Abstract
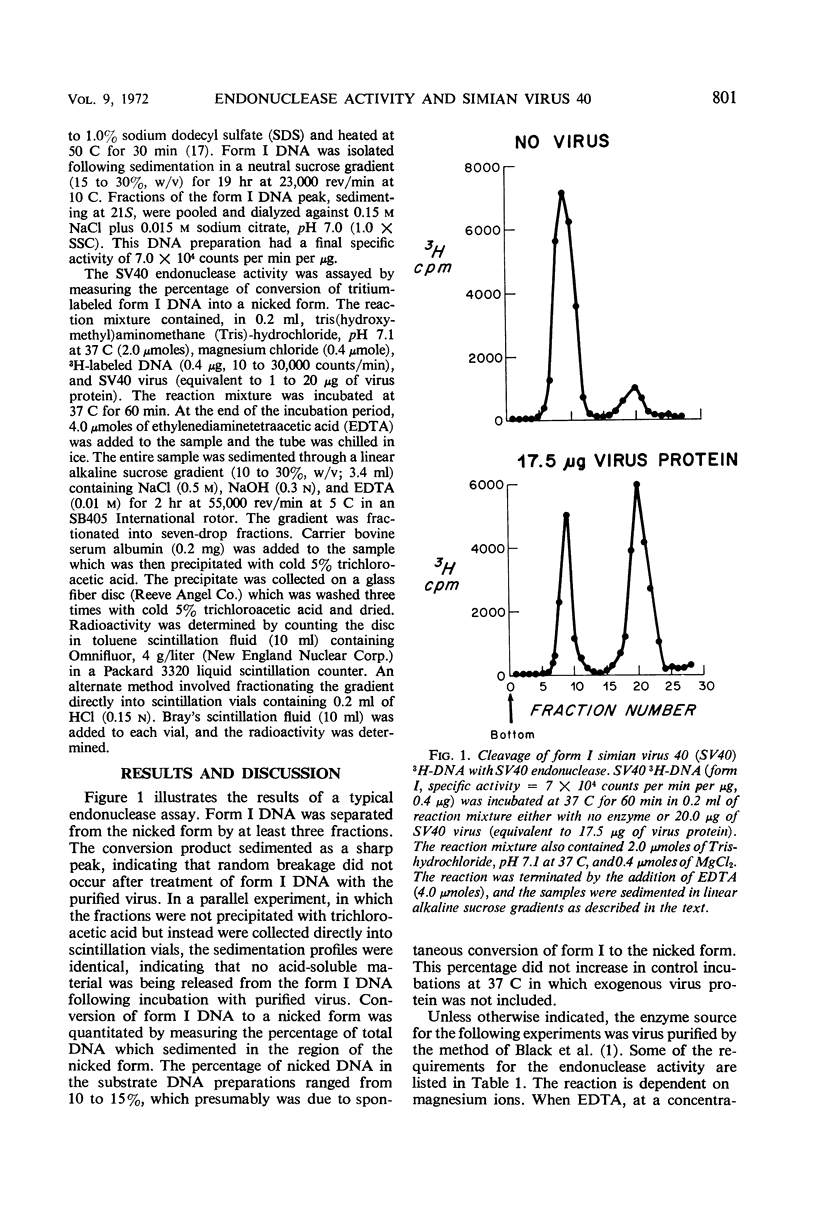
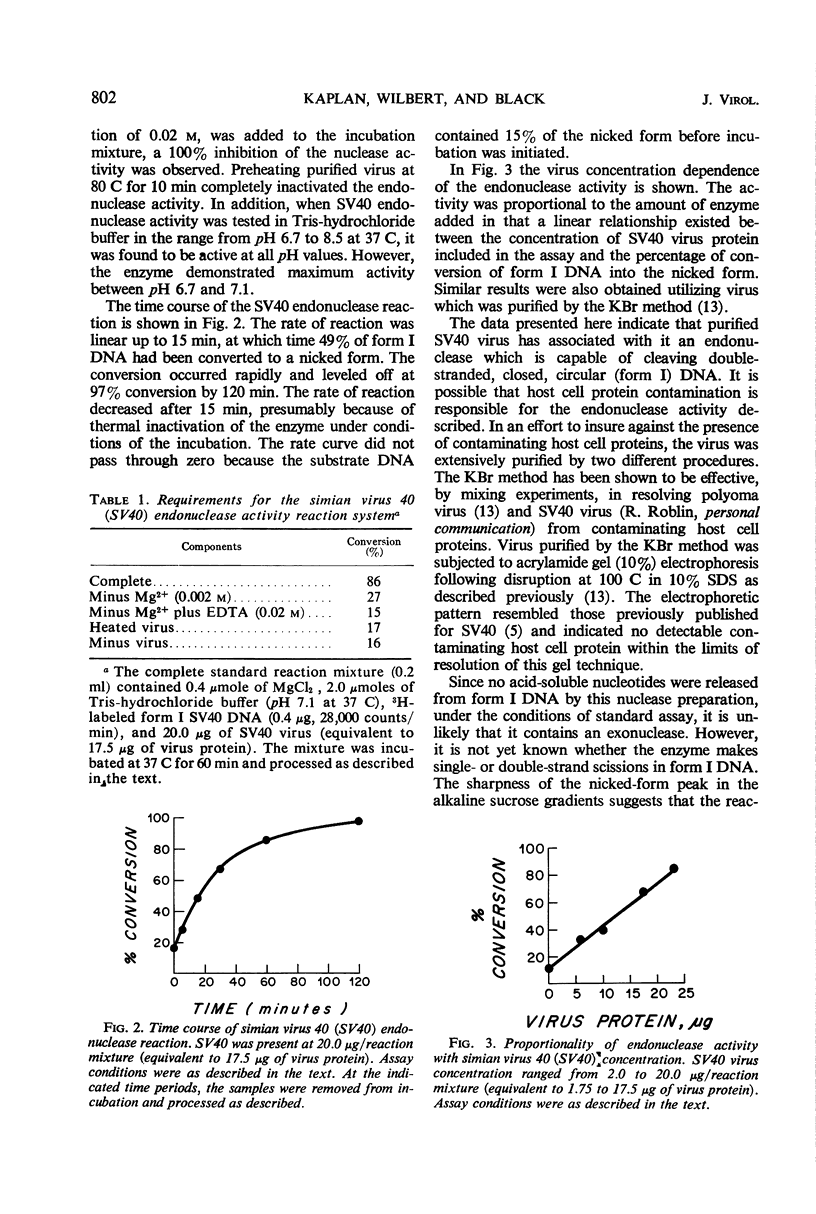
Purified simian virus 40 has associated with it an endonuclease activity which converts form I (double-stranded, circular) simian virus 40 deoxyribonucleic acid to a nicked form that sediments as a homogeneous peak in alkaline sucrose gradients. The enzyme is dependent on magnesium ions for activity and is completely inhibited by ethylenediaminetetraacetic acid (0.02 m) or heat (80 C for 10 min). In tris(hydroxymethyl)aminomethane-hydrochloride buffer it exhibits optimal activity between pH 6.7 and 7.1 at 37 C. Gel electrophoretic analysis of purified, disrupted virus indicates the absence of detectable host cell protein contamination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., CRAWFORD E. M., CRAWFORD L. V. THE PURIFICATION OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:381–387. doi: 10.1016/0042-6822(64)90175-8. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W., Pettersson U., Philipson L. Adenovirus endonuclease: association with the penton of adenovirus type 2. J Mol Biol. 1971 Aug 28;60(1):45–64. doi: 10.1016/0022-2836(71)90446-3. [DOI] [PubMed] [Google Scholar]
- Burns W. H., Black P. H. Analysis of SV40-induced transformation of hamster kidney tissue in vitro. VI. Characteristics of mitomycin C induction. Virology. 1969 Dec;39(4):625–634. doi: 10.1016/0042-6822(69)90001-4. [DOI] [PubMed] [Google Scholar]
- Burns W. H., Black P. H. Analysis of simian virus 40-induced transformation of hamster kidney tissue in vitro. V. Variability of virus recovery from cell clones inducible with mitomycin C and cell fusion. J Virol. 1968 Jun;2(6):606–609. doi: 10.1128/jvi.2.6.606-609.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Blangy D., Rouget P. Activité endonucléasique de préparations purifiées du virus du Polyome. C R Acad Sci Hebd Seances Acad Sci D. 1971 Dec 20;273(25):2650–2653. [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Lehman J., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of primary infected Chinese hamster cells. J Virol. 1971 Nov;8(5):708–715. doi: 10.1128/jvi.8.5.708-715.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Wilbert S. M., Black P. H. Analysis of simian virus 40-induced transformation of hamster kidney tissue in vitro. 8. Induction of infectious simian virus 40 from virogenie transformed hamster cells by amino acid deprivation or cycloheximide treatment. J Virol. 1972 Mar;9(3):448–453. doi: 10.1128/jvi.9.3.448-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. A., Eggers H. J., Anderer F. A., Schlumberger H. D., Frank H. Structure of simian virus 40. I. Purification and physical characterization of the virus particle. Virology. 1967 Jul;32(3):503–510. doi: 10.1016/0042-6822(67)90302-9. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Boettiger D., Temin H. M. A DNA-depenent DNA polymerase and a DNA endonuclease in virions of Rous sarcoma virus. Nature. 1970 Oct 31;228(5270):424–427. doi: 10.1038/228424a0. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M., Kodama M., Wells R. T. DNA ligase and exonuclease activities in virions of rous sarcoma virus. Nat New Biol. 1971 Apr 21;230(16):232–235. doi: 10.1038/newbio230232a0. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Fanshier L., Evans B., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase(s) of Rous sarcoma virus: effects of virion-associated endonuclease on the enzymatic product. J Virol. 1971 Jul;8(1):17–27. doi: 10.1128/jvi.8.1.17-27.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin R., Härle E., Dulbecco R. Polyoma virus proteins. 1. Multiple virion components. Virology. 1971 Sep;45(3):555–566. doi: 10.1016/0042-6822(71)90171-1. [DOI] [PubMed] [Google Scholar]
- Rothschild H., Black P. H. Analysis of SV40-induced transformation of hamster kidney tissue in vitro. VII. Induction of SV40 virus from transformed hamster cell clones by various agents. Virology. 1970 Sep;42(1):251–256. doi: 10.1016/0042-6822(70)90264-3. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster R. C., Weissbach A. Evidence for a new endonuclease synthesized by lambda bacteriophage. J Virol. 1968 Oct;2(10):1096–1101. doi: 10.1128/jvi.2.10.1096-1101.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne H. V. Electrophoretic characterization and fractionation of polyoma virus DNA. J Mol Biol. 1967 Mar 14;24(2):203–211. doi: 10.1016/0022-2836(67)90326-9. [DOI] [PubMed] [Google Scholar]


