SUMMARY
While missense mutations of von Hippel-Lindau disease (VHL) gene are the most common germline mutation underlying this heritable cancer syndrome, the mechanism of tumorigenesis is unknown. We found a quantitative reduction of missense mutant VHL protein (pVHL) in VHL-associated tumors associated with physiologic mRNA expression. While mutant pVHL is unstable and degraded contemporarily with translation, it retains its E3 ligase function, including hypoxia inducible factor degradation. The premature pVHL degradation is due to misfolding and imbalance of chaperonin binding. Histone deacetylase inhibitors (HDACis) can modulate this pathway by inhibiting the HDAC6-Hsp90 chaperone axis, stabilizing pVHL and restoring activity comparable to wild type protein in vitro and in animal models (786-O tumor xenografts). HDACi mediated stabilization of missense pVHL significantly attenuates the growth of 786-O rodent tumor model. These findings provide direct biologic insight into VHL-associated tumors and elucidate a new treatment paradigm for VHL.
INTRODUCTION
Germline von Hippel-Lindau disease (VHL) gene mutations underlie VHL pathogenesis (Latif et al., 1993; Richard et al., 2012; Seizinger et al., 1988; Shen and Kaelin, 2012). VHL protein (pVHL) is an E3 ligase in the VCB-Cul2 complex (Okuda et al., 2001), which is responsible for binding, targeting and degrading hypoxia inducible factor (HIF)-1α/HIF-2α via the ubiquitin proteasome pathway (Cockman et al., 2000; Maxwell et al., 1999; Ohh et al., 2000). Reduction or absence of pVHL function leads to a marked and unregulated amplification in HIF activity under non-hypoxic conditions, which is oncogenic via activation of multiple signaling pathways underlying cell proliferation, survival, motility, angiogenesis and metabolism (Cockman et al., 2000; Krieg et al., 2000; Maxwell et al., 1999; Ohh et al., 2000; Tanimoto et al., 2000). The precise mechanism underlying loss of pVHL function is not known.
Emerging evidence indicates abnormal protein conformation associated with germline mutations in heritable disorders may lead to mutated protein recognition and accelerated degradation (Lu et al., 2011; Yang et al., 2011). Specifically, missense mutations in VHL have been shown to affect protein conformation and folding, resulting in immediate degradation after translation in yeast and VHL-expressing mammalian cells (McClellan et al., 2005; Melville et al., 2003). These data suggest mutated pVHL degradation in VHL may be controlled by the binding of molecular chaperonin/co-chaperonins like Hsp70, Hsp90 and TRiC/CCT (McClellan et al., 2005; Melville et al., 2003). Altogether, these findings indicate that proteostasis alterations due to VHL mutations may have a role in accelerated pVHL mutant degradation and loss of function.
Currently, while anti-angiogenic agents have been investigated for treatment of renal cell carcinoma in VHL, therapy for other VHL-associated tumors (particularly hemangioblastomas) is surgery, which is linked to significant morbidity and mortality (Ali et al., 2012; Jonasch et al., 2011; Lonser et al., 2003). Consequently, new non-invasive, syndrome-specific, therapies are needed. Better understanding of the precise mechanism underlying VHL-associated oncogenesis is needed to develop these targeted treatments. We demonstrate missense mutations in VHL cause significant quantitative intracellular reduction of functional pVHL due to rapid degradation. Chaperonin and co-chaperonins are involved with the rapid degradation of mutant VHL protein. However, proteostasis modulators can prolong mutant protein half-life and ameliorate tumor progression.
RESULTS AND DISCUSSION
pVHL in VHL-derived tumors
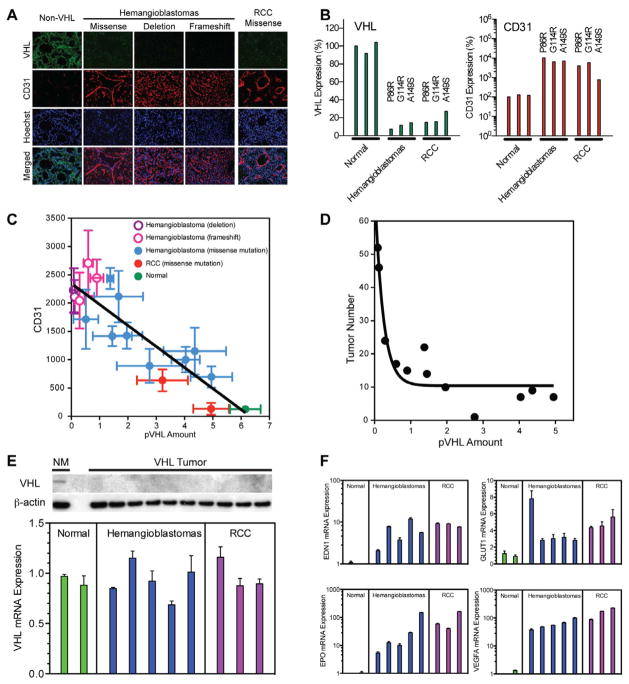
Prior studies have demonstrated loss of pVHL function in VHL-associated tumors underlies oncogenesis (Iliopoulos et al., 1995; Lubensky et al., 1996; Mehta et al., 2008). To begin to establish the cause of pVHL loss of function, we initially examined pVHL levels in VHL-associated tumors. VHL-associated tumors have significantly reduced or absent pVHL expression (hemangioblastomas=14; renal cell carcinomas=3) by immunofluorescence, including tumors with missense, frameshift or deletions of VHL (Figure 1A). Compared to controls, a 65 to 96% decrease in pVHL expression was found in missense VHL-associated tumors (Figure 1B). Alternatively, CD31, a marker for endothelium associated with deactivation of VHL (Wizigmann-Voos et al., 1995), was increased 90% to 890%. Moreover, consistent with an association between pVHL level and expression of VHL pathobiology, pVHL level was inversely related to CD31 expression (R2=0.8; Figure 1C) and central nervous system hemangioblastoma development in patients (R2=0.9; Figure 1D). Western blot analysis also confirmed the absence of pVHL in all VHL-associated tumor specimens (Figure 1E).
Figure 1. Quantitative loss of pVHL with intact mRNA expression of VHL in hemangioblastoma and RCC.
(A) Immunofluorescence staining for VHL (green) and CD31 (red) in VHL-associated hemangioblastoma and RCC specimen (scale bar=50 microns).(B) Quantitative analysis of VHL and CD31 protein expression in normal brain specimen (n=3), hemangioblastoma (n=3) and RCC (n=3) with missense VHL mutations, showing increased CD31 expression in hemangioblastomas and RCC.(C) Quantification of CD31 immunofluorescence compared to VHL immunofluorescence signal of patient samples (R2=0.8).(D) The total number of central nervous system VHL-associated hemangioblastomas found on magnetic resonance imaging was inversely related to pVHL immunofluorescence (R2=0.9).(E) Western blot analysis of pVHL from microdissected VHL-associated hemangioblastomas. Quantitative real-time PCR for VHL mRNA expression demonstrating maintenance of VHL mRNA in VHL-associated hemangioblastomas and RCCs.(F) Real-time PCR quantification of hypoxia-related gene expression in tumor specimens further confirming that classic hypoxia-related genes are upregulated in VHL-associated tumors. Data expressed as mean±SEM, n=6 fields/group for (C and D), n=3 real-time PCR reactions/group for (F).
Expression of VHL mRNA in tumors
To determine if the quantitative decrease in pVHL was the result of abnormal mRNA expression, we measured VHL mRNA transcript levels in microdissected hemangioblastomas (n=5) and RCCs (n=3) from VHL patients harboring germline missense mutations, which are the most common VHL germline mutation (Maher and Kaelin, 1997; Stolle et al., 1998). VHL mRNA was similarly expressed in the tumors and normal brain (Figure 1E). Consistent with lack of pVHL function, hypoxia-associated gene expression was profoundly up-regulated. Genes, including EPO (increased 131 to 643%), EDN1 (increased 200 to 1126%), VEGFα (increased 37 to 227%) and GLUT1 (increased by 206 to 2255%) were markedly elevated in VHL-associated tumors compared to control (Figure 1F) consistent with the abnormal hypoxia response seen with constitutively activated HIF-signaling in VHL (Keith et al., 2012).
Stability of pVHL in tumor-derived mutants
Because maintenance of physiologic levels of VHL mRNA expression indicated that the quantitative loss of pVHL occurs at the translational level or is determined by rate of degradation, we hypothesized that changes in protein stability underlie the loss of protein amount/function found in VHL-associated hemangioblastomas (McClellan et al., 2005). To test this theory, we examined mutant and wild type pVHL stability in vitro. Since missense mutations are the most frequent genetic changes in VHL-associated tumors, we analyzed pVHL kinetics in vitro from 3 VHL missense mutations (S68W, Y112N and A149S)(Atuk et al., 1998; Beroud et al., 2000; Nordstrom-O’Brien et al., 2010) found in VHL patients with either of the major forms of VHL, Type 1 (Y112N) or Type 2 (S68W and A149S) VHL (Lonser et al., 2003; van der Harst et al., 1998).
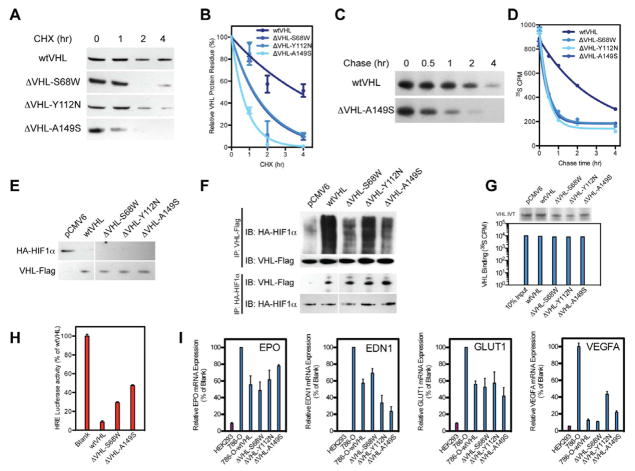
S68W, Y112N and A149S missense mutations were transfected into VHL-deficient cell line 786-O. Consistent with successful transfection, all pVHL mutants were successfully synthesized as full-length proteins. Protein expression between mutant and wild type pVHL was not different. To assess pVHL stability, we used cycloheximide (CHX)-mediated pulse chase assay Western blot analysis (Figure 2A). Quantitative analysis revealed that wild type pVHL has a half-life of 3.8 hours in physiologic conditions but tumor-derived mutant protein (ΔVHL-S68W, ΔVHL-Y112N and ΔVHL-A149S) half-life was significantly reduced by 1.2, 1.9 and 0.6 hours, respectively (Figure 2B). This is consistent with previous finding that pVHL with Type 1 VHL (surface type) mutations exhibit longer half-life than Type 2 VHL (deep type) mutations (Ong et al., 2007).
Figure 2. Mutant pVHL maintains intrinsic function but has shortened half-life.
(A) Cycloheximide (CHX) treatment of wild type and pVHL mutants at 0,1, 2 and 4 hours demonstrates rapid degradation of mutant pVHL versus wild type.(B) Calculation of protein degradation kinetic of wild type and pVHL mutants yielded pVHL mutants have shortened half-life.(C) Radioactive [35S] pulse chase assay for ΔVHL-A149S mutant and wild type at 0, 0.5, 1, 2 and 4 hr, confirming that pVHL have shortened half-life compared to wild type.(D) Scintillation analysis of [35S] labeled VHL demonstrating fundamental loss of pVHL stability in mutants compared to wild type.(E) Western blot for steady state expressed HA-tagged HIF1α with pVHL mutant reintroduction demonstrating successful degradation of HA-HIF1α by pVHL mutants.(F) Forward and reverse immunoprecipitation for HA-HIF1α binding with pVHL mutants demonstrates similar binding affinities of pVHL as compared to wild type VHL to HIF1α.(G) Autoradiography and radioactive scintillation for ex vivo binding of pVHL mutants to HIF2α derived peptides demonstrating similar affinity of mutant pVHL compared with wild type control (IVT VHL - in vitro translation of pVHL).(H) Luciferase assay for transcriptional activity of HRE-luciferase in 786-O cells with VHL mutant reintroduction reveals decreased HRE-luciferase activity in those with wild type and missense VHL, thus missense VHL maintains clearance of HIF1α consequently its target genes containing HRE.(I) Quantitative real-time PCR of hypoxia-related genes in 786-O cells with VHL mutant re-introduction further demonstrates missense VHL abilities to regulate HIF1α target genes (GLUT1, EDN1, EPO, and VEGFA) similarly to wild type VHL. Data expressed as mean±SEM, n=3 radioactive scintillation for (G), n=3 luciferase reaction/group for (H), and n=3 real-time PCR reactions/group for (I).
To confirm these results, we utilized [35S]-methionine mediated pulse chase analysis to the same missense mutant pVHL. Autoradiography confirmed that the mutants were synthesized in full length. Consistent with the prior results, newly synthesized mutant proteins exhibited significantly shortened half-life (0.3 hours) compared with wild type pVHL (2 hours) due to immediate degradation after synthesis (Figure 2C). Consequently, the fundamental loss of mutant pVHL stability, due to reduction in half-life, was confirmed by both Western blot analysis and liquid scintillation on [35S] radioactivity (Figure 2D).
Functional capacity of mutant pVHL
While the previous findings indicated that there is a quantitative loss of pVHL in VHL-associated tumors and that reduced protein stability underlies this reduction in protein, it was unclear if the intrinsic function of the missense mutant pVHL is also impacted, further impairing its E3 ligase activity. To determine whether tumor derived pVHL mutants exhibit impaired E3 ligase activity, we studied HIF1α degradation through transfections of both wild type and missense VHL in 786-O cells (HA-HIF1α-786-O stable transfects, Figure 2E) (Gnarra et al., 1994; Iliopoulos et al., 1995).
Western blot analysis revealed that HIF1α is efficiently degraded upon transfection of wild type or missense VHL indicating that missense pVHL can effectively degrade HIF1α in non-hypoxic conditions. To verify that missense pVHL retains E3 ligase intrinsic function and proper binding capacity to HIF1/2α, immunoprecipitation was performed both for VHL and HIF1α. Western blot analysis of the immunoprecipitate confirmed that the 3 missense mutants exhibit similar binding capacity and E3 ligase activity as wild type pVHL, which was demonstrated by HIF1agr; ubiquitination (Figure 2F). Binding of missense pVHL products was also confirmed by an ex vivo peptide binding assay that showed mutant pVHL had a similar affinity to HIF-derived peptide as wild type pVHL (Figure 2G).
We further established intact missense pVHL function via measuring luciferase activity driven by hypoxia regulated element (HRE) promoter. Upon co-transfection of wild type or missense VHL and HRE-luciferase plasmid into HA-HIF1α-786-O cells, HRE transcription activity was reduced by 91% in wild type VHL, while tumorigenic missense VHL mutants transfection reduced HRE transcription activity 54 to 81% (Figure 2H). Changes in transcription activity due to VHL mutant reintroduction were further established by measuring hypoxia-related gene expression using quantitative real-time PCR assay (Figure 2I) that revealed reduction of EDN1 (31 to 77%), EPO (22 to 52%), VEGFA (56 to 89%) and GLUT1 (48 to 58%) mRNA expression in wild type and missense mutant VHL. Our result confirmed previous finding that pVHL with missense mutation retain partial functional capacity as an E3 ligase(Chung et al., 2006; Li et al., 2007).
pVHL degradation
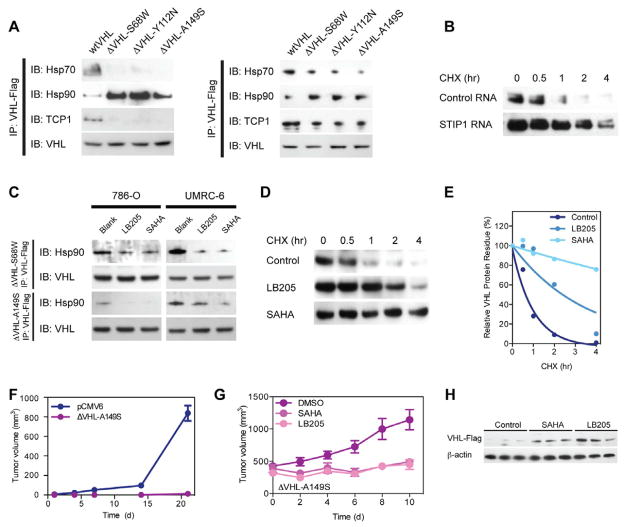
To define the mechanism underlying rapid turnover of mutant pVHL, we investigated the impact of protein quality control/degradation pathways on mutant and wild type pVHL. Specifically, co-translational degradation of VHL mutants in the nascent peptide stage is dependent on a quality control system that includes protein-folding machinery and chaperonin/co-chaperonins (Feldman et al., 1999). To elucidate the critical molecules that direct pVHL mutant into degradation, we performed immunoprecipitation assays to detect chaperonin binding to missense mutant pVHL (Figure 3A). Wild type pVHL bound robustly to Hsp70 and TCP1 but significantly less to Hsp90. Alternatively, missense mutant pVHL (ΔVHL-S68W, ΔVHL-Y112N and ΔVHL-A149S) exhibited decreased physical interaction to Hsp70 and TCP1 but increased affinity to Hsp90, consistent with abnormalities in chaperonin binding contributing to rapid degradation (McClellan et al., 2005). Involvement of Hsp70/90 appears critical for mutant pVHL degradation, since siRNA silencing on Hsp70/90 organizing protein STIP1 elongates ΔVHL-Y112N protein half-life (Figure 3B).
Figure 3. Increased survival VHL mutant protein after treatment with HDAC inhibitors and reduction of tumor growth corresponds to increased expression of VHL.
(A) Immunoprecipitation assay for chaperonin binding to mutant VHL protein demonstrating loss of Hsp70 and TCP1 but increased Hsp90 binding.(B) Western blots for protein stability change of ΔVHL-Y112N with STIP1 RNA interference, confirming the role of STIP1 in pVHL degradation pathway.(C) Immunoprecipitation assay for changes in chaperonin binding to mutant VHL protein after LB-205 or SAHA treatment, demonstrating that treatment with HDACi can alter chaperonin binding to pVHL.(D) Western blot for VHL-Y112N stability with proteostasis regulator treatments.(E) Quantification of protein half-life, exhibiting treatment with HDACi prolongs pVHL half-life.(F) Tumor growth of stably transfected ΔVHL-A149S 786-O (107) cells subcutaneous after 3 weeks treatment with vehicle in comparison to parental control, confirming that the presence of pVHL can inhibit tumor growth (P<0.0001).(G) Tumor growth of stably transfected ΔVHL-A149S 786-O (107) cells subcutaneous after reaching 300 mm3 and then treated with DMSO, SAHA or LB-205, HDACi halted tumor progression (P<0.0001). (I) Western blots for ΔVHL-A149S from tumors at the end of treatment, increased VHL expression by addition of HDACi correlated to attenuated tumor progression. Data expressed as mean±SEM, n=5 mice/group for (F) and n=3 to 5 mice/group for (G).
Modulation of missense pVHL stability
The chaperonin system role in rapid degradation of mutant pVHL highlighted a potential therapeutic strategy that exploits pharmacologic manipulation of this protein homeostatic pathway. Specifically, histone deacetylase inhibitors (HDACis) can actively modulate protein folding and degradation machinery by targeting chaperone and chaperonin system. SAHA and LB-205 are HDACis that affect molecular chaperone HSP90 binding to proteins, including mutant pVHL. SAHA, a Food and Drug Administration-approved small molecular weight HDACi, inhibits HDAC by binding the zinc atom in its catalytic site (Finnin et al., 1999). Similarly, LB-205 is a new small-molecule HDACi that also exploits a zinc-binding moiety that inhibits HDACs but has a longer half-life than SAHA (SAHA half-life, 4 to 8 hours; LB-205 half-life, 12 hours)(Lu et al., 2011).
To determine if HDACis reduce mutant pVHL degradation and minimize HIF1α-related gene expression, we examined the protein expression of the missense VHL products (ΔVHL-S68W, ΔVHL-Y112N and ΔVHL-A149S) in treated and untreated 786-O cells. We found that Hsp90 affinity to VHL mutants dramatically decreased with SAHA or LB-205 treatment (Figure 3C). To evaluate the impact of HDACis on pVHL stability, we performed protein half-life measurements based on CHX treatment (Figure 3D). Consistent with the in vitro results, mutant pVHL was rapidly degraded in physiologic conditions. LB-205 and SAHA stabilized the protein within the same CHX assay, increasing the half-life of mutant pVHL to 2.5 hours and 10.2 hours, respectively (Figure 3E).
Effect of HDACi on Tumor Growth
To determine if introduction of stable missense pVHL in tumor cells would have an effect on tumor growth, we subcutaneously injected stably transfected missense ΔVHL-A149S 786-O and empty vector cells into athymic nu/nu mice. Consistent with the in vitro data demonstrating maintenance of intrinsic function of missense mutant pVHL and previous data examining the effect of a VHL missense mutation in 786-O cells (Hansen et al., 2002), re-introduction of steady-state levels of missense VHL inhibited tumor growth in vivo (P<0.0001) compared to empty vector 786-O parental control cells. Specifically, there was 99% reduction in tumor growth with introduction of missense VHL compared to control (Figure 3F).
To determine if HDACi would have similar effects on stability of missense pVHL products in vivo, athymic nu/nu mice with stably transfected missense ΔVHL-A149S 786-O tumors were treated with HDACis (SAHA and LB-205). Treated mice had a 57% and 61% reduction in tumor growth (SAHA and LB-205, respectively) compared to vehicle treated ΔVHL-A149S 786-O tumors (P<0.0001; Figure 3G). The lack of a significant difference between SAHA and LB-205 for the effective amelioration of tumor progression (P>0.05) indicates that single daily dosing may be an effective strategy to inhibit hemangioblastoma growth. SAHA and LB-205 enable the maintenance of sufficient VHL enzyme activity to curtail tumor progression (Dollfus et al., 2002; Rini et al., 2009; Wanebo et al., 2003). Expression of the mutant protein was verified by Western blot of excised tumors demonstrating quantitative increases in the missense mutant pVHL with treatment of SAHA and LB-205 (Figure 3H).
These findings provide critical insights into understanding VHL pathogenesis and treatment. Specifically, protein degradation pathways that reduce missense mutant pVHL half-life appear to underlie the reduction in intracellular pVHL activity/amount. Moreover, the findings also increase the basic understanding of the nature of the primary biochemical abnormality with different VHL mutations and help to define a possible mechanism to explain the substantial phenotypic heterogeneity that characterizes the VHL disease. Therapeutic modification of the protease pathways involved in degradation of missense pVHL mutants with intrinsic E3 ligase function may provide new strategies for treatment of VHL.
EXPERIMENTAL PROCEDURES
VHL Tumor Samples
Tissues, imaging and clinical data were collected under institute-approved protocols (NIH #03-N-0164 and #00-N-0147). Tissue samples included VHL-associated hemangioblastomas and RCC, as well as normal brain. Dissection of tissues has been detailed previously (Zhuang et al., 1995).
Protein Extraction
Cell pellet or microdissected tissue specimens were extracted for protein in RIPA lysis buffer (Thermo Fischer Scientific Inc.) with Halt Protease Inhibitor (Thermo Fischer Scientific Inc.) and purified through centrifuge. The Bio-Rad Protein Assay kit (Bio-Rad) was used to quantify protein in the supernatant.
Western blot
Protein samples were resolved on 4–15% Bis-Tris gels (Invitrogen) and placed on polyvinylidene fluoride membranes (Millipore). Membranes were probed using antibodies against β-Actin (1:2000, Sigma), FLAG (1:2000, Origene), HA (1:1000, Origene), VHL (1:500, Cell Signaling Technology), CD31 (PECAM, 1:1000, Dako), ubiquitin (1:1000, Cell Signaling Technology), Hsp70 (1:1000, Sigma), Hsp90 (1:1000, Cell Signaling Technology) and TCP1 (1:1000, Sigma). Horseradish peroxidase-conjugated secondary antibodies (species-specific) that were visualized by enhanced chemiluminescence substrate (SuperSignal; Pierce).
Immunoprecipitation
Immunoprecipitation was performed by applying 200 μg of whole cell lysate to Dynabeads (Invitrogen) preincubated with monoclonal antibodies against FLAG or HA (1:200, Origene). Stringent washing and elutions were performed per manufacturer’s protocol.
Immunofluorescence Analysis
Frozen sections from tumor specimen were fixed and labeled overnight with primary antibodies against VHL (1:50, Cell Signaling Technology) and CD31 antibody (1:200, Dako). Cell nuclei were counterstained (Hoechst 33342; Invitrogen). Immunofluorescence was analyzed by confocal microscopy.
Real-Time PCR
Gene expression was measured by PCR on the Eco Real-time PCR Platform (Illumina). PCR was performed with primer sets for VHL, EPO, EDN1, GLUT1 and VEGFA (GAPDH internal control, Qiagen) using Power SYBR Green PCR Master Mix (Applied Biosystems).
Cell Culture and Transfection
VHL-deficient cell line 786-O (ATCC) were transfected with VHL or HIF1/2 vectors by using Xtremegene 9 transfection reagent (Roche). Stable transfection, were selected with G-418 (0.5 mg/ml) for 10 days followed by serial dilution, screening was verified by Western blot. Cells were exposed to 10 μg/mL CHX for protein stability measurement.
Mutagenesis and Cloning
Using site-directed mutagenesis kit (Agilent), VHL site-specific mutations were generated. Three known VHL patient missense mutations were selected (Beroud et al., 2000; Gallou et al., 1999). Mutants and wild type VHL were generated and sequenced in pCMV6-Entry vectors (Origene). HA-HIF1alpha-pcDNA3 and HA-HIF2alpha-pcDNA3 was a gift of Dr. William Kaelin (Addgene plasmid #18949 and #18950).
35S Metabolic Labeling and Pulse Chase Assessment
Pulse chase with [35S]-methionine was performed as described with modifications (McClellan et al., 2005). Seven hundred thousand 786-O cells were transfected with VHL vectors 12 hours before labeling. pVHL was immunoprecipitated with monoclonal FLAG antibody (Origene) using 200 μg from whole cell lysate.
In Vivo Tumor Model
Five-week-old athymic mice (Taconic) or NOD/SCID IL2R-gamma null mice (The Jackson Laboratory) were injected with 107 cells s.c. into the flanks. Tested clonal lines included 786-O, 786-O stable transfected with missense ΔVHL-A149S lines. Tumor volumes were assessed by measuring width and length (volume = width2 × length) with digital calipers. Daily treatment with SAHA (15 mg/kg) and LB-205 (15 mg/kg) were given intraperitoneal after reaching 300 mm3. Tumors were harvested at 10 days after treatments.
Highlights.
Loss of mutant pVHL in VHL-associated tumors is due to rapid degradation.
Mutant pVHL maintains its intrinsic function as an E3 ligase.
HDACis stabilize of mutant pVHL and ameliorate tumor growth in vivo.
HDACis represent a new targeted therapy for VHL-associated tumors.
Acknowledgments
This research was supported by the Intramural Research Program of NINDS and a cooperative research and development agreement between the NINDS and Lixte Biotechnology Holdings, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali T, Kandil D, Piperdi B. Long-term disease control with sunitinib in a patient with metastatic pancreatic neuroendocrine tumor (NET) associated with Von Hippel-Lindau syndrome (VHL) Pancreas. 2012;41:492–493. doi: 10.1097/MPA.0b013e31822a645e. [DOI] [PubMed] [Google Scholar]
- Atuk NO, Stolle C, Owen JA, Jr, Carpenter JT, Vance ML. Pheochromocytoma in von Hippel-Lindau disease: clinical presentation and mutation analysis in a large, multigenerational kindred. J Clin Endocrinol Metab. 1998;83:117–120. doi: 10.1210/jcem.83.1.4479. [DOI] [PubMed] [Google Scholar]
- Beroud C, Collod-Beroud G, Boileau C, Soussi T, Junien C. UMD (Universal mutation database): a generic software to build and analyze locus-specific databases. Hum Mutat. 2000;15:86–94. doi: 10.1002/(SICI)1098-1004(200001)15:1<86::AID-HUMU16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Chung J, Roberts AM, Chow J, Coady-Osberg N, Ohh M. Homotypic association between tumour-associated VHL proteins leads to the restoration of HIF pathway. Oncogene. 2006;25:3079–3083. doi: 10.1038/sj.onc.1209328. [DOI] [PubMed] [Google Scholar]
- Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- Dollfus H, Massin P, Taupin P, Nemeth C, Amara S, Giraud S, Beroud C, Dureau P, Gaudric A, Landais P, et al. Retinal hemangioblastoma in von Hippel-Lindau disease: a clinical and molecular study. Invest Ophthalmol Vis Sci. 2002;43:3067–3074. [PubMed] [Google Scholar]
- Feldman DE, Thulasiraman V, Ferreyra RG, Frydman J. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol Cell. 1999;4:1051–1061. doi: 10.1016/s1097-2765(00)80233-6. [DOI] [PubMed] [Google Scholar]
- Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- Gallou C, Joly D, Mejean A, Staroz F, Martin N, Tarlet G, Orfanelli MT, Bouvier R, Droz D, Chretien Y, et al. Mutations of the VHL gene in sporadic renal cell carcinoma: definition of a risk factor for VHL patients to develop an RCC. Hum Mutat. 1999;13:464–475. doi: 10.1002/(SICI)1098-1004(1999)13:6<464::AID-HUMU6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- Hansen WJ, Ohh M, Moslehi J, Kondo K, Kaelin WG, Welch WJ. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol Cell Biol. 2002;22:1947–1960. doi: 10.1128/MCB.22.6.1947-1960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos O, Kibel A, Gray S, Kaelin WG., Jr Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- Jonasch E, McCutcheon IE, Waguespack SG, Wen S, Davis DW, Smith LA, Tannir NM, Gombos DS, Fuller GN, Matin SF. Pilot trial of sunitinib therapy in patients with von Hippel-Lindau disease. Ann Oncol. 2011;22:2661–2666. doi: 10.1093/annonc/mdr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19:5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang L, Zhang X, Yan Q, Minamishima YA, Olumi AF, Mao M, Bartz S, Kaelin WG., Jr Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and type 2B VHL mutations. Mol Cell Biol. 2007;27:5381–5392. doi: 10.1128/MCB.00282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- Lu J, Yang C, Chen M, Ye DY, Lonser RR, Brady RO, Zhuang Z. Histone deacetylase inhibitors prevent the degradation and restore the activity of glucocerebrosidase in Gaucher disease. Proc Natl Acad Sci U S A. 2011;108:21200–21205. doi: 10.1073/pnas.1119181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubensky IA, Gnarra JR, Bertheau P, Walther MM, Linehan WM, Zhuang Z. Allelic deletions of the VHL gene detected in multiple microscopic clear cell renal lesions in von Hippel-Lindau disease patients. Am J Pathol. 1996;149:2089–2094. [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Kaelin WG., Jr von Hippel-Lindau disease. Medicine (Baltimore) 1997;76:381–391. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- McClellan AJ, Scott MD, Frydman J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell. 2005;121:739–748. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Mehta GU, Shively SB, Duong H, Tran MG, Moncrief TJ, Smith JH, Li J, Edwards NA, Lonser RR, Zhuang Z, et al. Progression of epididymal maldevelopment into hamartoma-like neoplasia in VHL disease. Neoplasia. 2008;10:1146–1153. doi: 10.1593/neo.08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville MW, McClellan AJ, Meyer AS, Darveau A, Frydman J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Lindau tumor suppressor complex. Mol Cell Biol. 2003;23:3141–3151. doi: 10.1128/MCB.23.9.3141-3151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom-O’Brien M, van der Luijt RB, van Rooijen E, van den Ouweland AM, Majoor-Krakauer DF, Lolkema MP, van Brussel A, Voest EE, Giles RH. Genetic analysis of von Hippel-Lindau disease. Hum Mutat. 2010;31:521–537. doi: 10.1002/humu.21219. [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Okuda H, Saitoh K, Hirai S, Iwai K, Takaki Y, Baba M, Minato N, Ohno S, Shuin T. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J Biol Chem. 2001;276:43611–43617. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- Ong KR, Woodward ER, Killick P, Lim C, Macdonald F, Maher ER. Genotype-phenotype correlations in von Hippel-Lindau disease. Hum Mutat. 2007;28:143–149. doi: 10.1002/humu.20385. [DOI] [PubMed] [Google Scholar]
- Richard S, Gardie B, Couve S, Gad S. Von Hippel-Lindau: How a rare disease illuminates cancer biology. Semin Cancer Biol. 2012 doi: 10.1016/j.semcancer.2012.05.005. (epub) [DOI] [PubMed] [Google Scholar]
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Farmer GE, Lamiell JM, Haines J, Yuen JW, Collins D, Majoor-Krakauer D, et al. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988;332:268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- Shen C, Kaelin WG., Jr The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2012 doi: 10.1016/j.semcancer.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolle C, Glenn G, Zbar B, Humphrey JS, Choyke P, Walther M, Pack S, Hurley K, Andrey C, Klausner R, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12:417–423. doi: 10.1002/(SICI)1098-1004(1998)12:6<417::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Harst E, de Krijger RR, Dinjens WN, Weeks LE, Bonjer HJ, Bruining HA, Lamberts SW, Koper JW. Germline mutations in the vhl gene in patients presenting with phaeochromocytomas. Int J Cancer. 1998;77:337–340. doi: 10.1002/(sici)1097-0215(19980729)77:3<337::aid-ijc5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- Wizigmann-Voos S, Breier G, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. 1995;55:1358–1364. [PubMed] [Google Scholar]
- Yang C, Asthagiri AR, Iyer RR, Lu J, Xu DS, Ksendzovsky A, Brady RO, Zhuang Z, Lonser RR. Missense mutations in the NF2 gene result in the quantitative loss of merlin protein and minimally affect protein intrinsic function. Proc Natl Acad Sci U S A. 2011;108:4980–4985. doi: 10.1073/pnas.1102198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Bertheau P, Emmert-Buck MR, Liotta LA, Gnarra J, Linehan WM, Lubensky IA. A microdissection technique for archival DNA analysis of specific cell populations in lesions < 1 mm in size. Am J Pathol. 1995;146:620–625. [PMC free article] [PubMed] [Google Scholar]