Abstract
Purpose
Dual blockade of HER2 with trastuzumab with lapatinib or with pertuzumab is a superior treatment approach compared to single agent HER2 inhibitors. However, many HER2-overexpressing breast cancers still escape from this combinatorial approach. Inhibition of HER2 and downstream phosphatidylinositol-3 kinase (PI3K)/AKT causes a transcriptional and post-translational upregulation of HER3 which, in turn, counteracts the antitumor action of the HER2-directed therapies. We hypothesized that suppression of HER3 would synergize with dual blockade of HER2 in breast cancer cells sensitive and refractory to HER2 antagonists.
Experimental Design
Inhibition of HER2/HER3 in HER2+ breast cancer cell lines was evaluated by western blot. We analyzed drug-induced apoptosis and 2- and 3-dimensional growth in vitro. Growth inhibition of PI3K was examined in vivo in xenografts treated with combinations of trastuzumab, lapatinib, and the HER3 neutralizing monoclonal antibody U3-1287.
Results
Treatment with U3-1287 blocked the upregulation of total and phosphorylated HER3 that followed treatment with lapatinib and trastuzumab and, in turn, enhanced the anti-tumor action of the combination against trastuzumab-sensitive and -resistant cells. Mice bearing HER2+ xenografts treated with lapatinib, trastuzumab, and U3-1287 exhibited fewer recurrences and better survival compared to mice treated with lapatinib and trastuzumab.
Conclusions
Dual blockade of HER2 with trastuzumab and lapatinib does not eliminate the compensatory upregulation of HER3. Therapeutic inhibitors of HER3 should be considered as part of multi-drug combinations aimed at completely and rapidly disabling the HER2 network in HER2-overexpressing breast cancers.
Keywords: HER2, HER3, lapatinib, trastuzumab, breast cancer
Introduction
Gene amplification and/or overexpression of the receptor tyrosine kinase (RTK) HER2 occur in about 20% of human breast cancers and are associated with poor patient prognosis (1, 2). The antibodies trastuzumab and pertuzumab and the tyrosine kinase inhibitor (TKI) lapatinib are approved by the FDA for the treatment of HER2-overexpressing breast cancer. Trastuzumab is a humanized monoclonal antibody that binds domain IV in the extracellular region of HER2. Mechanisms of action of the antibody include blockade of ligand-independent HER2-HER3 dimerization (3) and induction of antibody-dependent cellular cytotoxicity and innate immunity to HER2 (4, 5). Trastuzumab in combination with chemotherapy significantly improves the survival of patients with early HER2+ breast cancer (6-8). The small molecule lapatinib is an ATP-mimetic that reversibly binds the ATP pocket in the HER2 tyrosine kinase domain, thus inhibiting its catalytic activity (9). It is active as first line monotherapy in patients with HER2+ metastatic breast cancer and in combination with chemotherapy improves progression free survival compared to chemotherapy alone (10, 11). Pertuzumab is a humanized antibody that binds the heterodimerization loop in subdomain II of HER2 and, as such, prevents HER2 from dimerizing with ligand-bound HER3 (12). In two recent seminal studies, the combination of trastuzumab and pertuzumab was superior to trastuzumab in patients with operable and metastatic HER2+ breast cancer (13, 14). Several preclinical and clinical reports have already suggested that dual blockade of HER2 with a combination of HER2 antagonists with complementary mechanisms of action, such as trastuzumab and lapatinib, is a more robust approach to inhibit the HER2 signaling network and, in turn, induce an antitumor effect (15-17).
In patients with HER2+ metastatic breast cancer, resistance to trastuzumab and/or lapatinib, either as single-agents or in combination with chemotherapy, commonly occurs within months of starting therapy. Only a fraction of patients with HER2+ metastatic breast cancer respond to single agent trastuzumab (18, 19), suggesting de novo mechanisms of resistance in advanced cancers. These mechanisms include signaling from other HER (ErbB) receptors (20, 21), compensatory signaling from RTKs outside of the HER family (22, 23), aberrant phosphatidylinositol 3-kinase (PI3K) signaling as a result of mutations in this pathway (24, 25) and the presence of truncated forms of HER2 (26), among few others. Mechanisms of resistance to lapatinib also point to increased (PI3K) signaling, derepression/activation of compensatory survival pathways (27, 28) and defects in pro-apoptosis molecules such as BIM (29).
HER2 (ErbB2) is a member of the ErbB family of transmembrane RTKs, which also includes the epidermal growth factor receptor (EGFR, ErbB1), HER3 (ErbB3), and HER4 (ErbB4). Binding of ligands to the extracellular domain of EGFR, HER3 and HER4 induces the formation of kinase active homo- and heterodimers to which activated HER2 is recruited as a preferred partner (30). HER3, which lacks potent intrinsic kinase activity, is able to strongly activate the PI3K/Akt via its six docking sites for the p85 regulatory subunit of PI3K, whereas HER2 is unable to directly bind to and activate PI3K-Akt. Loss of HER3 inhibits viability of HER2-overexpressing breast cancer cells (31, 32) and HER2-overespressing cells are particularly sensitive to apoptosis induced by PI3K inhibitors (33), thus suggesting the HER3-PI3K axis is essential for survival of HER2-dependent cells.
We and others have shown that inhibition at multiple levels of the PI3K pathway results in FoxO-dependent feedback reactivation of several RTKs which, in turn, limit the sustained inhibition of PI3K and attenuates the action of PI3K pathway antagonists (34-36). In a clinical trial where patients with HER2+ breast cancer were treated with lapatinib, we showed there was upregulation of HER3 protein and maintenance of active AKT in tumor core biopsies obtained at 2 weeks of treatment (34, 37). These studies suggest that treatment approaches aimed at disabling the reactivation of HER3 should improve the antitumor effect of HER2/PI3K-directed therapies.
In this study, we examined whether the neutralizing HER3 monoclonal antibody U3-1287, currently in clinical development, would prevent the upregulation of active HER3 after dual blockade of HER2 with lapatinib and trastuzumab in HER2-overexpressing cells sensitive and refractory to HER2 inhibitors. U3-1287 has been shown to inhibit ligand-induced P-HER3 and cause growth inhibition of pancreatic, NSCLC, and colorectal xenograft tumors (38, 39). It has recently completed safety and dose-finding studies in patients with advanced cancer (40). Herein we demonstrate U3-1287 downregulates HER3 from the cell surface and blocks the upregulation of HER3 that follows the inhibition of HER2. Moreover, U3-1287 in combination with the HER2 inhibitors enhanced apoptosis in vitro, partially restored sensitivity to trastuzumab in trastuzumab-resistant xenografts and improved the survival of mice bearing BT474 tumors compared to lapatinib and trastuzumab. These studies suggest that 1) dual blockade of HER2 does not eliminate HER3 function completely, and 2) HER3 inhibitors sensitize HER2-overexpressing breast cancers to dual blockade of HER2.
Materials and Methods
Cells and reagents
BT474, SKBR3, and MDA453 cells were obtained from ATCC. HR6 cells were derived from a trastuzumab-resistant BT474 xenograft in our laboratory and have been described previously (20). Cells were grown as described (20, 34). The following drugs were used: lapatinib (GW-572016, LC Laboratories), trastuzumab, pertuzumab (Vanderbilt University Hospital Pharmacy) and U3-1287 (kindly provided by Johannes Bange at U3 Pharma).
Immunoblot analysis
Cells were prepared as described (34). Lysates were separated by 7% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes (Bio-Rad). Primary antibodies included: Y1197 and Y1289 P-HER3, S473 and T308 P-Akt, total Akt, T202/Y204 PErk, total Erk, P-GSK3α/β, P-S6 (Cell Signaling), HER3 (Santa Cruz Biotechnology), and β-actin (Sigma). Immunoreative bands were detected by enhanced chemiluminescence after incubation with horseradish peroxidase-conjugated secondary antibodies (Promega).
Cell-surface biotinylation
Cells were treated with a receptor saturating concentration of U3-1287 (20 μg/ml) alone or in combination with the HER2 inhibitors at 37°C for 24 h; cells were treated and lysed as described (41). Equal amounts of protein extracts (500 μg) were precipitated using immobilized Neutravidin gel (Pierce) followed by SDS-PAGE and HER3 immunoblot analysis.
Terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling
To measure apoptosis, adherent cells were serum-starved for 24 h and then treated ± 1.0 μM lapatinib, 20 μg/ml trastuzumab and/or 20 μg/ml U3-1287. After 48 h, both detached and adherent cells were pooled and subjected to terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling analysis using the APO-bromodeoxyuridine kit (Phoenix Flow Systems) following the manufacturer's protocol.
Monolayer and three-dimensional growth assays
Cells were seeded in 6-well plates (5×104/well) in 10% FBS-containing medium followed by treatment with inhibitors. Media and inhibitors were replenished every 2-3 days until 60-80% confluence was achieved in untreated wells. Cells were then fixed and stained with 20% methanol/79.5% water/0.5% crystal violet for 3 min, washed with water and dried. Crystal violet staining intensity was quantified by scanning plates using an Odyssey Infrared Imaging System (LI-COR Biosciences), followed by analysis using manufacturer's software. For growth in 3D, cells were seeded on growth factor-reduced Matrigel (BD Biosciences) in 48-well plates following published protocols (42). Inhibitors were added to the medium at the time of cell seeding; 12 to 16 days later, the plates were scanned, and colonies measuring ≥25 μm were counted using GelCount software (Oxford Optronix). Colonies were photographed using an Olympus DP10 camera mounted in an inverted microscope. In Figure 3B the mean colony size was determined using the imaging software ImageJ (NIH).
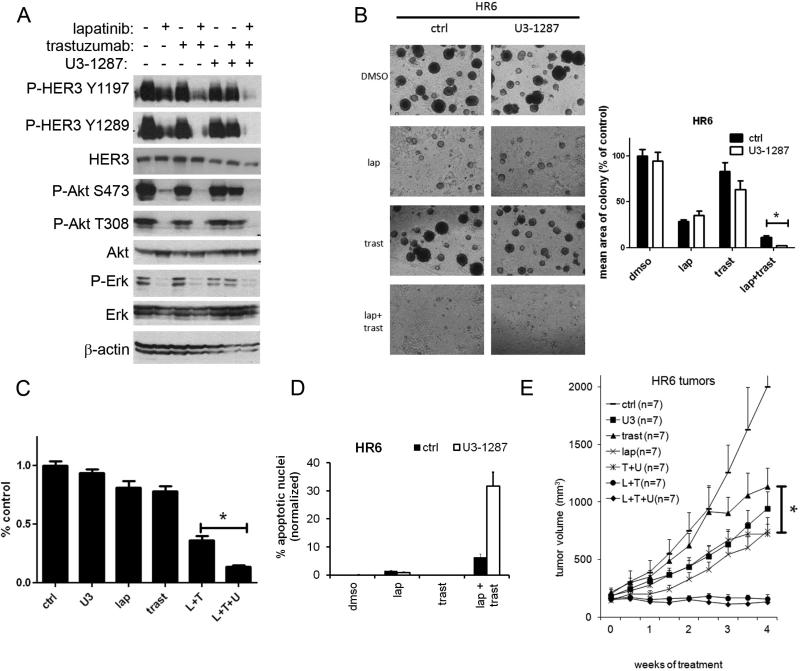
Figure 3. Trastuzumab resistant cells remain sensitive to HER2-HER3 blockade.
A. Trastuzumab-resistant HR6 cells were treated with U3-1287, trastuzumab, lapatinib or the indicated combinations for 24 h. Whole cell lysates were prepared and separated by 7% SDS-PAGE followed by immunoblot analysis with the indicated antibodies. B. HR6 cells were seeded in Matrigel and allowed to grow in the absence or presence of lapatinib, trastuzumab and/or U3-1287 as indicated. Medium and inhibitors were replenished every 3 days. Images shown were recorded 15 days after cell seeding. Each bar graph represents the mean ± S.E.M. of triplicate wells (*, p<0.05, t test). C. Cells were seeded in triplicate and treated with DMSO, 20 μg/ml trastuzumab, 0.1μM lapatinib, 20 μg/ml U3-1287 or the indicated combinations. Media and inhibitors were replenished every 3-4 days. The monolayers were stained with crystal violet when the untreated cells became confluent after 14-21 days. Quantification of integrated intensity (% control) was measured as describe in Methods (*, p<0.05, t test). D. HR6 cells were seeded in 60-mm plates in growth medium containing 10% FCS. The following day, cells were changed to serum-free media and treated with lapatinib (1 μM), trastuzumab (20 μg/ml) and U3-1287 (20 μg/ml) as indicated. Forty-eight h later, adherent and detached cells were collected and subjected to TUNEL assay using the APO-BrdU kit. Each bar represents the mean ± SEM of cells with apoptotic nuclei for each treatment group (n=3; *, p<0.05, t test). E. Female athymic mice were injected with HR6 cells as indicated in Methods; once tumors reached a volume ≥200 mm3, mice were randomized to the indicated treatments. Tumor volumes were measured overtime. Each data point represents the mean tumor volume + SEM(*, p<0.05, trastuzumab versus trastuzumab + U3-1287).
Xenograft studies
A 21-day 17β-estradiol pellet (Innovative Research of America) was inserted subcutaneously (s.c.) in the dorsum of 4- to 6-week-old female athymic mice (Harlan Sprague Dawley Inc.) one day before cell injection. Approximately 5×106 BT474 or HR6 cells were injected s.c. into the mouse right flank. Once tumors reached a volume ≥200 mm3, mice were randomized to different treatments including U3-1287, trastuzumab, and/or lapatinib using doses as described (34). Tumor xenografts were measured as described (34). After 5 days of treatment, some of the animals were anesthetized with 1.5% isoflurane-air mixture and sacrificed by cervical dislocation. All mouse experiments used in this study were approved by the Institutional Animal Care Committee of Vanderbilt University.
Immunohistochemistry (IHC)
Tumor fragments were harvested and immediately fixed in 10% buffered neutral formalin for 24 h at room temperature, then dehydrated and paraffin-embedded. Five-μm sections were used for H&E staining and IHC using an antibody against S473 P-AKT (Cell Signaling). The intensity of cytoplasmic P-AKT staining were graded by an expert breast pathologist (M.G.K.) using a score of 0-300 as described (34). Scoring was blinded to treatment arms.
Results
U3-1287 downregulates HER3 and blocks HER3 phosphorylation following inhibition of HER2
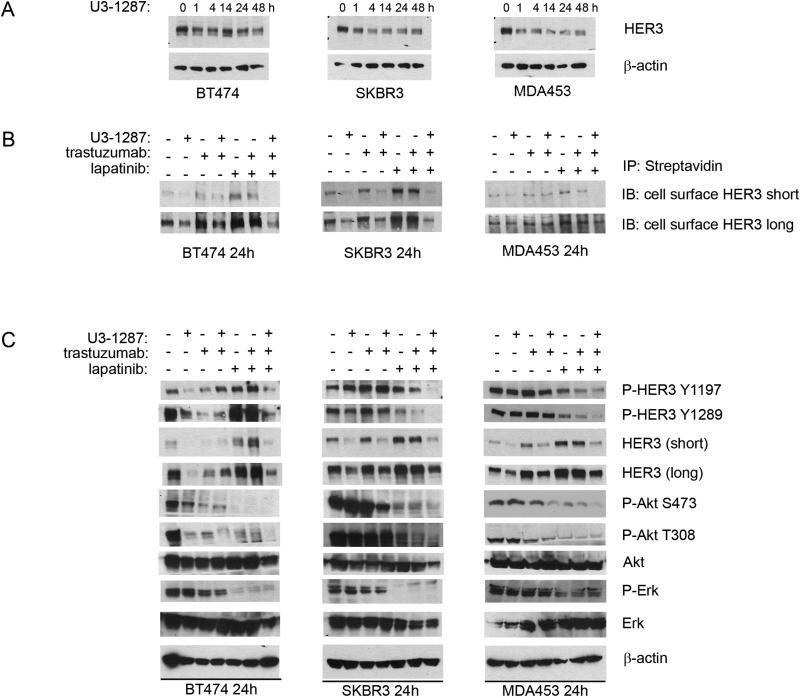
We initially examined the temporal effect the HER3 neutralizing antibody U3-1287 (38, 39) on HER3 protein levels in the HER2-overexpressing human breast cancer cell lines BT474, SKBR3, and MDA453. In all 3 cell lines, there was a modest decrease in HER3 protein levels starting at 1 h and lasting through 48 h of treatment (Fig 1A). Treatment with U3-1287 induced a modest dose-dependent downregulation of HER3, more evident in SKBR3 cells than in BT474 cells (Suppl. Fig. 1). We and others have reported that inhibition of the HER2 kinase and downstream PI3K/AKT with lapatinib results in increased FoxO-dependent HER3 transcription followed by recovery of HER3 phosphorylation (34). Thus, the potent inhibition of PI3K/Akt with the combination of lapatinib and trastuzumab should still result in an increase in FoxO-dependent HER3 transcription. Trastuzumab has been shown to dampen the recovery of HER3 phosphorylation (and hence recovery of PI3K/AKT) by inhibiting ligand-independent HER2-HER3 dimers (3). However, as HER3 can be phosphorylated by other kinases including EGFR, MET (43), Src (44) and FGFR2 (45), it is possible that P-HER3 may still recover, thus engaging PI3K and decreasing the action of the anti-HER2 combination.
Figure 1. U3-1287 downregulates HER3 and blocks HER3 phosphorylation following inhibition of HER2.
A. BT474, SKBR3 and MDA453 were treated with 20 μg/ml of U3-1287 over the indicated time course. Whole cell lysates were prepared and separated in a 7% SDS gel followed by immunoblot analysis with HER3 and β-actin antibodies. B. Cells were treated with U3-1287 (20 μg/ml), trastuzumab (20 μg/ml), lapatinib (1 μM) or the indicated combinations for 24 h and then biotinylated on their cell surface as described in Methods. Cell lysates were precipitated with immobilized Neutravidin gel; eluates were separated by SDS-PAGE and subjected to HER3 immunoblot analysis. C. Cells were treated with U3-1287, trastuzumab, lapatinib or the indicated combinations for 24 h. Whole cell lysates were prepared and separated in a 7% SDS gel followed by immunoblot analysis with the indicated antibodies.
Therefore, we examined the effect of U3-1287 on total, phosphorylated and cell surface HER3, and P-AKT. There was moderate to marked upregulation of cell surface HER3 in all 3 cell lines treated with both HER2 inhibitors (Fig. 1B). Trastuzumab is overall a weak inhibitor of PI3K/Akt. Since this inhibition is required to derepress FoxO-mediated transcription of HER3 mRNA (34), we did not observe a consistent increase in HER3 levels in cells treated with trastuzumab alone. The addition of U3-1287 to lapatinib and trastuzumab blocked this increase and almost completely downregulated cell surface HER3 levels. P-HER3 as measured with site specific antibodies was lower in cells treated with the triple combination. Treatment with lapatinib inhibited both S473 and T308 P-Akt levels. In SKBR3 and MDA453 cells, the addition of U3-1287 to lapatinib and trastuzumab enhanced the inhibition of S473 P-Akt. Further, in BT474 and SKBR3 cells, phosphorylation of the PIP3-dependent, PDK-1 site in AKT, as measured by immunoblot with a T308 P-AKT antibody, was lower in cells treated with all 3 inhibitors compared to the other treatments (Fig. 1C).
Neutralizing HER3 antibody sensitizes to combination of HER2 inhibitors
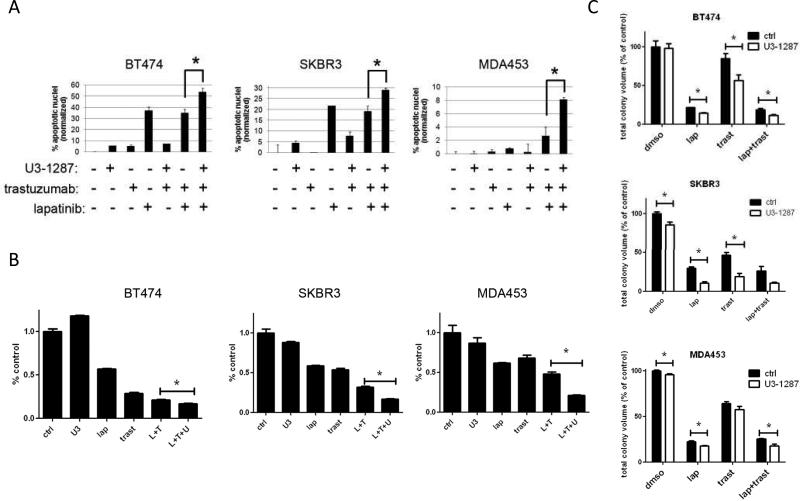
We next examined the apoptotic effect of lapatinib, trastuzumab and U3-1287 using the TUNEL assay. Lapatinib and the combination of lapatinib and trastuzumab induced BT474 and SKBR3 cell apoptosis whereas trastuzumab or U3-1287 did not (Fig 2A). Addition of the HER3 antibody to lapatinib and trastuzumab-treated cells resulted in a clear increase in apoptosis in all of the cell lines. We next performed a two-dimensional focus forming assay as readout for cell growth. The combination of lapatinib and trastuzumab was variably effective against all cell lines. BT474 cells are most sensitive to lapatinib and trastuzumab and the addition of U3-1287 resulted in a statistical, albeit small decrease in growth compared to lapatinib and trastuzumab. However SKBR3 and MDA453 cells, which are less sensitive to lapatinib and trastuzumab, showed a more obvious decrease in cell growth upon the addition of U3-1287 (Fig. 2B). In 3-dimensional growth assays in Matrigel, the addition of U3-1287 to lapatinib and trastuzumab resulted in a statistical decrease in total BT474 and MDA453 colony formation compared to that in the presence of both HER2 inhibitors (Fig 2C). These data suggest the HER3 antibody sensitizes breast cancer cells to dual HER2 blockade.
Figure 2. Neutralizing HER3 antibody sensitizes to combination of HER2 inhibitors.
A. BT474, SKBR3 and MDA453 cells were seeded in 60-mm plates in growth medium containing 10% FCS. The following day, cells were changed to serum-free media and treated with lapatinib (1 μM), trastuzumab (20 μg/ml) and U3-1287 (20 μg/ml) as indicated. Forty-eight h later, adherent and detached cells were collected and subjected to TUNEL assay using the APOBrdU kit. Each bar represents the mean ± SEM of cells with apoptotic nuclei (n=2-4; *, p<0.05, t test). B. Cells were seeded in 6-well plates in triplicate and treated with DMSO, 20 μg/ml trastuzumab, 0.1 μM lapatinib, 20 μg/ml U3-1287 or the indicated combinations. Media and inhibitors were replenished every 3-4 days. The monolayers were stained with crystal violet when the untreated cells became confluent after 14-21 days. Quantification of integrated intensity (% control) is shown (*, p<0.05, t test). C. BT474, SKBR3 and MDA453 cells were seeded in Matrigel and allowed to grow in the absence or presence of lapatinib (1 μM), trastuzumab (20 μg/ml) and/or U3-1287 (20 μg/ml). Media and inhibitors were replenished every 3 days. Mean total colony volume was quantified using the GelCount system. Each bar graph represents the mean ± S.E.M. of triplicate samples (*, p<0.05, t test).
Trastuzumab-resistant cells remain sensitive HER2-HER3 blockade
We next expanded our studies to trastuzumab-refractory breast cancer cells. HR6 cells are derived from a BT474 xenograft that was rendered resistant to trastuzumab in vivo (20). In these cells, the addition of U3-1287 to lapatinib and trastuzumab reduced levels of P-HER3, total HER3, and S473 P-AKT (Fig 3A). Moreover, the triple combination resulted in a statistical reduction of colony formation in 3D-Matrigel (Fig 3B), two-dimensional growth (Fig 3C) and an increase in apoptosis compared to that induced by the combination of both HER2 antagonists (Fig 3D).
We next tested whether the addition of U3-1287 would sensitize HR6 xenografts to trastuzumab and lapatinib. Mice bearing established HR6 xenografts were randomized to therapy with vehicle (control), trastuzumab, U3-1287, lapatinib, trastuzumab and U3-1287, lapatinib and trastuzumab or the combination of all 3 drugs. Trastuzumab as a single-agent had no antitumor activity (Fig 3E), whereas single-agent lapatinib, U3-1287, or the combination of trastuzumab and U3-1287 showed statistical reduction compared to vehicle treated mice. Tumors treated with the combination of trastuzumab and U3-1287 exhibited a statistical reduction in tumor volume compared to the trastuzumab arm. The addition of U3-1287 to lapatinib/trastuzumab did not reduce tumor volume further compared to lapatinib/trastuzumab. HR6 cells overexpress EGFR and ligands for EGFR including EGF, TGFα, and HB-EGF (20). Thus, being an EGFR TKI, lapatinib would have a significant antitumor effect but U3-1287 would be unable to significantly add to the combination since it cannot completely block ligand-induced EGFR-HER3 dimers nor prevent EGFR signaling through the MAPK pathway. There was no apparent drug-related toxicity in any of the treatment arms.
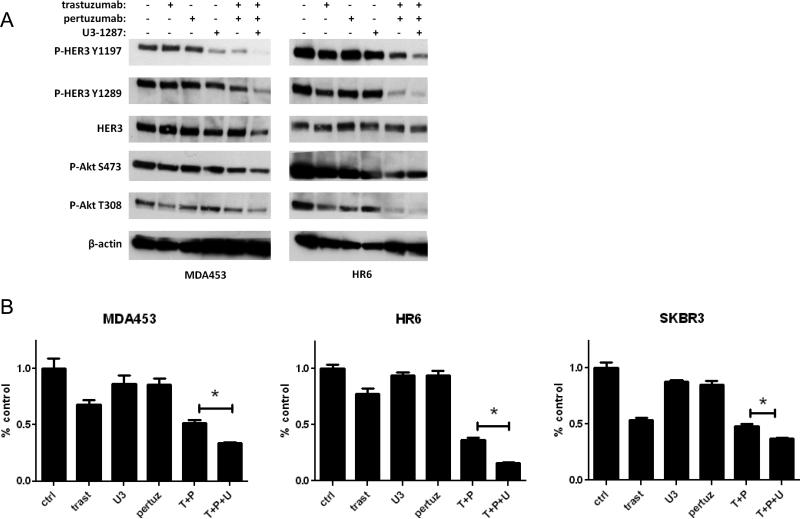
Addition of U3-1287 to the combination of trastuzumab and pertuzumab enhances inhibition of HER3/PI3K signaling and tumor growth
We next considered a second approach for dual blockade of HER2: the combination of trastuzumab and pertuzumab. This combination has recently been approved for patients with metastatic HER2+ breast cancer (14, 46). Indeed, the addition of U3-1287 to trastuzumab and pertuzumab resulted in further inhibition of Y1197 and Y1289 P-HER3 as well as enhanced inhibition of T308 P-Akt in both MDA453 and HR6 cells (Fig. 4A). We next performed monolayer focus forming assay as readout for cell growth. The combination of pertuzumab and trastuzumab was variably effective against MDA453, HR6 and SKBR3 cells. However, in each case, the addition of the HER3 antibody resulted in a statistical decrease in cell growth compared to both HER2 inhibitors (Fig 4B).
Figure 4. Addition of U3-1287 to the combination of trastuzumab and pertuzumab enhances inhibition of HER3/PI3K signaling and tumor growth.
A. Cells were treated with U3-1287 (20 μg/ml), trastuzumab (20 μg/ml), pertuzumab (20 μg/ml) or the indicated combinations for 24 h. Whole cell lysates were prepared and separated in a 7% SDS gel followed by transfer to nitrocellulose and immunoblot analysis with the indicated antibodies. B. Cells were seeded in in triplicate and treated with vehicle, U3-1287 (20 μg/ml), trastuzumab (20 μg/ml), pertuzumab (20 μg/ml) or the indicated combinations. Media and inhibitors were replenished every 3-4 days. The monolayers were stained with crystal violet when the untreated cells became confluent after 14-21 days. Quantification of integrated intensity (% control) is shown (*, p<0.05, t test).
Dual blockade of HER2 in combination with HER3 antibody reduces tumor growth
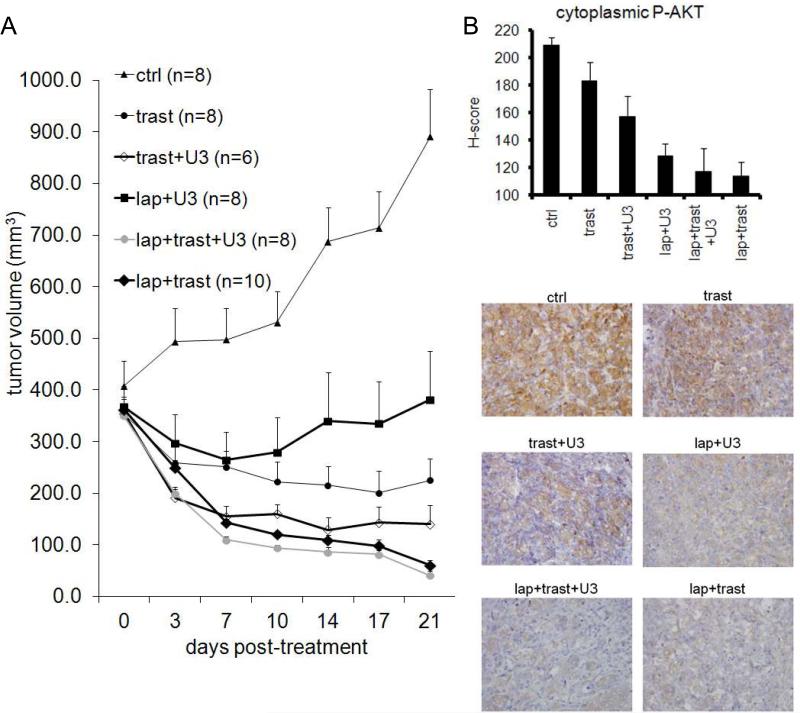
To expand our findings to an in vivo setting, we determined the activity of lapatinib, trastuzumab, U3-1287, or combinations of these against trastuzumab-sensitive BT474 xenografts. We had previously reported that U3-1287 (previously called AMG-888) does not inhibit BT474 xenograft growth and single agent lapatinib has a modest antitumor effect (34). Mice bearing BT474 xenografts measuring ≥350 mm3 were treated with trastuzumab, trastuzumab + U3-1287, lapatinib + U3-1287, lapatinib + trasuzumab or lapatinib + trastuzumab + U3-1287. All treatments significantly inhibited BT474 xenograft growth, particularly the combinations of lapatinib + trastuzumab, U3-1287 + trastuzumab and the triple combination (Fig. 5A). Mice treated with lapatinib + trastuzumab or lapatinib + trastuzumab + U3-1287 had almost no palpable tumor (mean tumor volume 60 mm3 and 41 mm3, respectively) remaining after 3 weeks of treatment.
Figure 5. Dual blockade of HER2 in combination with HER3 inhibitor reduces tumor growth.
A. Female athymic mice were injected with BT474 cells; once xenografts reached a volume of 350-400 mm3, they were randomized to the indicated treatments. Each data point represents the mean tumor volume in mm3 + SEM. B. Some mice were sacrificed after 5 days of treatment, receiving the last dose of lapatinib at 1h and/or trastuzumab and/or U3-1287 at 24 h before sacrifice. IHC analysis of S473 P-Akt in formalin-fixed, paraffin-embedded tumor sections from mice on treatment for 5 days (n=4-6). Top: Quantitative comparison of P-Akt histoscore for intensity of cytoplasmic staining. Bottom: Representative images from tumor sections are shown.
To document pharmacodynamic biomarkers of PI3K pathway inactivation, some xenografts (n=4-6) were harvested after 5 days of treatment. All treatment groups had a reduction in S473 P-Akt as assessed by IHC (Fig 5B). Mice treated with trastuzumab alone exhibited the weakest reduction in S473 P-AKT levels, consistent with the weak anti-signaling effect of trastuzumab (47). All the remaining treatment groups exhibited a statistical reduction in S473 P-Akt compared to vehicle treated (control) tumors.
Dual HER2 blockade in combination with HER3 antibody improves survival
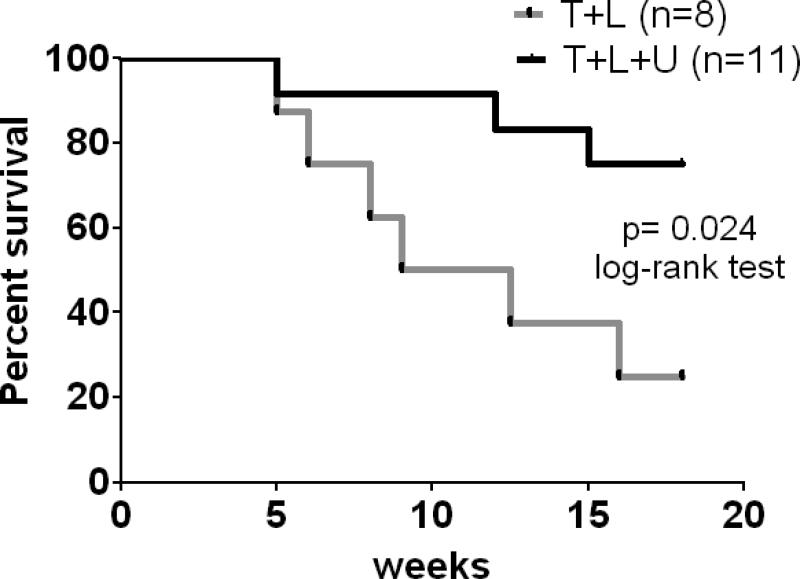
Finally, we sought to determine if combined inhibition of HER2 and HER3 will improve long-term survival over dual HER2 blockade with trastuzumab and lapatinib. To test this, we treated mice bearing large BT474 xenografts with 1) lapatinib and trastuzumab, or 2) lapatinib, trastuzumab and U3-1287. Treatment was initiated when tumors were ≥350 mm3; after 3 weeks of treatment tumors were undetectable in both groups (data not shown). Treatment was stopped at this time and tumor re-growth was monitored. Eighteen weeks after treatment was discontinued, 6/8 (75%) mice treated with lapatinib and trastuzumab whereas only 3/11 (27%) mice treated with lapatinib, trastuzumab and U3-1287 had to be euthanized due to tumors measuring ≥2000 mm3. This translated to a significant increase in survival in mice treated with the triple therapy compared to mice treated without the HER3 antibody (Fig. 6).
Figure 6. Dual HER2 blockade in combination with HER3 antibody improves survival.
Female athymic mice were injected with BT474 cells as described in Methods. Once tumors reached a volume ≥350 mm3, mice were randomized to lapatinib and trastuzumab or lapatinib, trastuzumab and U3-1287. Treatment was administered for 21 days after which time treatment stopped and mice were monitored for tumor regrowth. The x-axis indicates weeks after drug treatment stopped. Mice were sacrificed once recurrent tumors were ≥2000 mm3.
Discussion
There is clear evidence to suggest that the HER3 co-receptor is essential for HER2-mediated transformation and tumor progression as well as for the acquired resistance to HER2 inhibitors in HER2-overexpressing breast cancers. For example, loss of HER3 prevents HER2-mediated transformation of mammary epithelium (48). We show herein that a HER3 neutralizing antibody blocks the upregulation of total and activated HER3 that follows inhibition of PI3K/AKT upon treatment with lapatinib and trastuzumab. In turn, the HER3 antibody enhanced the antitumor effect of the anti-HER2 drug combination. Finally, mice treated with lapatinib, trastuzumab, and U3-1287 exhibited an increase in overall survival compared to mice treated with lapatinib and trastuzumab. These data suggest that HER3 antagonists should be an integral part of treatment strategies aimed at completely disabling the output of the HER2 network to PI3K in HER2-overexpressing cancers.
Inhibition of HER2/PI3K/AKT has been to shown to induce an upregulation of several RTKs including HER3, IGF-1R, and insulin receptor among others (34-36). In turn, this compensatory feedback dampens the antitumor effect of HER2/PI3K/AKT antagonists. Treatment of HER2+ cells with lapatinib and trastuzumab resulted in an increase in both cell-surface and total HER3 while not completely eliminating HER3 phosphorylation (Fig. 1B,C). However, addition of the HER3 antibody U3-1287 decreased cell-surface and total HER3 as well as phosphorylated HER3 below basal levels. In BT474 and SKBR3 cells, phosphorylation of the PIP3-dependent, PDK-1 site in AKT, as measured by immunoblot with a T308 P-AKT antibody, was lower in cells treated with all 3 inhibitors compared to the other treatments. This suggests that a more complete and sustained inhibition of HER3 is necessary to block PI3K function and the subsequent production of PIP3, which maintains PDK-1 and AKT at the plasma membrane. Pulsatile and less frequent higher doses of lapatinib have been proposed as a means of sustained inhibition of HER3 in HER2+ tumors (49). This schedule is currently under investigation. However, higher concentrations than the one we are using (1 μM) against cells in culture are not achieved in patients with the current daily schedule and may introduce off-target effects.
The combination of trastuzumab and U3-1287 was particularly effective against BT474 xenografts, significantly more so than the combination of lapatinib and U3-1287. Of note, the antitumor action of this combination was associated with moderate inhibition of tumor P-AKT levels (Fig 5A), not as potent as that seen in lapatinib-treated tumors. Trastuzumab is thought to work mainly via blockade of ligand-independent HER2-HER3 dimerization (3) and induction of antibody-dependent cellular cytotoxicity and innate immunity to HER2 (4, 5). Therefore, this result suggests the immune effector mechanisms of trastuzumab are central to its antitumor action.
It is also possible that U3-1287 and trastuzumab block ligand-induced (U3-1287) and ligand-independent HER2-HER3 dimers (trastuzumab). This speculation is consistent with modest inhibition of P-AKT, a downstream effect of HER2-HER3 dimerization and activation of PI3K. This result is reminiscent of the proposed mechanism of synergy between trastuzumab and pertuzumab (16). Thus, we investigated the addition of the HER3 inhibitor to dual blockade of HER2 with pertuzumab and trastuzumab. The combination of U3-1287, trastuzumab and pertuzumab induced a greater inhibition of PI3K signaling and tumor growth in vitro compared to trastuzumab and pertuzumab (Fig. 4). This suggests that dual blockade of ligand-induced and ligand-independent HER2-HER3 dimers (by pertuzumab and trastuzumab, respectively) is not completely effective at removing HER3 function in HER2-dependent tumors.
In summary, dual blockade of HER2 does not eliminate HER3 function completely. Targeted inhibition of HER3 improved the response to HER2 antagonists in several HER2-dependent models of breast cancer. Based on these data, we conclude therapeutic inhibitors of HER3 should be considered as part of multi-drug combinations aimed at completely and rapidly disabling the HER2 network in HER2-overexpressing breast cancers. Further, the combination with of HER3 antibodies with trastuzumab represent another potential strategy for dual blockade of HER2.
Supplementary Material
Statement of Translational Relevance.
There is mounting data that dual blockade of HER2 with inhibitors such as trastuzumab with lapatinib or with pertuzumab is a superior treatment approach. Although these anti-HER2 therapies have improved outcome for patients with HER2+ breast cancer, patients frequently develop acquired drug resistance. Recent reports suggest that inhibition of HER2/PI3K/AKT causes upregulation in HER3 which may attenuate the antitumor action of these inhibitors. We show herein that the neutralizing HER3 antibody, U3-1287, in combination with dual blockade of HER2 further suppresses the growth of HER2+ human breast cancer cells and xenografts. Furthermore, treatment with lapatinib, trastuzumab, and U3-1287 in HER2+ human breast cancer xenografts in vivo results in a statistical increase in survival compared to treatment with lapatinib and trastuzumab. These findings support the use of combinations of HER2 and HER3 inhibitors for the treatment of patients with HER2+ breast cancer.
Acknowledgments
Financial support: This work was supported by R01 Grant CA80195 (CLA), American Cancer Society (ACS) Clinical Research Professorship Grant CRP-07-234 (CLA), The Lee Jeans Translational Breast Cancer Research Program, Breast Cancer Specialized Program of Research Excellence (SPORE) P50 CA98131, Susan G. Komen for the Cure Foundation grant SAC100013 (CLA). C. Arteaga is supported by a Stand Up to Cancer Dream Team Translational Cancer Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209). J.T. Garrett is supported by ACS 118813-PF-10-070-01-TBG and DOD BC093376 postdoctoral fellowship awards.
Footnotes
Conflicts of Interest: The authors declare no financial conflicts of interest.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15(8):2894–904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 3.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 5.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets [see comments]. Nat Med. 2000;6(4):443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 7.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 9.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 10.Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26(18):2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 11.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 12.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–37. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 366(2):109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–14. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 16.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–6. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 379(9816):633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 19.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13(16):4909–19. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 21.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62(11):3151–8. [PubMed] [Google Scholar]
- 22.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65(23):11118–28. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 23.Shattuck DL, Miller JK, Carraway KL, 3rd, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68(5):1471–7. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 24.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 27.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68(22):9221–30. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rexer BN, Ham AJ, Rinehart C, Hill S, Granja-Ingram Nde M, Gonzalez-Angulo AM, et al. Phosphoproteomic mass spectrometry profiling links Src family kinases to escape from HER2 tyrosine kinase inhibition. Oncogene. 30(40):4163–74. doi: 10.1038/onc.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faber AC, Corcoran RB, Ebi H, Sequist LV, Waltman BA, Chung E, et al. BIM Expression in Treatment-Naive Cancers Predicts Responsiveness to Kinase Inhibitors. Cancer Discov. 1(4):352–65. doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 31.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68(14):5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 32.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100(15):8933–8. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106(52):22299–304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 108(12):5021–6. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 29(2):166–73. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freeman D, Ogbagabriel S, Rothe M, Radinsky R, Treder M. Fully human Anti-HER3 monoclonal antibodies (mAbs) have unique in vitro and in vivo functional and antitumor activities versus other HER family inhibitors. AACR Meeting Abstracts. 2008 2008(1_Annual_Meeting):LB-21- [Google Scholar]
- 39.Treder M, Hartmann S, Ogbagabriel S, Borges E, Green L, Kang J, et al. Fully human Anti-HER3 monoclonal antibodies (mAbs) inhibit oncogenic signaling and tumor cell growth in vitro and in vivo. AACR Meeting Abstracts. 2008 2008(1_Annual_Meeting):LB-20- [Google Scholar]
- 40.Berlin J, Keedy VL, Janne PA, Yee L, Rizvi NA, Jin X, et al. A first-in-human phase I study of U3-1287 (AMG 888), a HER3 inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2011;29(200s) [Google Scholar]
- 41.Ghosh R, Narasanna A, Wang SE, Liu S, Chakrabarty A, Balko JM, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 71(5):1871–82. doi: 10.1158/0008-5472.CAN-10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30(3):256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 43.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 44.Contessa JN, Abell A, Mikkelsen RB, Valerie K, Schmidt-Ullrich RK. Compensatory ErbB3/c-Src signaling enhances carcinoma cell survival to ionizing radiation. Breast Cancer Res Treat. 2006;95(1):17–27. doi: 10.1007/s10549-005-9023-9. [DOI] [PubMed] [Google Scholar]
- 45.Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, et al. FGFR2- amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008;68(7):2340–8. doi: 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 46.Pertuzumab (Perjecta) for HER2-positive metastatic breast cancer. Med Lett Drugs Ther. 54(1395):59–60. [PubMed] [Google Scholar]
- 47.Mohsin SK, Weiss HL, Gutierrez MC, Chamness GC, Schiff R, Digiovanna MP, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23(11):2460–8. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 48.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, et al. HER3 is required for HER2-induced pre-neoplastic changes to the breast epithelium and tumor formation. Cancer Res. doi: 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amin DN, Sergina N, Ahuja D, McMahon M, Blair JA, Wang D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med. 2(16):16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.