Abstract
Acute lymphoblastic leukemia (ALL) is the most common cancer in children and adolescents. Clinical presentation often reflects bone marrow (BM) involvement and consequences of BM failure. Microscopic involvement of the testis is rare, occurring in about 2% of cases. We present a case of a 3-year-old child who displayed unilateral macroorchidism as the only clinical symptom of ALL. Although the patient presented with localized disease, he was treated with systemic chemotherapy without recurrence. In this report, we review the current literature on ALL testicular involvement, diagnosis and treatment.
Keywords: ALL, B lymphoblastic lymphoma, testicle, unilateral macroorchidism, testicular infiltration
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common type of cancer in children. It is estimated that 2500 to 3500 new cases of B-ALL are diagnosed annually with an incidence of 2.8 cases per 100,000 [1]. In the most recent WHO classification of hematologic malignancies, precursor lymphoid neoplasms are divided into two broad categories based on cell lineage: B lymphoblastic leukemia/lymphoma (B-ALL/B-LBL) and T lymphoblastic leukemia/lymphoma (T-ALL/T-LBL) [2].
B-ALL is a neoplasm of precursor cells committed to the B-cell lineage (B-lymphoblasts) involving BM and peripheral blood (PB), which is the most typical presentation. Clinically, a case is defined as B-ALL if there are > 25% BM lymphoblasts with or without involvement of nodal or extranodal sites. In contrast, B-LBL is confined to a mass lesion with no or minimal evidence of PB and BM involvement. However, extramedullary involvement is frequent, with particular predilection for central nervous system, lymph nodes, soft tissue, skin, and spleen. Mediastinal masses are found infrequently.
Diagnosis and classification of leukemias are based on BM examination to determine the immunophenotype (lineage) and cytogenetic/molecular abnormalities [3, 4]. Leukemic cells can infiltrate various extramedullary tissues during the disease process [5]. In particular, microscopic testicular infiltration occurs at variable rates ranging from 1.9 to 21% [6,7]. Testicular infiltration more commonly occurs later during the course of disease and at relapse [8,9]. A debate continues with regards to the possibility of the testis as a sanctuary site [6]. Initial presentation with primarily testicular disease is exceedingly rare [10]. Here we present a case of isolated primary testicular B-ALL presenting in a 3-year-old boy with unilateral macroorchidism.
CASE REPORT
A 3-year-old male born in Singapore first presented to his pediatrician with pain and enlargement of his left testicle. The parents reported that the swelling fluctuated in size without notable inguinal swelling. The patient had no history of fever, trauma, lymphadenopathy, sick contacts, change in appetite, weight loss, or rash. After a course of antibiotics, the mass showed no improvement and he was referred for surgical evaluation.
Examination of the scrotum demonstrated swelling and tenderness of the left testicle. The testicle was firm with homogenous enlargement and no discrete mass was appreciated. A scrotal ultrasound revealed an asymmetrically enlarged hypoechoic left testicle with increased vascularity without focal mass (Fig. 1). Reexamination 3 weeks later showed no change in symptoms or testicular size. The patient underwent a transinguinal exploration, which revealed an enlarged left testis without focal mass. An incisional biopsy revealed infiltration with small round blue cells concerning for a hematological malignancy (Fig. 2).
Figure 1.
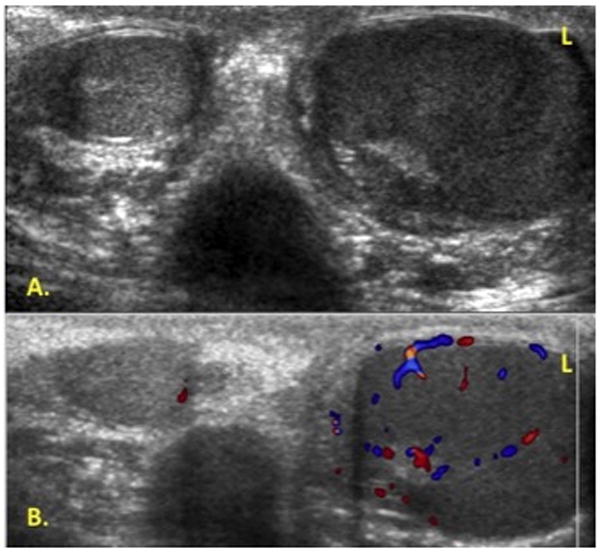
Scrotal doppler ultrasound. A. Asymmetrically enlarged hypoechoic left testicle compared to right. B. Left testicle with increased vascularity without focal mass.
Figure 2.
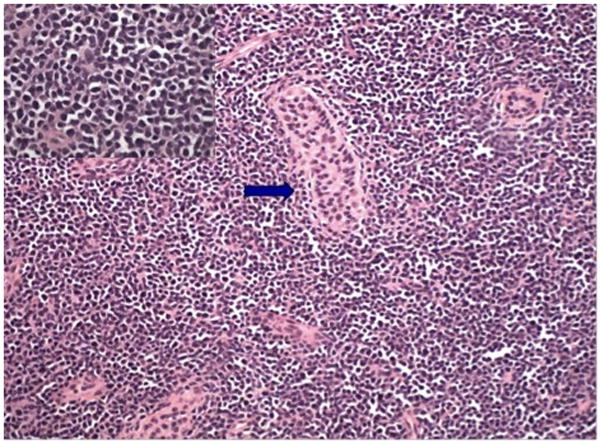
Testicular biopsy demonstrating extensive and diffuse infiltrate of lymphoblasts, residual tubule, arrowed, (Haematoxylin & Eosin stain, ×100). Higher magnification, insert, H&E stain, x400
Flow cytometry revealed a major population of immature precursor B-cells with the following immunophenotype: CD34+, CD19+, CD20−, Cyt.CD79a+, CD10+, Cyt. IgM+, HLA-DR+, TdT+, CD38+, CD43+, CD13−, CD33−, CD117−, and CytMPO−; this immunophenotype is diagnostic of B lymphoblastic lymphoma/leukemia (Fig. 3). Cytogenetics/FISH analysis showed p16 heterozygous deletion and evidence of trisomy 21 and trisomy 10, consistent with a hyperdiploid karyotype. Fluorescent PCR was positive for clonal Immunoglobulin Heavy Chain (IgH) gene rearrangement. Whole body PET scan revealed several subcentimeter lymph nodes in the paratracheal and hilar lung regions (SUV 1.5–2) and uptake in the left testis (SUV 5.0). BM aspirate with biopsy and lumbar puncture were negative for leukemia.
Figure 3.
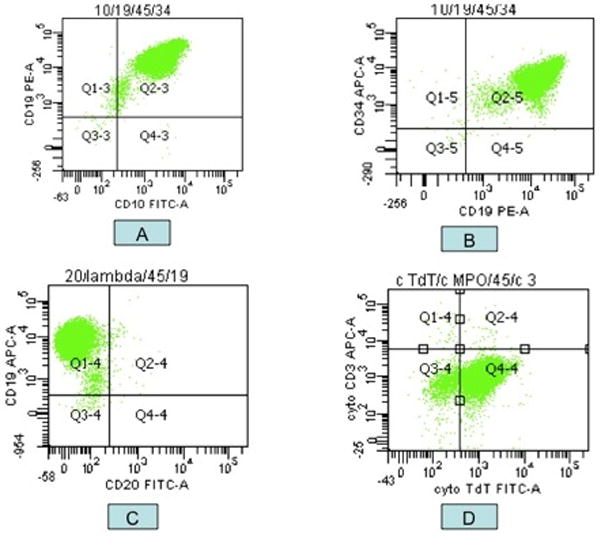
Flow cytometric analysis shows a population of lymphoblasts: CD19+/CD10+ (A), CD34+ (B), CD20− (C), and TdT+ (D).
Despite the isolated testicular involvement and absence of bone marrow infiltration or other mass lesions we chose to treat the patient with a leukemia-like therapy. Treatment was initiated in accordance with the Dana Farber Cancer Institute protocol for children with ALL, DFCI 05-001. The regimen consists of the following: induction (vincristine, prednisone, doxorubicin, methotrexate and intrathecal metotrexate, hydrocortisone, cytarabine); consolidation (vincristine, decadron, intrathecal metotrexate, hydrocortisone, cytarabine, high dose methotrexate, and L-asparaginase administered weekly for 30 weeks); and continuation (vincristine, decadron, methotrexate, 6-mercaptopurine).
At the completion of the consolidation phase of treatment a repeat PET scan was normal without avidity in the left testicle. A left testicular biopsy revealed no morphologic or phenotypic evidence of residual disease. Fluorescent PCR was negative for clonal Immunoglobulin Heavy Chain (IgH) gene rearrangement. Two years after the initial diagnosis, the patient’s disease remains in remission.
DISCUSSION
Acute lymphoblastic leukemia is the most common type of childhood cancer. Although leukemic cell infiltration can be seen in extramedullary tissues, it is rare to find testicular infiltration at the time of diagnosis. We found only one other case in the literature describing bilateral macroorchitis at presentation in an adolescent [10].
The differential diagnosis for left testicular swelling in a 3-year-old child includes inguinal hernia, hydrocele, varicocele, orchitis, abscess, torsion, Fragile X syndrome, trauma, and neoplastic disorders. In this case, symptoms persisted despite an appropriate course of antibiotics. Physical examination and ultrasound ruled out hernia and hydrocele. Testicular biopsy and evaluation confirmed B-ALL infiltration. If clinical suspicion for a testicular neoplastic process is present a transinguinal biopsy should be preformed to avoid transscrotal lymphatic spread.
Distinguishing neoplastic processes from orchitis on clinical grounds is challenging. Scrotal ultrasound is a useful modality in the evaluation of testicular enlargement and can aid in differentiating intratesticular from extratesticular abnormalities. Gray scale sonograms of testicular lymphoma and leukemia typically show diffuse or focal regions of decreased echogenicity with normal gross testicular shape. Color Doppler sonography can highlight increased vascularity present within these tumors [11,12]. Although not diagnostic of a neoplastic process, ultrasound can be used to support clinical findings of lymphoma or leukemia. Scrotal ultrasound can be used for initial evaluation and follow-up after treatment to assess for recurrence.
The diagnosis of B-ALL first requires clinical suspicion, since many of the presenting symptoms are non-specific. Even so, this case presentation suggests B-ALL may occur in the absence of the common symptoms such as fatigue and general malaise. Laboratory results from a complete blood count and blood smear may reveal anemia, elevated white blood cell counts, and blasts. A conclusive diagnosis is generally made from a bone marrow biopsy/aspiration and flow cytometry analysis to identify the presence of leukemic cells. In some cases, patients will present with a mass lesion, and involved tissue should also be biopsied. The diagnosis must indicate the type of leukemia in order to properly assess prognosis and selection of treatment for the patient.
Prognosis is dependent on leukemia phenotype and genotype, and other various prognostic factors such as patient’s age at diagnosis. Appropriate therapy for children presenting with localized disease is uncertain and current regimens balance efficacy with toxicity while addressing individual risk of relapse [13]. Systemic treatment of localized disease is an evolving treatment alternative that may reduce the rate of recurrence. A study examining CHOP therapy and no radiation for localized lymphoblastic lymphoma in children found a relapse rate of 30% after receiving 8 months of chemotherapy [14]. Despite the presentation with localized disease, B-ALL is likely a systemic disease and treatment should be geared to minimize relapse.
This case report describes a unique clinical picture of B-ALL wherein the patient did not present with the usual non-specific generalized symptoms, but rather isolated unilateral testicular involvement. Despite having localized disease he was treated with systemic chemotherapy, which allowed preservation of testicular function instead of surgery or radiation.
Footnotes
Financial disclosure: None
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Sherdlow SH, Campo E, Harris NL, et al. World Health Organization classification of tumors of haematopoietic and lymphoid tissues. IARC; Lyon: 2008. [Google Scholar]
- 3.Harrison CJ. The detection and significance of chromosomal abnormalities in childhood acute lymphoblastic leukemia. Blood Rev. 2001;15:49–59. doi: 10.1054/blre.2001.0150. [DOI] [PubMed] [Google Scholar]
- 4.Felix CA, Poplack DG, Reaman GH, et al. Characterization of immunoglobulin and T-cell receptor gene patterns in B-cell precursor acute lymphoblastic leukemia of childhood. J Clin Oncol. 1990;8:431–442. doi: 10.1200/JCO.1990.8.3.431. [DOI] [PubMed] [Google Scholar]
- 5.Hustu HO, Aur RJ. Extramedullary leukaemia. Clin Haematol. 1978;7:313–337. doi: 10.1016/s0308-2261(78)80008-3. [DOI] [PubMed] [Google Scholar]
- 6.Kay HEM. Testicular infiltration in acute lymphoblastic leukaemia. Br J Haematol. 1983;53:537–542. doi: 10.1111/j.1365-2141.1983.tb07305.x. [DOI] [PubMed] [Google Scholar]
- 7.Hijiya N, Liu W, Sandlund JT, et al. Overt testicular disease at diagnosis of childhood acute lymphoblastic leukemia: lack of therapeutic role of local irradiation. Leukemia. 2005;19:1399–1403. doi: 10.1038/sj.leu.2403843. [DOI] [PubMed] [Google Scholar]
- 8.Ortega JJ, Javier G, Torán N. Testicular infiltrates in children with acute lymphoblastic leukemia: a prospective study. Med Pediatr Oncol. 1984;12:386–393. doi: 10.1002/mpo.2950120606. [DOI] [PubMed] [Google Scholar]
- 9.Eden OB, Rankin A, Kay HEM. Isolated testicular relapse in acute lymphoblastic leukaemia of childhood. Arch Dis Child. 1983;58:128–131. doi: 10.1136/adc.58.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas A, Sahana PK. A case of Acute Lymphoblastic Leukemia presenting with Macroorchidism in a fourteen-year-old boy: A rare presentation. The Internet Journal of Hematology. 2009:5. [Google Scholar]
- 11.Mazzu D, Jeffrey RB, Rall PW. Lymphoma and leukemia involving the testicles: findings on gray-scale and color Doppler sonography. AJR. 1995;164:645–647. doi: 10.2214/ajr.164.3.7863887. [DOI] [PubMed] [Google Scholar]
- 12.Hamm B. Differential diagnosis of scrotal masses by ultrasound. Eur Radiol. 1997;7:668–679. doi: 10.1007/BF02742924. [DOI] [PubMed] [Google Scholar]
- 13.Link MP, Shuster JJ, Donaldson SS, et al. Treatment of children and young adults with early stage non-Hodgkin’s lymphoma. N Engl J Med. 1997;337:1259–1266. doi: 10.1056/NEJM199710303371802. [DOI] [PubMed] [Google Scholar]
- 14.Patte C, Auperin A, Michon J, et al. The Societe Francaise d’Ongologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]


