, Hassan SS, Kim JS. Peripheral CD300a+CD8+ T Lymphocytes with a Distinct Cytotoxic Molecular Signature Increase in Pregnant Women with Chronic Chorioamnionitis. Am J Reprod Immunol. 2011 doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dinarello CA. The biological properties of interleukin-1. Eur Cytokine Netw. 1994;5:517–531. [PubMed] [Google Scholar]
- 87.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 88.Dinarello CA. Interleukin-18, a proinflammatory cytokine. Eur Cytokine Netw. 2000;11:483–486. [PubMed] [Google Scholar]
- 89.Dunne A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 90.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 91.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP, Mitchell MD. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–238. [PubMed] [Google Scholar]
- 93.Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009;89:924–936. doi: 10.1038/labinvest.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitchell MD, Romero RJ, Edwin SS, Trautman MS. Prostaglandins and parturition. Reprod Fertil Dev. 1995;7:623–632. doi: 10.1071/rd9950623. [DOI] [PubMed] [Google Scholar]
- 95.Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359–366. doi: 10.1016/s0002-9378(99)70562-4. [DOI] [PubMed] [Google Scholar]
- 96.Mitchell MD, Chang MC, Chaiworapongsa T, Lan HY, Helliwell RJ, Romero R, Sato TA. Identification of 9alpha,11beta-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J Clin Endocrinol Metab. 2005;90:4244–4248. doi: 10.1210/jc.2004-2496. [DOI] [PubMed] [Google Scholar]
- 97.Han YM, Romero R, Kim JS, Tarca AL, Kim SK, Draghici S, Kusanovic JP, Gotsch F, Mittal P, Hassan SS, Kim CJ. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod. 2008;79:954–961. doi: 10.1095/biolreprod.108.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romero R, Sepulveda W, Mazor M, Brandt F, Cotton DB, Dinarello CA, Mitchell MD. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992;167:863–872. doi: 10.1016/s0002-9378(12)80003-2. [DOI] [PubMed] [Google Scholar]
- 99.Fidel PL, Jr, Romero R, Ramirez M, Cutright J, Edwin SS, LaMarche S, Cotton DB, Mitchell MD. Interleukin-1 receptor antagonist (IL-1ra) production by human amnion, chorion, and decidua. Am J Reprod Immunol. 1994;32:1–7. doi: 10.1111/j.1600-0897.1994.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 100.Romero R, Gomez R, Galasso M, Mazor M, Berry SM, Quintero RA, Cotton DB. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol. 1994;171:912–921. doi: 10.1016/s0002-9378(94)70058-3. [DOI] [PubMed] [Google Scholar]
- 101.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 102.Fidel PL, Jr, Romero R, Cutright J, Wolf N, Gomez R, Araneda H, Ramirez M, Yoon BH. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J Soc Gynecol Investig. 1997;4:22–26. doi: 10.1177/107155769700400104. [DOI] [PubMed] [Google Scholar]
- 103.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 104.Wang X, Hagberg H, Mallard C, Zhu C, Hedtjarn M, Tiger CF, Eriksson K, Rosen A, Jacobsson B. Disruption of interleukin-18, but not interleukin-1, increases vulnerability to preterm delivery and fetal mortality after intrauterine inflammation. Am J Pathol. 2006;169:967–976. doi: 10.2353/ajpath.2006.050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 107.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 108.Kurowska-Stolarska M, Hueber A, Stolarski B, McInnes IB. Interleukin-33: a novel mediator with a role in distinct disease pathologies. J Intern Med. 2011;269:29–35. doi: 10.1111/j.1365-2796.2010.02316.x. [DOI] [PubMed] [Google Scholar]
- 109.Pineles B, Romero R, Montenegro D, Tarca AL, Than NG, Hassan S, Gotsch F, Draghici S, Espinoza J. “The Inflammasome” in Human Parturition. Reprod” Sci. 2007;14:59A–60A. [Google Scholar]
- 110.Montenegro D, Romero R, Pineles B, Tarca AL, Madsen-Bouterse SA, Hassan S, Kusanovic JP, Draghici S, Espinoza J, Kim CJ. Differential Expression of the Inflammasome Components in the Fetal Inflammatory Response Syndrome. Reprod Sci. 2007;14:59A–60A. [Google Scholar]
- 111.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, Hassan SS. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lamkanfi M, Kanneganti TD, Van DP, Vanden BT, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Nunez G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 116.Hayakawa M, Hayakawa H, Matsuyama Y, Tamemoto H, Okazaki H, Tominaga S. Mature interleukin-33 is produced by calpain-mediated cleavage in vivo. Biochem Biophys Res Commun. 2009;387:218–222. doi: 10.1016/j.bbrc.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 117.Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 122.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 124.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 125.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS. One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 -cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 127.Lamkanfi M, Dixit VM. IL-33 raises alarm. Immunity. 2009;31:5–7. doi: 10.1016/j.immuni.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 128.Romero R, Chaiworapongsa T, Alpay SZ, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Than NG, Kim SS, Abbas A, Han YM, Hotra J, Tarca AL, Erez O, Wildman DE, Kusanovic JP, Pineles B, Montenegro D, Edwin SS, Mazaki-Tovi S, Gotsch F, Espinoza J, Hassan SS, Papp Z, Romero R. Chorioamnionitis and increased galectin-1 expression in PPROM --an anti-inflammatory response in the fetal membranes? Am. J Reprod Immunol. 2008;60:298–311. doi: 10.1111/j.1600-0897.2008.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–7. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 131.Kim SY, Romero R, Tarca AL, Bhatti G, Lee J, Chaiworapongsa T, Hassan SS, Kim CJ. miR-143 regulation of prostaglandin-endoperoxidase synthase 2 in the amnion: implications for human parturition at term. PLoS One. 2011;6:e24131. doi: 10.1371/journal.pone.0024131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marin V, Kaplanski G, Gres S, Farnarier C, Bongrand P. Endothelial cell culture: protocol to obtain and cultivate human umbilical endothelial cells. J Immunol Methods. 2001;254:183–190. doi: 10.1016/s0022-1759(01)00408-2. [DOI] [PubMed] [Google Scholar]
- 133.Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007;2:481–485. doi: 10.1038/nprot.2007.54. [DOI] [PubMed] [Google Scholar]
- 134.Pinheiro J, Bates D, DebRoy S, Sarkar D. nlme: Linear and nonlinear mixed effects models. 2006:3.1–79. [Google Scholar]
- 135.Granne I, Southcombe JH, Snider JV, Tannetta DS, Child T, Redman CW, Sargent IL. ST2 and IL-33 in pregnancy and pre-eclampsia. PLoS One. 2011;6:e24463. doi: 10.1371/journal.pone.0024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H, Haraldsen G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173:1229–1242. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 139.Mor G, Koga K. Macrophages and pregnancy. Reprod Sci. 2008;15:435–436. doi: 10.1177/1933719108317253. [DOI] [PubMed] [Google Scholar]
- 140.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 141.Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci. 2010;17:209–218. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- 142.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187:3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 143.McIntire RH, Petroff MG, Phillips TA, Hunt JS. In vitro models for studying human uterine and placental macrophages. Methods Mol Med. 2006;122:123–148. doi: 10.1385/1-59259-989-3:123. [DOI] [PubMed] [Google Scholar]
- 144.Renaud SJ, Graham CH. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol Invest. 2008;37:535–564. doi: 10.1080/08820130802191375. [DOI] [PubMed] [Google Scholar]
- 145.Nile CJ, Barksby E, Jitprasertwong P, Preshaw PM, Taylor JJ. Expression and regulation of interleukin-33 in human monocytes. Immunology. 2010 doi: 10.1111/j.1365-2567.2009.03221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sakashita M, Yoshimoto T, Hirota T, Harada M, Okubo K, Osawa Y, Fujieda S, Nakamura Y, Yasuda K, Nakanishi K, Tamari M. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy. 2008;38:1875–1881. doi: 10.1111/j.1365-2222.2008.03114.x. [DOI] [PubMed] [Google Scholar]
- 148.Pushparaj PN, Tay HK, H’ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 149.Seidelin JB, Bjerrum JT, Coskun M, Widjaya B, Vainer B, Nielsen OH. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett. 2010;128:80–85. doi: 10.1016/j.imlet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 150.Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, Finckh A, Smith DE, Gabay C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 151.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 153.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 154.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 155.Balogh A, Pozsgay J, Matko J, Dong Z, Kim CJ, Varkonyi T, Sammar M, Rigo J, Jr, Meiri H, Romero R, Papp Z, Than NG. Placental protein 13 (PP13/galectin-13) undergoes lipid raft-associated subcellular redistribution in the syncytiotrophoblast in preterm preeclampsia and HELLP syndrome. Am J Obstet Gynecol. 2011;205:156–14. doi: 10.1016/j.ajog.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Than NG, Romero R, Kim CJ, McGowen MR, Papp Z, Wildman DE. Galectins: guardians of eutherian pregnancy at the maternal-fetal interface. Trends Endocrinol Metab. 2012;23:23–31. doi: 10.1016/j.tem.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Than NG, Erez O, Wildman DE, Tarca AL, Edwin SS, Abbas A, Hotra J, Kusanovic JP, Gotsch F, Hassan SS, Espinoza J, Papp Z, Romero R. Severe preeclampsia is characterized by increased placental expression of galectin-1. J Matern Fetal Neonatal Med. 2008;21:429–442. doi: 10.1080/14767050802041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 159.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63:587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





