Abstract
A highly monodispersed hetero-nanostructure with two different functional nanomaterials (gold (Au) and iron oxide (Fe3O4, IO)) within one structure was successfully developed as Affibody based trimodality nanoprobe (positron emission tomography, PET; optical imaging; and magnetic resonance imaging, MRI) for imaging of epidermal growth factor receptor (EGFR) positive tumors. Unlike other regular nanostructures with a single component, the Au-IO hetero-nanostructures (Au-IONPs) with unique chemical and physical properties have capability to combine several imaging modalities together to provide complementary information. The IO component within hetero-nanostructures serve as a T2 reporter for MRI; and gold component serve as both optical and PET reporters. Moreover, such hetero-nanoprobes could provide a robust nano-platform for surface-specific modification with both targeting molecules (anti-EGFR Affibody protein) and PET imaging reporters (radiometal 64Cu chelators) in highly efficient and reliable manner. In vitro and in vivo study showed that the resultant nanoprobe provided high specificity, sensitivity, and excellent tumor contrast for both PET and MRI imaging in the human EGFR-expressing cells and tumors. Our study data also highlighted the EGFR targeting efficiency of hetero-nanoparticles and the feasibility for their further theranostic applications.
Keywords: Au-Fe3O4, Hetero-nanoparticles, Affibody, EGFR, PET, MRI, Optical
Introduction
Advancement on the research of different imaging modalities such as positron emission tomography (PET), single photon emission computed tomography (SPECT), optical imaging (OI), magnetic resonance imaging (MRI), computed tomography (CT), ultrasound, etc. has greatly improved their imaging sensitivity, specificity, and spatial and temporal resolution, which render them powerful tools for diseases detection. More recently, it has been widely recognized that combining certain functional imaging techniques (PET, OI, etc.) with anatomic imaging modalities (MRI, CT, etc.) together can provide complementary information and thus offer synergistic advantages over any single modality alone. For example, PET/CT combines functional PET and three-dimensional high resolution CT [1, 2]. It has shown many applications in cancer imaging and been fully integrated into routine clinical practice [3, 4]. Comparing to CT, MRI offers even higher soft tissue resolution while without any radiation exposure to patients. As encouraged by the success of PET/CT, combination of PET and MRI (PET/MRI) has been actively pursued in the imaging community for the last several years. Several research groups have reported development of PET/MRI systems for small-animal studies [5, 6]. More importantly, PET/MRI in a single device with simultaneous imaging acquisition has also been introduced into clinical applications recently [7–9]. Comparing with PET/CT, PET/MRI provides better soft-tissue contrast and more functional information with lower radiation exposure, thereby raising it to be an attractive choice of future imaging modality.
With the rapid development of imaging instrumentations, there are urgent demands in developing multimodal molecular probes such as dual-labeled probes for PET/MRI pre-clinically and clinically. This type of agents could be biomolecules labeled with both PET radionuclide and MRI metal or nanoparticle (NPs) that are capable of providing specific dual-modal imaging. Recently iron oxide nanoparticle (IONP) has been modified with the Arg-Gly-Asp (RGD) peptide and radiometal chelators and labeled with 64Cu for both PET and MRI of integrin αvβ3 expression in tumor [10, 11]. These pioneer studies demonstrate the feasibility of preparation of PET/MRI probes and further use of them for cancer imaging in vivo. However, until now the majority of studies on PET/MRI probe development have simply focused on the use of IONP which is composed by a single component and could suffer from certain limitations as listed below. First, coupling of different chemical functionalities on a mono-component NP surface could be a low yield synthetic process; Second, the presence of targeting biomolecules, functional labeling moieties and drugs on the same NP surface may compromise the targeting and imaging capability and the subsequent in vivo performance of the NP conjugates; Lastly, modification of a single component NP with multiple different ligands simultaneously or sequentially is a relative complex process that could have low reproducibility on the product preparation. It usually generates heterogeneous products which could be consisted by different ratios of ligands on the NPs. This problem could severely hamper their further translation into clinic.
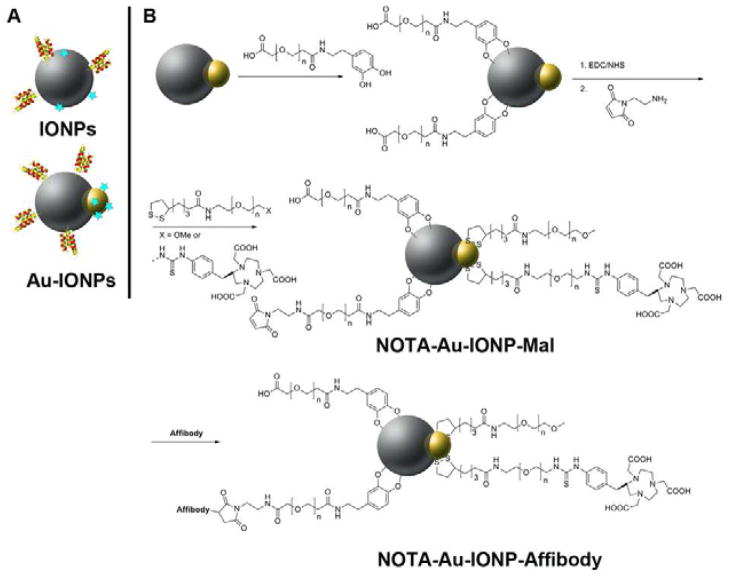
One of the promising solutions is to use multicomponents NPs, which have at least two different NPs within one structure [12, 13]. Unlike regular single-component nanospheres, dumbbell-like NPs (DBNPs) have two distinctive NP surfaces (Figure 1A), so that many biological targeting molecules, drug molecules, or labeling moieties can be specifically anchored on each of NP surface, eliminating their intimate contact and interference. The recent progress in synthesis and application of Au–Fe3O4 DBNPs (Au-IONPs) had attracted much attention for its potential as multifunctional probes in diagnosis and therapy [13–17]. Furthermore, Au-IONPs are constructed with both a magnetic (IO) and an optically active plasmonic (Au) unit, which is also suitable for simultaneous optical and magnetic detection [12]. But all the previous reported studies regarding Au-IONPs are solely focused on in vitro studies. Moreover, the average sizes of those whole hetero-nanostructures varied in the range from 15 to 20 nm with a broad size distribution. There was not much attention paid to the size and quality control of hetero-nanostructures when they were used as probes or delivery vehicles in vitro studies. To achieve highly sensitive and efficient tumor cell detection in vivo, the hetero-nanostructures need to be monodisperse so that each individual NP has nearly identical physical and chemical properties for controlled biodistribution, bioelimination and contrast effects. Moreover, it is important to systematically optimize various nanostructure parameters (such as size, surface chemistry) at a time to enhance the detection sensitivity and tumor accumulation of those engineered hetero-nanostructures. Considering the size, shape, and surface modification have profound impact to the in vivo performance of the NPs and smaller size NPs are usually preferred for in vivo targeted imaging, herein, we report the development and use of a highly monodispersed dumbbell-shaped Au–IONP (size: ~4 nm in diameter for Au NP and 8 nm in diameter for IONP, characterized by TEM) for the development of a PET/Optical/MRI probe for cancer imaging in small living animals.
Figure 1.
A: Schematic three dimensional illustration of the Affibody binding domain with conventional single-component IONP (top) and dumbbell Au-IONP (bottom). B: Schematic illustration of Au-IONPs surface functionalization and conjugation with Affibody.
The diagnosis and treatment of cancers at the molecular level would be greatly enhanced by the ability to deliver contact agents and/or potentially therapeutic agents into specific cancer cells and cellular compartments in living systems. Such monodisperse Au–IONP provides a robust nanoplatform for surface-specific modification with both targeting molecules and PET reporters in a highly efficient and reliable manner. Specifically, the Fe3O4 component in the Au–IONP can serve as a T2 reporter for MRI, and the IO surface was first coated with the polyethylene glycolated (PEGylated) dopamine linkers, followed by specifically conjugating anti-epidermal growth factor receptor (EGFR) Affibody proteins (for example: Ac-Cys-ZEGFR: 1907, amino acid sequence, Ac-C-VDNKFNKEMWAAWEEIRNLPNLNGWQMTAFIASLVDDPS -QSANLLAEAKKLNDAQAPK) (Figure 1B). Affibody proteins are a new class of engineered scaffold proteins with a three-helix bundle structure. Comparing to antibodies, they have certain advantages for tumor-targeted imaging, including their much smaller size and lower molecular weight (58-amino acid residues, 7 kDa), faster tumor targeting ability, more well-defined structure which could potentially be site-specifically modified and can be chemically synthesized. Affibodies have shown high promise for development of tumor targeting agents [18–24]. Several anti-EGFR Affibody proteins including ZEGFR:1907 with high affinities in nM ranges have been reported and used for tumor imaging and potential radionuclide therapy [25- 30]. Since EGFR is a well established tumor biomarker and overexpressed in a wide range of human tumors [31]. modification of the Au–IONP with anti-EGFR Affibody molecules, like Ac-Cys-ZEGFR:1907 [30], makes the resulting nanoprobe capable for early diagnosis of various EGFR positive tumors. To further prepare the multimodal nanoprobe, the Au component of the Au-IONP was surface-specifically modified with thiolated PEG linkers via Au-S bonds, followed by coupling with the radiometal chelators, 2-S-(4-isothiocyanatobenzyl)-1, 4, 7-triazacyclononane-1, 4, 7-triacetic acid (p-SCN-Bn-NOTA). Radiolabeling the NOTA and Ac-Cys-ZEGFR:1907 modified Au–IONP (NOTA-Au-IONP-Affibody) with PET radionuclide, 64Cu, results in an EGFR targeted PET/Optical/MR Imaging probe, 64Cu-NOTA-Au-IONP-Affibody (Figure 1B). We therefore consider that Affibody molecules are promising affinity ligands for making Au-IONPs targetable without adverse effect on their in vivo performance and giving those NPs targeting ability toward EGFR-positive tumors.
Materials and methods
Instrumentation and Materials
Hydrogen tetrachloroaurate (III) hydrate was ordered from Strem Chemicals, Inc.. N-hydroxysuccinimide (NHS), N-hydroxysulfosuccinimide (sulfo-NHS), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) were purchased from Pierce Biotechnology. PEG bisamine [Molecular weight (MW) = 2000 and 3000] and methoxyPEG amine 2000 (m-PEG-2000-NH2) were ordered from Sigma/Aldrich. The metal chelator, p-SCN-Bn-NOTA, was obtained from Macrocyclics. Unless otherwise mentioned, all other chemicals were purchased from Sigma/Aldrich. All solvents and chemicals were used as received. 64Cu was provided by the Department of Medical Physics, University of Wisconsin at Madison (Madison, WI).
A CRC-15R PET dose calibrator (Capintec Inc., Ramsey, NJ) was used for all radioactivity measurements. Mass spectra of synthetic polymers were recorded by a time-of-flight (TOF) mass spectrometer (AB SCIEX TOF/TOF 5800, Applied Biosystems) equipped with a matrix-assisted laser desorption ionization (MALDI) ion source. UV/vis absorption spectra were measured by a PerkinElmer Lambda 35 UV/vis spectrometer. The fluorescence spectra were acquired on Fluoromax 4 (Horiba Jobin Yvon Inc.) spectrofluorometer. Fluorescence confocal scanning laser microscopy (CSLM) was performed using a Zeiss LSM510 Meta CLSM. Graphical image analysis was performed using Adobe Photoshop. The elemental analyses were performed using inductively coupled plasma mass spectrometer (ICP-MS, Thermo Scientific Xseries 2 Quadrupole). The NP samples were suspended in freshly prepared aqua regia (trace metal grade 70% nitric acid HNO3:36% hydrochloric acid HCl (Fisher Scientific), 1:3/v:v) and heated until completely dissolved, and then diluted up to 8 ml with double-distilled water. The cells pallets, homogenized tissues and organs (no more than 500 mg) were digested in a microwave (CEM MarsXpress Microwave Digester with Teflon microwave-safe vessels) before ICP analysis. The high-resolution transmission electron microscope (HRTEM) and scanning transmission electron microscope (STEM) images were recorded with a FEI Tecnai G2 F20 X-TWIN transmission electron microscope operating at 200 kV. Samples were deposited and dried on copper grids covered with a Formvar/carbon support film, followed by plasma cleaning.
Synthesis of Functional PEG linkers
Two different PEG linkers were synthesized for coating the Au and IONP surface. O-(3- aminopropyl) polyethylene glycol 2000 lipoate amide (LPA-PEG-2000-NH2): To a stirred solution of the PEG-2000-bisamine (400 mg, 0.2 mmol) and triethylamine (20 mg, 0.2 mmol) in 10 mL of 1,4-dioxane was added dropwise a solution of lipoate-NHS ester (0.8 eq. 0.16 mmol) in 2 mL of 1,4-dioxane over 1 h. The lipoate-NHS ester was synthesized according to the literature.[32] The reaction mixture was allowed to stand overnight until thin layer chromatography (TLC) analysis showed no lipoate-NHS ester remaining. After the solvent was evaporated, the crude product was purified by alumina column (dichloromethane/methanol, 95:5). The resultant product was recrystallized from a solution of methylene chloride/ethyl ether as a white solid (yield = 65%).
Monomethoxyl polyethylene glycol 2000 lipoate amide (LPA-mPEG-2000)
Similarly, to a stirred solution of the methoxyPEG amine 2000 (m-PEG-2000-NH2) (400 mg, 0.2 mmol) and triethylamine (20 mg, 0.2 mmol) in 10 mL of 1,4-dioxane was added dropwise a solution of lipoate-NHS ester (1.1 eq. 0.22 mmol) in 2 mL of 1,4-dioxane over 1 h. The reaction mixture was allowed to stand overnight until TLC analysis showed no free amine remaining (Kaiser Test). After the solvent was evaporated, the crude product was purified by a flash column. The resultant product was recrystallized from a solution of methylene chloride/ethyl ether as a white solid (yield = 87%).
LPA-PEG-2000-NOTA
PEG-NOTA conjugate was prepared under standard SCN-amine reaction condition. Briefly, a solution of 2.5 μmol of LPA-PEG-2000-NH2 (5 mg) was mixed with 5 μmol of p-SCN-Bn-NOTA (2.8 mg) in 0.4 mL of 20 mM sodium bicarbonate buffer (pH = 8.5). After stirring at room temperature for 4 hrs, the NOTA conjugated PEG was purified by HPLC. The collected fractions were combined and lyophilized to afford the final product as a white powder.
Synthesis of Au-IONPs and Surface Modification
The water soluble Au-IONPs were synthesized according to the procedures described in the previous publications [12, 14]. The surface modification of Au-IONPs was optimized according to previous works [13, 21, 23]. Briefly, PEG-3000-diacid (60 mg), EDC (4 mg) and NHS (3 mg) were dissolved in 2 mL of choloroform. After the mixture was stirred at room temperature for 30 min, dopamine hydrochloride (4 mg) in 1 mL of DMF was added into the solution in the present of 5 mg of sodium carbonate. The resultant mixture was stirred at room temperature for 2 hours. Au-IONPs (20 mg) pre-dissolved in 1 mL of choloroform was transferred into the mixture. The resulting mixture was kept shaking for overnight at room temperature under nitrogen. The resultant NPs were precipitated by adding hexane (15 mL), collected by a brief centrifuge or a permanent magnet, and dried under the nitrogen gas flow. The PEGylated NPs were then dispersed in water. The unbound PEG and any other excess reagents were removed by dialysis against water or phosphate-buffered saline (PBS) (0.01 M, pH 7.4) by tubing (Spectrum Spectra/Por dialysis membrane tubing, MWCO = 12 kDa). Any impurity or precipitate was removed by a 0.22 μm syringe filter. The final iron or gold concentration of PEGylated NPs was measured by inductively coupled plasma mass spectrometry (ICP-MS) analysis.
Determination of molar concentrations of Au-IONPs
The molar atom weight of dumbbell-like Au-IONPs was calculated based on the actual shapes identified by TEM (Figure S3). The ratio of gold to iron weight of Au-IONPs was determined by ICP-MS. The weight of single NP was determined by its ideal geometry shape and the density of individual component. The molar atom weight of Au-IONPs is 1.23 × 106 g/mol. The actual weight concentrations of Au-IONPs (based on iron or gold) were determined by ICP-MS. Based on their molar atom weight, the molar concentrations of Au-IONPs were calculated accordingly.
Synthesis of NOTA-Au-IONP-Mal
The water-soluble Au-IONPs (1 mg of Fe in 2 mL of water, 0.4 nmol of NPs) in water were mixed with EDC (1.0 mg, 5 μmol) and sulfo-NHS (2.2 mg, 10 μmol) for 30 min at room temperature. Then activated NPs run through a PD-10 column pre-washed with PBS to remove excessive EDC and sulfo-NHS. The 2-maleimidoethylamine (MEA) (1.25 mg, 5 μmol) in 50 μL of DMSO was added into the PBS solution of activated Au-IONPs. The resultant solution was stirred for 4 hrs at room temperature. The final conjugates were separated from unbound MEA by dialysis (MWCO = 12 kDa) against PBS. The final iron or gold concentration of thiol-active Au-IONPs (Au-IONP-Mal) was measured by ICP-MS analysis. The Au-IONP-Mal was then linked with functional PEG linkers, LPA-PEG-2000-NOTA and LPA-mPEG-2000. As-synthesized Au-IONP-Mal (0.5 mg of Fe in 1 mL of PBS, 0.2 nmol of nanoparticles) was directly mixed with functional PEG linkers (LPA-mPEG-2000, 1 mM in 0.5 mL of PBS, 500 nmol; LPA-PEG-2000-NOTA, 1 mM in 0.5 mL of PBS, 500 nmol) at room temperature overnight. The uncoupled ligand was removed through a PD-10 column. The final product was concentrated by a centrifugal-filter (Millipore, MWCO = 30 kDa). The final iron or gold concentration of NOTA-Au-IONP-Mal was measured by ICP-AES analysis.
Bioconjugation of NOTA-Au-IONP-Mal with Affibody
An Affibody derivative, Ac-Cys-ZEGFR:1907 (Ac-CVDNKFNKEMWAAWE EIRNLPNLNGWQMTAFIASLVDDPSQSANLLAEAKKLNDAQAPK-NH2) with 59 amino acid residues and a cysteine at the N terminal, was successfully synthesized using solid phase peptide synthesis as previously reported by our group [23, 24, 27]. The as-prepared NOTA-Au-IONP-Mal (0.5 mg of Fe in 1 mL of PBS, 0.2 nmol of nanoparticles) was added to the Affibody solution in PBS (0.35 mg, 50 nmol). The resultant mixture was stirred at room temperature for overnight. The uncoupled Affibody was removed by a PD-10 column. Similarly, the final product NOTA-Au-IONP-Affibody was concentrated by a centrifugal-filter (Millipore, MWCO = 30 kDa), and iron or gold concentration of NOTA-Au-IONP-Affibody was measured by ICP-AES analysis.
The average number of Affibody on each NOTA-Au-IONP-Affibody NP was estimated during the modification. Briefly, unbound Affibody after conjugation reaction was collected. Compared with the initial concentration of Affibody, the concentrations of unbound Affibody in the filtrate were measured with HPLC. The coupled Affibody was calculated from the difference between initial concentration and final one. The ratio of Affibody to Au-IONP was 213 ± 47:1 (n = 3).
Characterization of Dumbbell NPs and Dumbbell-Affibody compounds
The size, surface charge, morphology of the Au-IONPs and the modified NPs including NOTA-Au-IONP, NOTA-Au-IONP-Affibody were characterized by dynamic light scattering (DLS), transmission electron microscopy (TEM), and scanning transmission electron microscopy (STEM). DLS was used to examine the hydrodynamic diameters and zeta potential of Au-IONPs before and after modification using a Malvern Zeta Sizer Nano S-90 DLS instrument (Malvern Instruments Ltd, UK). The TEM and STEM images were recorded with a FEI Tecnai G2 F20 X-TWIN transmission electron microscope operating at 200 kV (see previous section). Samples were dispersed on carbon films supported on copper grids. STEM was performed to confirm the component of dumbbell structure NPs. High-angle annular dark field and annular bright field images were observed to detect different incoherent quasi-elastic electron scattering of the Au-IONPs, depending on the variation in nuclear atoms.
MRI Phantom Study
To prove the feasibility of Au-IONPs as an MRI contrast agent, transverse T2 weighted MR images of both Feridex and Au-IONPs were acquired on a 7.0-T small-animal MRI system (GE Healthcare, USA), with gradient iron concentrations from 0 to 0.5 mM. T2-weighted MRI images were acquired on a GE 7.0 T small animal MRI system with the following parameters: TR 3000 ms; TE 40 ms; flip angle 30°; Field of view (FOV) 6 × 6, 256 × 256 matrix; slice thickness 1 mm. Particles were suspended in 0.5% agarose gel in 300 μL PCR tubes. The tubes were embedded in a home-made tank, which was designed to fit the MRI coil and was filled with 0.5% agarose gel.
Cell culture and tumor xenograft model
EGFR positive A431 (human epithelial carcinoma cell line) cells were purchased from ATCC [27, 28]. The A431 cell line was cultured in DMEM [high glucose, 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin] in T25 flasks (Cornell Corp., Cornell, WI). The cells were expanded in tissue culture dishes and kept in a humidified atmosphere of 5% CO2 at 37°C. The media was changed every other day. A confluent monolayer was detached with 0.5% Trypsin-ethylenediaminetetraacetic acid (EDTA), PBS (0.01M, pH 7.4) and dissociated into a single-cell suspension for further cell culture and assays. All animal studies were carried out in compliance with federal and local institutional rules for the conduct of animal experimentation. Approximately 5×106 cultured A431 cells were suspended in PBS and subcutaneously implanted in the right shoulders of nude mice. Tumors were allowed to grow to a size of 0.5–1.0 cm in diameter (2–4 weeks) before imaging experiments.
Cell Study of NOTA-Au-IONP-Affibody and Optical Imaging
A431 cells were placed on the glass bottom Petri dishes (MatTek Corp.) and grown to ~80–90% confluence. NOTA-Au-IONP-Affibody at concentration of 20 μg[Fe]/mL3in DMEM was added to the culture dishes and incubated with A431 cells at 37°C in the 5% CO2 incubator for 1 hr. For the blocking experiment, A431 cells were pretreated with 20 μM Ac-Cys-ZEGFR:1907 for 30 min prior to adding NOTA-Au-IONP-Affibody. After incubation, the cells were washed with PBS three times and then fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-100 followed by 4′-6-Diamidino-2-phenylindole (DAPI) staining. Reflection images were taken on a Leica inverted epifluorescence/reflectance laser scanning confocal microscope. The wavelength used for the cell imaging was 633 nm. Similarly, A431 cells in 12-well plates were also incubated with 20 μg [Fe]/mL of NOTA-Au-IONP-Affibody for 1 h or 2 hrs at 37°C. After washed with PBS three times, they were detached by trypsine and harvested by centrifuge. Cells were counted with a hemocytometer. The cell pellets were then lysed by nitric acid and aqua regia. The cellular uptake of NPs was quantified by measuring Fe and Au content in each sample using ICP-MS.
Radiolabeling of NOTA-Au-IONP-Affibody and Serum Stability Study
The 64Cu labeling procedure was conducted according to the methods previously described.[21] Briefly, the stock solution of 64CuCl2 was diluted with 200 μL of 0.1 M sodium acetate buffer (pH = 6.0). 64Cu stock solution (1 mCi, 37 MBq) was then added to 200 μL of NOTA-Au-IONP-Affibody in 0.1 M sodium acetate buffer (pH = 6.0). After the resultant solution was incubated for 1 hr at 40 °C, the radiolabeled NPs were applied to a PD-10 column and eluted with PBS. The product was used for further in vitro and animal experiments. The radiolabeling yield was generally ~60%. The purification of radiolabeling solution using a PD-10 column afforded 64Cu-NOTA-Au-IONP-Affibody with modest specific activity (74~222 MBq/nmol particle, 1~3 μCi/μg Fe, see more Information in the concentration conversion section).
The serum stability of 64Cu labeled NOTA-Au-IONP-Affibody was performed according to our previous report [21]. Briefly, 64Cu-NOTA-Au-IONP-Affibody was incubated in mouse serum at 37°C for up to 24 hrs. Aliquots of the mixture were removed from the solution at predetermined time intervals and filtered through 30 kDa cutoff filters. The radioactivity of filtrates was then measured. The stability of radiolabels NPs was calculated by the percentages of retained 64Cu on the NPs to the total activity. Samples were assayed in triplicate.
Quantification Analysis of number of NOTA groups on NOTA-Au-IONP-Affibody
The average number of NOTA chelators on each NOTA-Au-IONP-Affibody NP was measured using a modified procedure [33, 34]. Briefly, 100 μCi of 64CuCl2 in 10 μl of 100 mM 0.1 M sodium acetate buffer (pH = 6.0) was added into a known amount of non-radioactive CuCl2 in sodium acetate buffer (200 fold excess of Au-IONP concentration, 25 μl of 20 μM). Twenty picomolars of NOTA-Au-IONP-Affibody in 50 μl of sodium acetate buffer were added to the above solution. The resultant mixture was incubated at 40°C for 1 hour with constant shaking. The Cu-chelated NOTA-Au-IONP-Affibody were then purified using a centrifugal filter (Amicon centrifugal filter device, MWCO = 30 kDa). The decay-corrected radio-activities of both collected filtrate and filtered tripods were measured to calculate the radio labeling yield. The average number of NOTA groups on NOTA-Au-IONP-Affibody was determined as ratio of the moles of (non-radioactive Cu2+ × yield) to moles of Au-IONPs. Typically, the average number of NOTA per Au-IONP equals 13.7 ± 2.4 (n = 3).
In vivo Small Animal PET/MRI of Tumor Mice
64Cu-NOTA-Au-IONP-Affibody (100 μCi in 150 μL PBS) was injected via tail vein into the A431 tumor-bearing mice at a dose of 10 mg Fe/kg of mouse weight (n=3 for each group). For in vivo blocking study, equivalent amount of 64Cu-NOTA-Au-IONP-Affibody spiked with Ac-Cys- ZEGFR:1907 (20 mg/Kg) and was injected intravenously into tumor mice for PET and MRI. In vivo small-animal PET was carried out on a microPET R4 rodent model scanner (Siemens Medical Solution, cite information if it is needed) according to the previous publications [21, 27, 28]. PET scans were performed at 1, 4, 24 and 48 hr post-injection (p.i.). Mice were anesthetized with isoflurane (5% for induction and 2% for maintenance in 100% O2). With the help of a laser beam attached to the scanner, mice were placed in the prone position and near the center of the field of view (FOV) of the scanner and 3-min static scans were obtained. All the small animal PET images were reconstructed by a two-dimensional ordered-subsets expectation maximization (OSEM) algorithm. No background correction was performed. Region of interests (ROIs; 2.5 pixels for coronal and transaxial slices) were drawn over the tumor on decay-corrected whole-body coronal images. Values in three to ten adjacent slices (depending on the size of the tissue or organ) were averaged to obtain a reproducible value of radioactivity concentration. The maximum counts per pixel per minute were obtained from the ROIs and were converted to counts per milliliter per minute using a calibration constant. ROIs were then converted to counts per gram per minute based on the assumption of a tissue density of 1 gram/ml, and image ROI-derived percentage of the injected radioactive dose per gram of tissue (%ID/g) values were determined by dividing counts per gram per minute by injected dose. No attenuation correction was performed.
Immediately after PET scans, T2-weighted fast spin-echo MR images were acquired on a 7.0-T small animal MRI system (GE Healthcare) under the following parameters: repetition time (TR) = 3000 ms, TE =40 ms, echo train length = 8, FOV = 4×4 cm2, section thickness = 1mm, flip angle = 90°. MR images were acquired in both transverse and coronal direction pre-injection and at 4 hr and 48 hr after injection. Transversal and coronal MR images were acquired and the signal intensities were measured in defined ROIs using OsiriX imaging software (OsiriX version 3.2; Apple Computer). The NIH standard was used for tumor imaging processing. The ROI analysis was performed according to the previous publications [10, 11, 13].
In vivo Biodistribution Study
For the mice imaged with PET/MRI, at the end of the 48 h scan, they were sacrificed and the major organs or tissues were collected and wet weighed. The radioactivity in the tissues or organs was measured using a γ-counter (Packard/Perkin Elmer), and calibrated against a known aliquot of the injection and normalized with body weights. The radioactivity uptake in the tumor and normal tissues was expressed as a % ID/g. Finally, after radioactivity decay, the tumor and other collected tissues were digested for ICP-MS analysis of Fe and Au contents from 64Cu-NOTA-Au-IONP-Affibody.
Statistical Method
Statistical analysis was performed using the Student’s t-test for unpaired data. A 95% confidence level was chosen to determine the significance between groups, with P < 0.05 being designated as significantly different.
Result
NOTA-Au-IONP-Affibody Preparation and Characterization
First, the highly monodisperse Au-IONPs were synthesized according to the modified procedures described in the previous publications [12, 14], in which the injection condition of iron precursor (iron pentacarbonyl) and subsequent heating rate played important roles in determining the quality of obtained NPs (including shape and size distribution). The size of iron oxide component within Au-IONPs was dependant on the ratio of gold seeds to iron precursor. A constant heating rate at 8°C/min resulted in the Au-IONPs with high quality shape and narrow size distribution.
In order to maximize blood circulation half-life and enhance the tumor uptake, the dumbbell Au-IONPs can be made suitable for linking different functional molecules or moieties to each end of the structure through surface modification. After ligand exchange, PEGylated dopamine (Dop-PEG2000, supporting information) was strongly attached onto the IONPs and rendered particles with great stability against agglomeration in physiological environment. With this onestep surface coating procedure, the PEGylated NPs were water-dispersible with amine terminal groups and further modified with the anti-EGFR small Affibody protein which is an engineered small protein with 58-amino acid residues, 7 kDa with a three-helix bundle structure. Metal chelator, NOTA, can then be immobilized on the gold NPs by disulfide bonds and followed with 64Cu raidolabeling. Figure 1 illustrated the surface functionalization of the Au–IONPs and the conjugation with Affibody. Affibody was linked to the PEGylated Au-IONPs via the maleimide bonds. The Au NP was protected with thiolated PEG via Au-S bond. The coupled Affibody was measured from the difference between initial concentration of Affibody and final one during the surface modification (Supporting Information) The ratio of Affibody to Au-IONP was 213 ± 47:1 (n = 3).
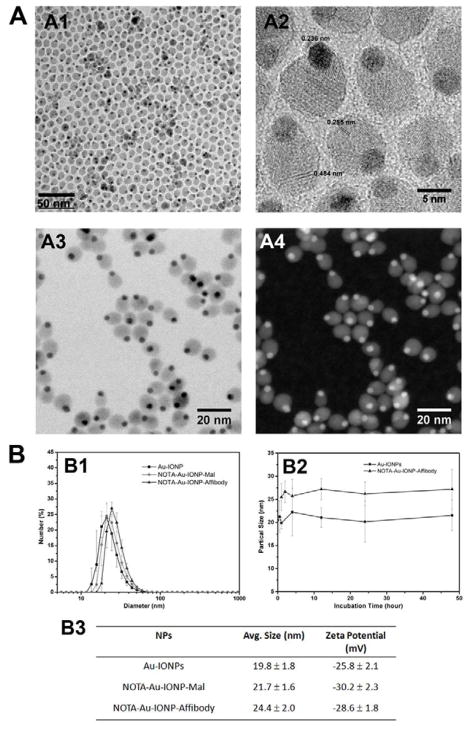
Figure 2 illustrated the characterization of dumbbell-like Au–IONPs that were constructed and functionalized in this study. TEM images (Figure 2A1) showed homogeneous dispersed dumbbell-shaped NPs with a narrow size distribution. The lattice at [111] was 0.235 nm of Au, and 0.484 nm of Fe3O4. The lattice of Fe3O4 at 311 was 0.255 nm (Figure 2A2). To assess the constitution of Au-IONP particles, STEM was performed. In the high-angle annular dark field (Z contrast) image (Figure 2A4), the Au particles, which have high atomic numbers, were represented as bright dots. STEM results confirmed the Au and IO components in the dumbbell-shaped nanostructure. DLS study results showed the average hydrodynamic diameter of Au-IONPs, Au-IONP-NOTA and NOTA-Au-IONP-Affibody were 19.8 ± 1.8 nm, 21.7 ± 1.6 nm and 24.4 ± 2.0 nm, respectively (Figure 2B1 and 2B3). The increase of size from 19.8 nm to 24.4 nm was due to the conjugation of Affibody with NPs, which confirmed the successful surface coating and Affibody conjugation. The zeta potential detected by DLS was -25.8 ± 2.1 mV, -30.2 ± 2.3mV and -28.6 ± 1.8 mV (Figure 2B3), which showed that the surface charge of Au-IONPs was quite stable after surface modification. The particle sizes of Au-IONPs and NOTA-Au-IONP-Affibody were very stable with not much variation after incubation in 10% fetal bovine serum (FBS) in PBS at 37°C for 48 hours, as evidenced by their unchanged hydrodynamic sizes (Figure 2B2).
Figure 2.
A shows the TEM and STEM images of Au-IONPs. TEM reveals that the average size of Au-IO nanoparticles is approximately 10 nm (A1–2). The Au particles represented as dark dots in the bright field (A3) and bright dots in the high-angle annular dark field (Z contrast) image (A4). B demonstrates the hydrodynamic size (B1), zeta potential (B3) and stability results (B2) of Au-IONPs.
MRI Phantom study
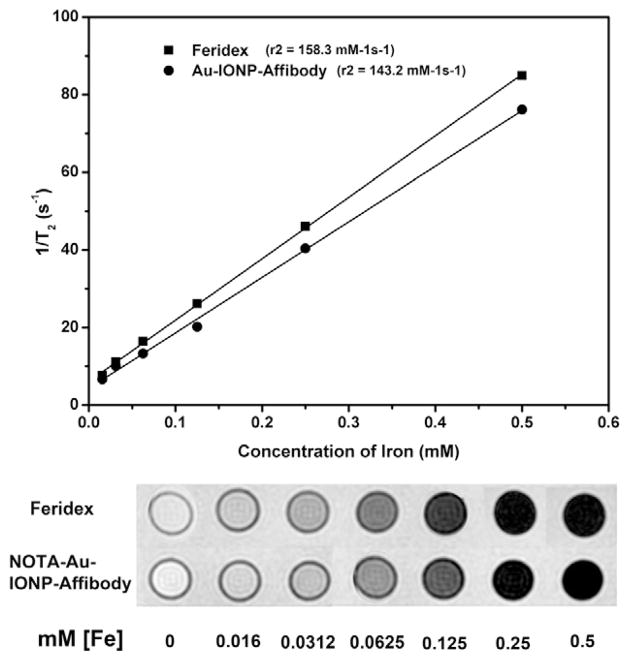
The iron oxide component in the Au-IONP structure can serve as a contrast enhancement agent for MRI. The contrast-enhancement effect of T2-weighted MR images of NOTA-Au-IONP- Affibody and Feridex in a same concentration gradient ranging from 0 mM to 0.5 mM was performed in a MRI phantom study. MRI analysis revealed that Au-IONPs could shorten the T2 relaxation of the water molecules with their relaxation rate (r2 relaxivities) being 143.2 (s·mM)−1, which was close to that of the commercial available MRI contrast agent, Feridex, 158.3 (s·mM)− 1, suggesting that Au-IONP could serve as an efficient MR contrast agent (Figure 3).
Figure 3.
Magnetic properties of NOTA-Au-IONP-Affibody. T2-weighted MR images of NOTA-Au-IONP-Affibody and Feridex in a same concentration gradient ranging from 0 mM to 0.5 mM, the relaxation rate is similar between the two contrast agents [143.2(s·mM)−1 versus 158.3(s·mM)−1].
Cell Study of NOTA-Au-IONP-Affibody
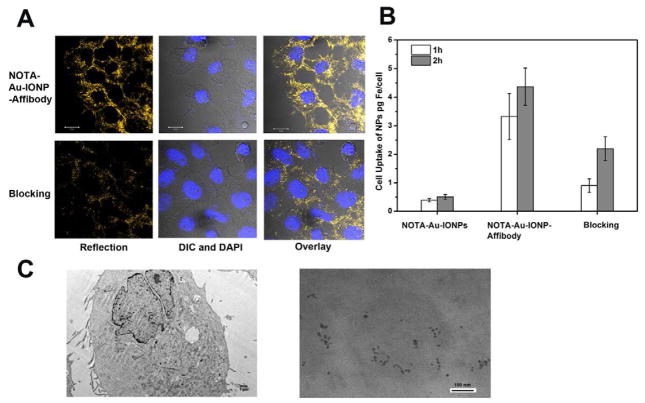
Specific targeting of NOTA-Au-IONP-Affibody were visualized with an inverted epifluorescence/reflectance laser scanning confocal for reflection and fluorescence imaging after 2 h incubation of the nanoprobe (Figure 4A). Due to plasmon enhancement, the gold strongly scatters the light. The wavelength used was 633 nm, and it was found that the region where the NPs could show strong reflection. The signal detected from the reflection of Au-IONPs could be clearly seen from the reflection images. As we found from the overlay of DAPI and reflection images, after 2 h incubation, most of the NOTA-Au-IONP-Affibody probes were internalized in the EGFR-positive tumor A431cell. In comparison, the reflection signal of the A431 cells from the Affibody blocking group was much weaker (Figure 4A). The result confirmed that the nanoprobe could selectively target EGFR positive tumor cells. Moreover, after incubation with the NOTA-Au-IONP-Affibody and Au-IONP with and without the blocking agent at 1 and 2 h,, the concentrations of Fe and Au in A431 cells were determined by ICP. The quantification results demonstrated that, NOTA-Au-IONP-Affibody had a significantly higher cellular uptake in A431 cells at both 1h and 2h time points, comparing with the untargeted and blocking samples (Figure 4B). Lastly, the TEM images also showed the internalization of NOTA-Au-IONP-Affibody, which directly confirmed the intracellular uptake of the NPs (Figure 4C).
Figure 4.
A. Reflection and confocal images show that A431 cells labeled with NOTA-Au–IONP-Affibody can be visualized with a scanning confocal microscope at 633 nm. The reflection signals from the NOTA-Au–IONP-Affibody incubated A431 cells are much stronger than that of the blocking group. B. Quantification analysis of the cellular uptake in A431 cells after incubation with NOTA-Au-IONP, and NOTA-Au-IONP-Affibody with and without Affibody blocking. NOTA-Au-IONP-Affibody had a significantly higher cellular uptake at both 1 h and 2 h time points than that of the other two groups. C. TEM images for NOTA-Au-IONP-Affibody internalization. Figure 5C-left shows the A431 tumor cell morphology, while Figure 5C-right demonstrates the NOTA-Au-IONP-Affibody in the endosome of A431 cell as the evidence of cell internalization.
In vivo PET of 64Cu-NOTA-Au-IONP-Affibody
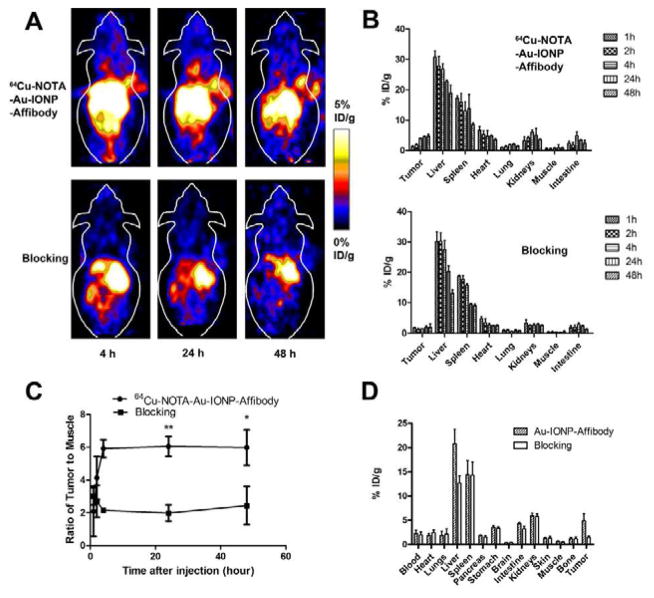
Anti-EGFR Affibody was used as a targeting reagent to modify the surface of Au-IONPs. NOTA-Au-IO-Affibody can be easily labeled with 64Cu with a radiolabeling yield of around 60%. The average number of NOTA chelators on each NOTA-Au-IONP-Affibody NP was measured using a modified procedure (Supporting Information) [33, 34]. Typically, the average number of NOTA per Au-IONP equals 13.7 ± 2.4 (n = 3). Figure 5A showed the representative coronal and transverse PET images of A431-tumor–bearing mice at different time points after injecting 64Cu-NOTA-Au-IONP-Affibody. The A431 tumor was clearly visualized with high contrast relative (the tumor-to-muscle ratio was about 6 after 24 hours) to the background from 4 h to 48 h after injection of radiolabeled NPs. Comparing with the 64Cu-NOTA-Au-IO-Affibody specific targeted imaging, the blocking group showed significantly lower tumor uptake (4.6% ID/g for targeting one vs. 1.9% ID/g for blocking one at 24 h p.i., P < 0.05), with no obvious tumor accumulation at 4 h, 24 h and 48 h after injecting of free Affibody (20 mg/Kg) (Figure 5A).
Figure 5.
A: In vivo decay-corrected whole body coronal PET images of A431 tumor bearing mice acquired 4h, 24h and 48h after injection of 64Cu-NOTA-Au-IONP-Affibody and the blocking dose of Affibody (n = 3/group). 64Cu-NOTA-Au-IONP-Affibody shows excellent and specific tumor imaging ability (n = 3/group). B: Quantitative analysis of PET images of major tissues and organs for control and blocking mice. C: The tumor to muscle ratios of 64Cu-NOTA-Au-IONP-Affibody and blocking one during the imaging (** p < 0.01, * p < 0.05). D: Quantitative radioactive results for 64Cu-NOTA-Au-IONP-Affibody with or without Affibody as blocking.
The targeted tumor accumulation of 64Cu-NOTA-Au-IONP-Affibody was further confirmed by both PET quantification analysis and quantitative radioactive biodistribution study, and the results were shown in Figure 5B–5D. Tumor accumulation at 4 h after injection of 64Cu-NOTA-Au-IONP-Affibody was 3.5 (%ID/g) and peaked at 4.6 (%ID/g) at 24h after injection. Quantitative biodistribution studies also revealed that the targeted 64Cu-NOTA-Au-IONP-Affibody were accumulated in the A431 tumor more efficiently than in the Affibody blocking group. The biodistribution results of tissue and organ uptakes were consistent with the PET quantification analysis results (Figure 5D). Both PET quantification analysis result and biodistribution data confirmed the nonspecific uptake of the NPs by liver and spleen, but little or no accumulation in kidney, muscle or other major organs, which indicated that the most of the injected 64Cu-NOTA-Au-IONP-Affibody were mainly taken up by the reticuloendothelial system (RES). Moreover, the EGFR targeted group and blocking group all showed relatively high liver uptake and spleen uptake (Figure 5A), and the liver uptake of 64Cu-NOTA-Au-IONP-Affibody was obviously higher than that of the blocking group, mainly due to the EGFR expression of murine liver (Figure 5B and 5D) [27]. This result also confirmed the EGFR targeted specificity of the nanoprobe.
In vivo MRI of 64Cu-NOTA-Au-IONP-Affibody
To investigate the MRI visibility of 64Cu-NOTA-Au-IONP-Affibody, in vivo T2-weighted fast spin-echo MRI was performed with mice bearing A431 tumors immediately after their PET scans (Figure 6A). In the in vivo MRI, at 4 h after tail vein injection of 64Cu-NOTA-Au-IONP-Affibody into the A431 bearing tumor mice, the tumor signal was getting darker than that of the pre-injection group. The quantification analysis of the signal intensity showed a signal drop of 44.0% at the A431 tumor sites at 48 h after injection. This decrease was obviously in both transversal and coronal T2-weighted spin echo images (Figure 6A). To evaluate the specific EGFR targeting ability, the Affibody blocking study was also performed. It was found that the MRI signal intensity of EGFR-blocking group had no significant decrease of MRI signal before injection and at 4 h and 48 h after injection (Figure 6B).
Figure 6.
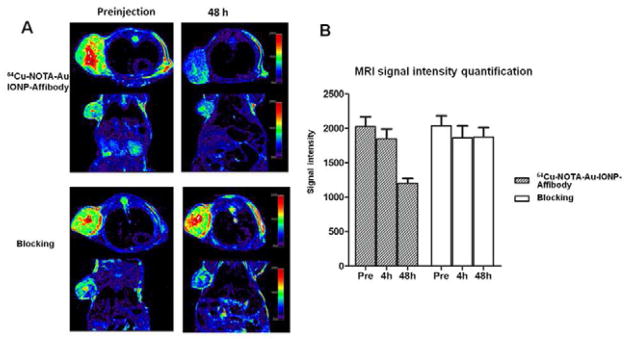
A: In vivo T2-weighted MR images of A431 tumor bearing mice acquired before and at 48h after injection of 64Cu-NOTA-Au-IONP-Affibody and blocking dose of Affibody. B: MRI signal intensity quantification before and at 4h and 48h after injection of 64Cu-NOTA-Au-IONP-Affibody and Affibody.
Discussion
Nanoparticles based molecular probes for imaging have attracted enormous attentions because of their unique properties and multifunctionalities as nanoscale platforms. With the rapid development of imaging instrumentations, PET/MRI in a single device with simultaneous imaging acquisition has been introduced into clinical applications recently [1, 3, 10, 35]. There are urgent demands in developing multimodal molecular probes such as probes for PET/MRI. Among the current well-developed magnetic nanomaterials, IONPs show notable superparamagnetic features in the magnetic field and have been widely applied as an approved MRI a contrast agent for diagnosis, especially for cancers [36, 37]. Several studies have focused on the application of IONPs as PET/MRI imaging probes with targeting capabilities [10, 23, 35]. Compared with conventional single-component nanoparticles, nanoprobes with dual-functional nanomaterials have distinct advantages for multimodal molecular imaging. Hence, in this study, we constructed dumbbell-like Au-IONP nanoplatform for receptor targeted PET/Optical/MRI, with IONPs acting as both a MRI reporter and a functional nanoplatform for biomodification, while Au-NPs serving as an optical imaging reporter and a nanoplatform for radiolabeling (Figure 1). As expected, Figure 4 showed the in vitro phantom comparison study of magnetic properties between Au-IONPs and Feridex. The dumbbell-like Au-IONPs did show a quite similar relaxation rate to Feridex, which confirmed its contrast ability for MRI imaging. Also Figure 4 showed that the Au-IONP could be optically imaged for cell assays.
The characterization of nanoprobes using TEM, SEM, DLS and ICP-MS confirmed that we had successfully constructed the dumbbell particles with homogeneous size distribution, favorable stability and well-shaped dual-functional Au and IONP nano-compositions. This dumbbell NPs can serve as a perfect platform for further surface modification and conjugation. TEM is a highly versatile technique for providing quantitative and morphologic information from nanostructured samples. The TEM images in Figure 2 demonstrated that the Au-IONPs prepared showed excellent characteristics in terms of particle morphology and the corresponding size distribution. The Au-IONPs also displayed favorable stability and biocompatibility before and after surface modification, as evidenced by their unchanged hydrodynamic sizes after incubation in PBS containing 10% FBS at 37°C for 48 h.
The synthesis and applications of Au-IONPs have only been pursued in in vitro studies recently, and also the size of those Au-IONPs is much larger (15–20 nm) than what we prepared [13, 14]. Therefore, in our study, we systematically performed the synthesis, characterization, in vitro studies, and in vivo evaluation to explore the value of Au-IONPs for bioimaging. Moreover, we developed targeted PET/Optical/MRI nanoprobe based on Au-IONPs. In the well-constructed Au-IONPs, there is a difference of surface area between the Au and IO particles based on the size difference of the two particles (Au = 4 nm, IO = 8 nm). IO NP has about 4-time-larger surface area than that of Au-NP, which can provide more surface area for modification and consequently offer more binding position for further functionalization. Therefore, we chose the IO functional side to bioconjugate with the biomolecules such as Affibody for achieving better targeting efficiency, while Au-NP is only used for modification with radiometal chelators such as NOTA because of the low chemical quantity of the 64Cu used for the radiolabeling
EGFR has become an attractive target for cancer molecular imaging and therapy. As reported in our previous work, we designed and chemically synthesized ZEGFR:1907 and its derivatives [21, 23, 24, 27, 28, 38]. ZEGFR:1907 site-specifically labeled with 111In and 64Cu have shown specific targeting ability, good tumor accumulation and tumor-to-normal tissue imaging contrast in EGFR positive tumor models. These results have encouraged us to further explore the application of anti-EGFR Affibody in targeted multimodality imaging. In this study, we thus applied a simple one-step surface modification method by synthesis of maleimide-terminated Au-IONPs, which is feasible for bio-conjugation with various types of biomolecules, including Affibody. To precisely conjugate the Affibody molecules with maleimide-terminated Au-IONPs, we incorporated a single cysteine residue into the N-terminus of the synthetic Affibody molecules (Ac-Cys-ZEGFR: 1907). The thiolated Affibody molecule provides a well-defined conjugation site when we use 4-maleimidobutyric acid N-succinimidyl ester as a heterodimeric cross-linker. It leads to an opportunity to site-specifically conjugate Affibody with the maleimide-terminated NPs through their N-terminus cysteine residue [23, 27], as shown in Figure 1B. Comparing with the conventional non-specific bioconjugation of antibody with NPs, the preparation of NP-Affibody conjugates is more reliable with well-defined structure [39]. Moreover, as reported previously, multiple Affibody molecules are conjugated with a NP, and this kind of modification may potentially enhance the targeting ability of the resulting nanoprobe comparing with the conventional radiolabeled Affibody molecules, because the binding effect in a multivalent reaction could be stronger than that of a monovalent binding [40, 41].
On the basis of successfully prepared 64Cu-NOTA-Au-IONP-Affibody, we performed both in vitro cell uptake study and in vivo PET/MRI dual-modality targeted tumor imaging study. In vitro cell uptake experiments showed that 64Cu-NOTA-Au-IONP-Affibody had rapid accumulation in A431 cells. This accumulation was EGFR specific since the cellular uptake of control group was significantly higher than that of Au-IONPs group and Affibody blocking group at both 1 h and 2 h time points, according to the ICP-MS quantification results. Confocal fluorescence reflection imaging for cellular uptake also proved the specific EGFR targeting ability of NOTA-Au-IONP-Affibody, as it showed much stronger reflection signals from the NOTA-Au-IONP-Affibody incubated A431 cells than that of the blocking group. Overall, in vitro study results indicated that the Affibody-modified dumbbell nanoparticles had high specificity in binding with EGFR positive cells and they were good optical probe for imaging EGFR.
More importantly, in vivo results clearly showed that the Affibody-based Au-IONP nanoprobe had excellent in vivo performance. It had high targeting specificity to EGFR-expressing cell and tumor, with significant higher contrast enhancement in both MRI and PET comparing to the blocking group. In vivo MRI demonstrated the signal intensity at the A431tumor sites drops 44.0 % steadily from 4 h to 48 h after 64Cu-NOTA-Au-IONP-Affibody injection. While in the EGFR-blocking group, MRI signal intensity had no significant decrease before injection and at 4 h, 24, 48 h after injection. Consistently, in vivo small animal PET showed specific tumor targeting, high tumor accumulation (4.6% ID/g at 24 h p.i.), and good tumor-to-normal tissue contrast for 64Cu-NOTA-Au-IONP-Affibody. Both PET quantification analysis and radioactive biodistribution studies further confirmed that the nanoprobe had significantly higher tumor uptake comparing to the blocking group, with nonspecific uptake by the liver and the spleen, as an evidence of reticuloendothelial system (RES) uptake of the NPs. Higher liver accumulation can also be observed in 64Cu-NOTA-Au-IONP-Affibody group than that of the blocking control, which was caused by the high EGFR expression in the normal liver tissue. Based on these results, the PET and MRI images were well correlated with the probe biodistribution results. Thus, 64Cu-NOTA-Au-IONP-Affibody demonstrated high potential as an EGFR-targeted PET/MRI probe. With this nanoprobe, we can not only perform targeted PET/Optical/MR imaging, but also understand the in vivo distribution and clearance of biomolecules modified Au-IONP-Affibody in tumor mice, which could be valuable information for using the dumbbell NPs for potential applications in targeted drug delivery. Moreover, or with the aid of MRI and PET, tumors treated with targeted Au-IONPs drug delivery system could be monitored in real-time. The knowledge gained from this work can be applied to many cancers since EGFR is over-expressed on a wide range of human cancers, and allow for widespread preclinical and potential clinical applications of theranostics in cancers.
Conclusions
In summary, the Au-IONPs can not only offer two functional nanocompositions for the site-specific attachment of various targeting biomolecules, reporting moieties and drugs after surface modification, they also contain both IO and Au NPs, which could be served for simultaneous magnetic and optical theronostic purpose. We developed a simple surface modification method, which is feasible for bioconjugation with various types of biomolecules, allowing versatile conjugations between Au-IONPs and biomolecules of interest. Most importantly, our study results highlight the feasibility of this method by preparation of the nanoprobe, 64Cu-NOTA-Au-IONP-Affibody, and performing successful PET/Optical/MRI multimodality targeted cancer imaging. The results clearly demonstrate that Au-IONPs are a very promising nanoplatform for multimodality imaging. This Affibody-targeted Au-IONPs system is expected to improve the diagnosis and therapy of EGFR positive tumors by offering specific targeted multi-modality imaging information as well as to provide a promising nano-platform for targeted drug delivery.
Supplementary Material
Acknowledgments
This work was partially supported by DOE Stanford Molecular Imaging Research and Training Program (SMIRTP), NCI of Cancer Nanotechnology Excellence Grant CCNE-TR U54 CA119367, CA151459, and National High Technology Research and Development Program of China (863 Plan, 2006AA027Z4B3). We thank Dr. Laura Jean Pisani for assistance in obtaining MR images.
Footnotes
Experimental details; materials and methods; mass spectra and TEM images are all available free of charge via the Internet at http://pubs.acs.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–79. [PubMed] [Google Scholar]
- 2.Townsend DW. A combined PET/CT scanner: the choices. J Nucl Med. 2001;42:533–4. [PubMed] [Google Scholar]
- 3.Grassetto G, Groheux D, Marzola MC, Hindie E, Al-Nahhas A, Rubello D. FDG PET/CT in ovarian cancer: what about treatment response and prognosis? Clin Nucl Med. 2012;37:54–6. doi: 10.1097/RLU.0b013e3182336126. [DOI] [PubMed] [Google Scholar]
- 4.Naswa N, Sharma P, Kumar A, Nazar AH, Kumar R, Chumber S, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. AJR Am J Roentgenol. 2011;197:1221–8. doi: 10.2214/AJR.11.7298. [DOI] [PubMed] [Google Scholar]
- 5.Wehrl HF, Judenhofer MS, Thielscher A, Martirosian P, Schick F, Pichler BJ. Assessment of MR compatibility of a PET insert developed for simultaneous multiparametric PET/MR imaging on an animal system operating at 7 T. Magn Reson Med. 2011;65:269–79. doi: 10.1002/mrm.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delso G, Ziegler S. PET/MRI system design. Eur J Nucl Med Mol Imaging. 2009;36 (Suppl 1):S86–92. doi: 10.1007/s00259-008-1008-6. [DOI] [PubMed] [Google Scholar]
- 7.Schlemmer HP, Pichler BJ, Schmand M, Burbar Z, Michel C, Ladebeck R, et al. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology. 2008;248:1028–35. doi: 10.1148/radiol.2483071927. [DOI] [PubMed] [Google Scholar]
- 8.Delso G, Furst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52:1914–22. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- 9.Sauter AW, Wehrl HF, Kolb A, Judenhofer MS, Pichler BJ. Combined PET/MRI: one step further in multimodality imaging. Trends Mol Med. 2010;16:508–15. doi: 10.1016/j.molmed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, et al. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–9. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, et al. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31:3016–22. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Xu C, Zeng H, Sun S. Recent Progress in Syntheses and Applications of Dumbbell-like Nanoparticles. Adv Mater. 2009;21:3045–52. doi: 10.1002/adma.200900320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu C, Xie J, Ho D, Wang C, Kohler N, Walsh EG, et al. Au-Fe3O4 dumbbell nanoparticles as dual-functional probes. Angew Chem Int Ed Engl. 2008;47:173–6. doi: 10.1002/anie.200704392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Chen M, Rice PM, Wang SX, White RL, Sun S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005;5:379–82. doi: 10.1021/nl047955q. [DOI] [PubMed] [Google Scholar]
- 15.Hao R, Xing R, Xu Z, Hou Y, Gao S, Sun S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv Mater. 2010;22:2729–42. doi: 10.1002/adma.201000260. [DOI] [PubMed] [Google Scholar]
- 16.Buck MR, Bondi JF, Schaak RE. A total-synthesis framework for the construction of high-order colloidal hybrid nanoparticles. Nat Chem. 2012;4:37–44. doi: 10.1038/nchem.1195. [DOI] [PubMed] [Google Scholar]
- 17.Gu H, Yang Z, Gao J, Chang CK, Xu B. Heterodimers of nanoparticles: formation at a liquid-liquid interface and particle-specific surface modification by functional molecules. J Am Chem Soc. 2005;127:34–5. doi: 10.1021/ja045220h. [DOI] [PubMed] [Google Scholar]
- 18.Miao Z, Levi J, Cheng Z. Protein scaffold-based molecular probes for cancer molecular imaging. Amino Acids. 2011;41:1037–47. doi: 10.1007/s00726-010-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson FY, Tolmachev V. Affibody molecules: new protein domains for molecular imaging and targeted tumor therapy. Curr Opin Drug Discov Devel. 2007;10:167–75. [PubMed] [Google Scholar]
- 20.Cheng Z, De Jesus OP, Namavari M, De A, Levi J, Webster JM, et al. Small-animal PET imaging of human epidermal growth factor receptor type 2 expression with site-specific 18F-labeled protein scaffold molecules. J Nucl Med. 2008;49:804–13. doi: 10.2967/jnumed.107.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Z, De Jesus OP, Kramer DJ, De A, Webster JM, Gheysens O, et al. 64Cu-labeled affibody molecules for imaging of HER2 expressing tumors. Mol Imaging Biol. 2010;12:316–24. doi: 10.1007/s11307-009-0256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jokerst JV, Miao Z, Zavaleta C, Cheng Z, Gambhir SS. Affibody-functionalized gold-silica nanoparticles for Raman molecular imaging of the epidermal growth factor receptor. Small. 2011;7:625–33. doi: 10.1002/smll.201002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Chen K, Miao Z, Ren G, Chen X, Gambhir SS, et al. Affibody-based nanoprobes for HER2-expressing cell and tumor imaging. Biomaterials. 2011;32:2141–8. doi: 10.1016/j.biomaterials.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoppmann S, Miao Z, Liu S, Liu H, Ren G, Bao A, et al. Radiolabeled affibody-albumin bioconjugates for HER2-positive cancer targeting. Bioconjug Chem. 2011;22:413–21. doi: 10.1021/bc100432h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman M, Orlova A, Johansson E, Eriksson TL, Hoiden-Guthenberg I, Tolmachev V, et al. Directed evolution to low nanomolar affinity of a tumor-targeting epidermal growth factor receptor-binding affibody molecule. J Mol Biol. 2008;376:1388–402. doi: 10.1016/j.jmb.2007.12.060. [DOI] [PubMed] [Google Scholar]
- 26.Nordberg E, Orlova A, Friedman M, Tolmachev V, Stahl S, Nilsson FY, et al. In vivo and in vitro uptake of 111In, delivered with the affibody molecule (ZEGFR:955)2, in EGFR expressing tumour cells. Oncol Rep. 2008;19:853–7. doi: 10.3892/or.19.4.853. [DOI] [PubMed] [Google Scholar]
- 27.Miao Z, Ren G, Liu H, Jiang L, Cheng Z. Small-animal PET imaging of human epidermal growth factor receptor positive tumor with a 64Cu labeled affibody protein. Bioconjug Chem. 2010;21:947–54. doi: 10.1021/bc900515p. [DOI] [PubMed] [Google Scholar]
- 28.Miao Z, Ren G, Liu H, Jiang L, Cheng Z. Cy5.5-labeled Affibody molecule for near-infrared fluorescent optical imaging of epidermal growth factor receptor positive tumors. J Biomed Opt. 2010;15:036007. doi: 10.1117/1.3432738. [DOI] [PubMed] [Google Scholar]
- 29.Hoppmann SQS, Miao Z, Liu H, Jiang H, Culter CS, Bao A, Cheng Z. 177Lu-DOTA-HSA-ZEGFR:1904: In vitro and in vivo characterization as a potential radiopharmaceutical for radionuclide therapy of EGFR-expressing head and neck carcinomas. Journal of Biological Inorganic Chemistry. 2012 doi: 10.1007/s00775-012-0890-3. [DOI] [PubMed] [Google Scholar]
- 30.Miao Z, Ren G, Liu H, Qi S, Wu S, Cheng Z. PET of EGFR expression with an 18F-labeled affibody molecule. J Nucl Med. 2012;53:1110–8. doi: 10.2967/jnumed.111.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Real FX, Rettig WJ, Chesa PG, Melamed MR, Old LJ, Mendelsohn J. Expression of epidermal growth factor receptor in human cultured cells and tissues: relationship to cell lineage and stage of differentiation. Cancer Res. 1986;46:4726–31. [PubMed] [Google Scholar]
- 32.Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, et al. Compact biocompatible quantum dots functionalized for cellular imaging. J Am Chem Soc. 2008;130:1274–84. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meares CF, McCall MJ, Reardan DT, Goodwin DA, Diamanti CI, McTigue M. Conjugation of antibodies with bifunctional chelating agents: Isothiocyanate and bromoacetamide reagents, methods of analysis, and subsequent addition of metal ions. Analytical Biochemistry. 1984;142:68–78. doi: 10.1016/0003-2697(84)90517-7. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nano. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 35.Choi JS, Park JC, Nah H, Woo S, Oh J, Kim KM, et al. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew Chem Int Ed Engl. 2008;47:6259–62. doi: 10.1002/anie.200801369. [DOI] [PubMed] [Google Scholar]
- 36.Taupitz M, Schnorr J, Wagner S, Abramjuk C, Pilgrimm H, Kivelitz D, et al. Coronary MR angiography: experimental results with a monomer-stabilized blood pool contrast medium. Radiology. 2002;222:120–6. doi: 10.1148/radiol.2221001452. [DOI] [PubMed] [Google Scholar]
- 37.Hahn PF, Stark DD, Lewis JM, Saini S, Elizondo G, Weissleder R, et al. First clinical trial of a new superparamagnetic iron oxide for use as an oral gastrointestinal contrast agent in MR imaging. Radiology. 1990;175:695–700. doi: 10.1148/radiology.175.3.2343116. [DOI] [PubMed] [Google Scholar]
- 38.Qi S, Miao Z, Liu H, Xu Y, Feng Y, Cheng Z. Evaluation of Four Affibody-Based Near-Infrared Fluorescent Probes for Optical Imaging of Epidermal Growth Factor Receptor Positive Tumors. Bioconjug Chem. 2012 doi: 10.1021/bc200596a. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Mao H, Wang YA, Cao Z, Peng X, Wang X, et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2009;5:235–43. doi: 10.1002/smll.200800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol. 2010;5:42–7. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–23. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.