Abstract
Fibroblasts incubated on 3D collagen matrices in serum or lysophosphatidic acid (LPA)-containing medium self-organize into clusters through a mechanism that requires cell contraction. However, in platelet-derived growth factor (PDGF)-containing medium, cells migrate as individuals and do not form clusters even though they constantly encounter each other. Here, we present evidence that a required function of cell contraction in clustering is formation of fibronectin fibrillar matrix. We found that in serum or LPA but not in PDGF or basal medium, cells organized FN (both serum and cellular) into a fibrillar, detergent-insoluble matrix. Cell clusters developed concomitant with FN matrix formation. FN fibrils accumulated beneath cells and along the borders of cell clusters in regions of cell-matrix tension. Blocking Rho kinase or myosin II activity prevented FN matrix assembly and cell clustering. Using siRNA silencing and function-blocking antibodies and peptides, we found that cell clustering and FN matrix assembly required α5β1 integrins and fibronectin. Cells were still able to exert contractile force and compact the collagen matrix under the latter conditions, which showed that contraction was not sufficient for cell clustering to occur. Our findings provide new insights into how procontractile (serum/LPA) and promigratory (PDGF) growth factor environments can differentially regulate FN matrix assembly by fibroblasts interacting with collagen matrices and thereby influence mesenchymal cell morphogenetic behavior under physiologic circumstances such as wound repair, morphogenesis and malignancy.
Keywords: 3D collagen matrix, Fibronectin, Integrin, Cell migration, Cell contraction, Wound repair, Tissue morphogenesis
INTRODUCTION
Mechanical interactions between cells and their extracellular environment play key roles in diverse aspects of normal cell physiology including cell migration, proliferation, and differentiation [1–5]. Changes in cell-matrix interactions contribute to the pathological features associated with scarring [6–9], aging [10], and tumor progression [11–15]. Understanding the biomechanics of cell-matrix interactions has become an important goal in the development of tissue engineering materials [16–20].
We and others have studied interactions between diverse types of tissue cells and 3D extracellular matrices as a biologically relevant platform to model cell behavior in tissue-like environments [21–25]. Recently, we reported that fibroblasts incubated on collagen matrices formed cell clusters depending on the growth factor environment. Clustering occurred in medium containing fetal bovine serum (FBS) or lysophosphatidic acid (LPA) [26], the latter a Rho-activating growth factor found in serum [27]. However, clustering did not occur in basal medium lacking growth factors or in medium containing platelet-derived growth factor (PDGF) [26].
Cell clustering also was observed to occur on soft 2D polyacrylamide gel substrates and found to require cell contraction, which was suggested to provide a mechanism by which cells came together on the compliant substrates [28, 29]. However, the studies all were carried out in serum-containing medium. We suspected that cell contraction might play an additional role besides promoting cell-cell interactions since, as mentioned above, clustering on collagen matrices did not occur in PDGF-containing medium even though the fibroblasts were moving and constantly encountering each other.
Fibroblasts form aggregates after culture on non-adhesive surfaces or in hanging drops and subsequently undergo a compaction process that requires fibronectin (FN) and integrin FN receptors [30–33]. Also, FN-null mouse embryo fibroblasts, which are unable to spread on collagen matrices, can utilize exogenously added FN to form a fibrillar FN matrix on the surface of the collagen matrix, and cells interacting with the FN matrix spread and proliferate [34]. Since FN fibrillar matrix formation in routine 2D cell culture requires Rho kinase and myosin II-dependent cell contraction [35, 36], we tested the possibility that in addition to bringing cells together, the function of cell contraction in fibroblast cluster formation on 3D collagen matrices was to organize FN into a fibrillar matrix that became a scaffold for clustering.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), CO2-independent DMEM, Opti-MEM, 0.25% Trypsin-EDTA, and antibiotic-antimycotic solutions were purchased from GIBCO (Grand Island, NY). Type collagen I (rat tail, high concentration) was obtained from BD Biosciences (Bedford, MA). FBS was obtained from Atlanta Biologicals (Lawrenceville, GA). Human plasma fibronectin (FN) was obtained from the New York Blood Center (New York, NY). BSA (fatty acid-free), lysophosphatidic acid (LPA), blebbstatin and monoclonal anti-actin antibody were obtained from Sigma (St. Louis, MO). BSA (fraction V) was obtained from Equitech (Kerrville, TX). Human recombinant PDGF-BB was purchased from Upstate Biotechnology (Lake Placid, NY). Cyclic peptides cyclo (-GRGDSP) and cyclo (-GRGESP) were obtained from Anaspec (Fremont, CA). Function-blocking monoclonal anti-FN (HFN 7.1) and polyclonal anti-FN were obtained from Abcam (Cambridge, MA). Anti-α5 integrin antibodies were purchased from Abcam and from Santa Cruz Biotechnology (Santa Cruz, CA). Function blocking antibodies against α5 and β1 integrin subunits (MAb16 and MAb13) were a generous gift from Dr. K. Yamada. Alexa 488-Phalloidin, propidium iodide (PI), Hoechst 33342, Alexa 488 and 568 conjugated antibodies against mouse and rabbit IgGs were obtained from Invitrogen-Molecular Probes (Eugene, OR). Fluoromount G was purchased from Southern Biotechnology (Birmingham, AL). RNAase (DNAase-free) was obtained from Roche Diagnostics (Indianapolis, IN). Lipofectamine was obtained from Invitrogen (Carlsbad, CA). siRNA oligonucleotide sequences (ON-TARGET plus siRNA) and a non-targeting siRNA (siGenome Control non-targeting siRNA) were purchased from Thermo Scientific-Dharmacon (Lafayette, CO). Horseradish peroxidase-conjugated goat anti-mouse and rabbit IgG were obtained from MP Biomedicals (Solon, OH) and Thermo Scientific (Pittsburgh, PA), respectively. Rhodamine FN (R-FN) was purchased from Cytoskeleton Inc (Denver, CO). Y27632 was obtained from EMD Millipore (Billerica, MA).
Cell culture
Use of human foreskin fibroblasts was approved by the University Institutional Review Board (Exemption #4). BR-5 cells (early passage, hTERT immortalized, human skin fibroblasts) [26] were cultured on DMEM supplemented with 10% FBS in a 37°C and 5% CO2 humidified incubator. Experimental incubation media consisted of DMEM supplemented with 10% FBS (DMEM/FBS), DMEM supplemented with 5mg/ml BSA (fatty acid free) and 10 μM LPA (DMEM/LPA), and DMEM supplemented with 5mg/ml BSA (fatty acid free) and 50 ng/ml PDGF (DMEM/PDGF). For time lapse microscopy, CO2-independent DMEM replaced regular DMEM medium.
Collagen matrix preparation and cell clustering
Collagen matrices (1 mg/ml) were prepared as described previously [26]. Briefly, 200 μl aliquots of collagen solution at physiological pH and ionic strength were polymerized within 12 mm circular scores made on the bottom of 24-multiwell plates. Polymerization occurred within 1h incubation at 37°C.
Cell clustering assays were carried out in 24-multiwell plates. Unless indicated otherwise, 2×104 cells in 1 ml of experimental incubation medium were cultured on collagen matrices for 18h at 37°C + 5% CO2. Cyclic peptides –GRGDSP and –GRGESP, function blocking antibodies MAb16, MAb13 and HFN 7.1, FN (soluble or rhodamine labeled), and pharmacologic inhibitors Y27632 and blebbistatin were added to the incubations as indicated in the Figure Legends. In some experiments, cell clustering assays were carried out with 96-multiwell plates, in which case proportional cell density and collagen volumes were used.
Microscopy
At the end of experimental incubation, cells were fixed with 3% paraformaldehyde in PBS and processed for immunofluorescence staining as described previously [37] except treatment with primary antibodies against anti-α5 integrin (1:200) and anti-FN (1:200) was carried out before permeabilization with Triton X-100. Staining for actin was accomplished after permeabilization using Alexa fluor 488 phalloidin (1:500) as indicated. Staining for nuclei was accomplished using propidium iodide (PI) (1:500) or Hoechst 33342 (2 μg/ml). After staining, samples were mounted on glass slides with Fluoromount G. Samples were observed in a Nikon Eclipse E600 and a Nikon Eclipse Ti using a 10X/0.45 PlanApo infinity corrected objective. Images were acquired with a Photometrics SenSys CCD camera and MetaVue acquisition and imaging software. For confocal microscopy, a Leica TCS SP5 microscope was used with a 63X immersion oil objective lens. Z-stack reconstructions were performed using 0.5 μm optical slices.
Time lapse microscopy was performed using Nikon Eclipse Ti microscope with a 10X/0.45 PlanApo infinity corrected objective and images were taken every 20 minutes during 18–20h as indicated in the Figure Legends using phase contrast and fluorescence. Images and the resultant picture sequences were processed using the NIS Elements software package. Final images were transferred to Image J for processing.
Samples were processed for scanning electron microscopy by standard methods as described previously [38]. Images were collected using a Philips XL30 ESEM scanning electron microscope using acceleration voltage of 15 kV.
siRNA transfection
siRNA transfection was accomplished beginning with trypsin rounded cells as described previously [37]. Semi-confluent (80%) cell cultures on 6-multiwell plates were washed twice in FBS-free, antibiotic-free DMEM medium and briefly trypsinized for 1 min. Trypsin was inactivated by adding 4 ml DMEM/FBS (antibiotic-free), followed by a careful wash with DMEM without FBS and antibiotics. Cells then were incubated with 800 μl of DMEM (serum and antibiotics-free) plus 200 μl of a mixed solution containing 0.1 μM of the desired α5 siRNA target sequence GAACGAGUCAGAAUUUCGA, 3% lipofectamine, and Opti-MEM for 24 h. After incubation, plates were expanded, adding 1.5 × 105 cells per well and incubating them for 72 and 96h. Preliminary experiments were initially performed using the 4 siRNA open reading frames provided by the manufacturer, all of which successfully silenced α5 integrin. Mock experiments used a control siRNA non-targeting sequence instead of specific siRNA.
Western Immunoblotting
Immunoblotting was performed as previously described [37]. Blocking consisted by incubating membranes for 1h at room temperature on 3% BSA (fraction V) TBS-Tween 20. Primary antibodies against actin (1:1000 dilution), and α5 integrin (1:1000) were incubated on PDVF membranes for 16h at 4°C. HRP-conjugated goat-anti-mouse (1:5000) or anti-rabbit (1:5000) were used as secondary antibodies.
RESULTS
Cell cluster formation
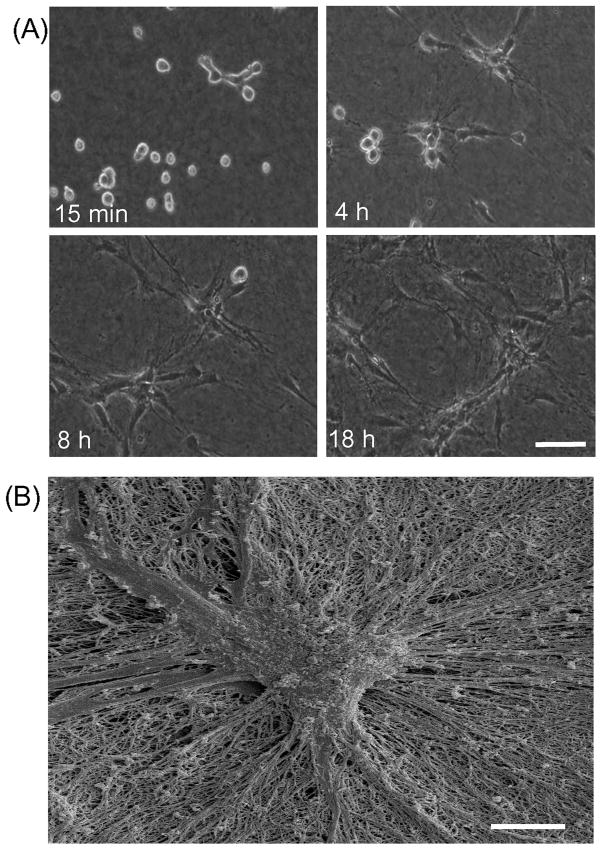
Figure 1A shows phase contrast time-lapse images of fibroblasts incubated in FBS-containing medium on the surface of a collagen matrix. After initial attachment (~15 min), cells appeared mostly round. Within four hours (4 h), cells extensions became visible. Local collagen matrix remodeling was observed based on the appearance of phase dark strands within the field of view (see Supplemental Movie 1). Cells nearby each other appeared to interact through their extensions. After 4h, most cells were spread in elongated morphology and cell clustering became evident. The size of the clusters and alignment of collagen matrix between clusters increased from 8h to 18h. Over that period, collagen matrix remodeling resulted in matrix compaction, which was observed as a decrease in matrix height [39].
Figure 1. Fibroblast clustering in DMEM medium containing FBS.
(A) Time-lapse phase contrast images (from Supplemental Movie 1) of cells cultured for 18h on collagen matrices in DMEM/FBS. Images shown are from the same microscopic field at the times indicated. Bar = 100 μm. (B) Scanning electron microscopic image of a collagen matrix incubated with fibroblasts in DMEM/FBS for 18h. At the end of the incubation, samples were fixed and processed for SEM. Bar = 10 μm.
Figure 1B shows the tightly packed appearance of a typical cell cluster visualized by scanning electron microscopy. Reorganization of collagen fibrils could be seen in close association with radially organized cell extensions. Fibrillar structures were not observed above the clusters.
Fibronectin matrix organization
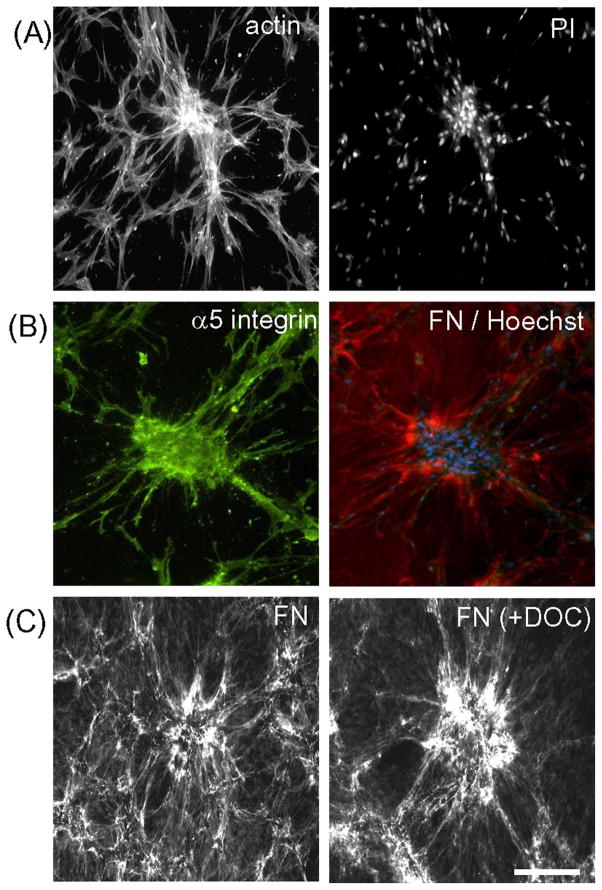
Figure 2A shows by fluorescence microscopy that actin stress fibers were prominent in cells within clusters and oriented towards nearby cells located outside of clusters. Nuclear staining with propidium iodide (PI) demonstrated that clusters often contained 10–30 cells. Experiments were then carried out to determine the location of fibronectin receptors (integrin α5β1) and fibronectin in relationship to cell clusters. Figure 2B shows the extracellular distribution of α5 integrin (green) and FN (red) for a typical cell cluster (blue = nuclei). Staining of α5 integrin appeared to occur over the cell bodies and extensions. FN staining was both diffuse and fibrillar with FN fibrils concentrated in regions surrounding the clusters and also present along cell extensions. Fibroblasts are known to organize FN into a pericellular fibrillar matrix that becomes resistant to deoxycholate extraction [40]. Figure 2C shows that the FN matrix organized during cell cluster formation was deoxycholate resistant.
Figure 2. Structural organization and molecular composition of cell clusters.
(A) Fluorescence microscopy image of a fibroblast cluster formed during 18h in DMEM/FBS fixed and stained for actin and nuclei (PI). (B) Same as “A” except stained for α5 integrin (green), FN (red), and cell nuclei (Hoechst, blue). (C) Same as “A” except washed once with cold PBS, and treated for 10 min at 37°C in PBS without or with 1% deoxycholate (DOC) as indicated after which the samples were washed three times and fixed and stained for fibronectin. Bar = 100 μm.
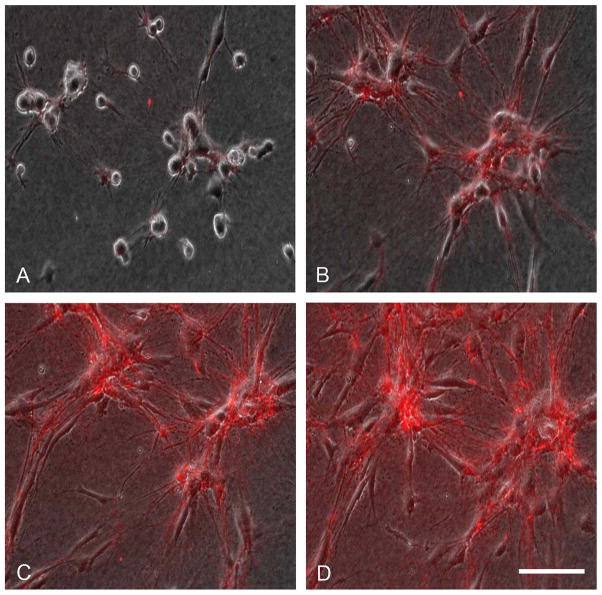
To observe directly the real-time dynamics of FN fibril organization during cell clustering, experiments were carried out with 5 μg/ml rhodamine-conjugated FN (R-FN) [41] added to the incubations. Figure 3 shows combined phase contrast fluorescence time-lapse images of fibroblasts undergoing cell clustering in FBS medium during 18h (see Supplemental Movie 2). Small amounts of R-FN could be seen to associate with the round cells as early as 1h (A). By 4h (B), two clusters had started to develop within the field of view. R-FN could be seen organizing along cell extensions and associated with the clusters. From 4h to 8 h (C) and 8 hr to 18h (D), cells continued to become associated with the two clusters resulting in an increased in their size and concomitant increase in R-FN matrix organization.
Figure 3. Rhodamine-fibronectin organization during cell cluster formation.
Time-lapse phase contrast/fluorescence images (from Supplemental Movie 2) of cells cultured 18h on collagen matrices in DMEM/FBS containing 5 μg/ml rhodamine-FN. Images shown are from the same microscopic field at the times indicated. (A) 1 h; (B) 4h; (D) 8h; (D) 18 h. Bar = 100 μm.
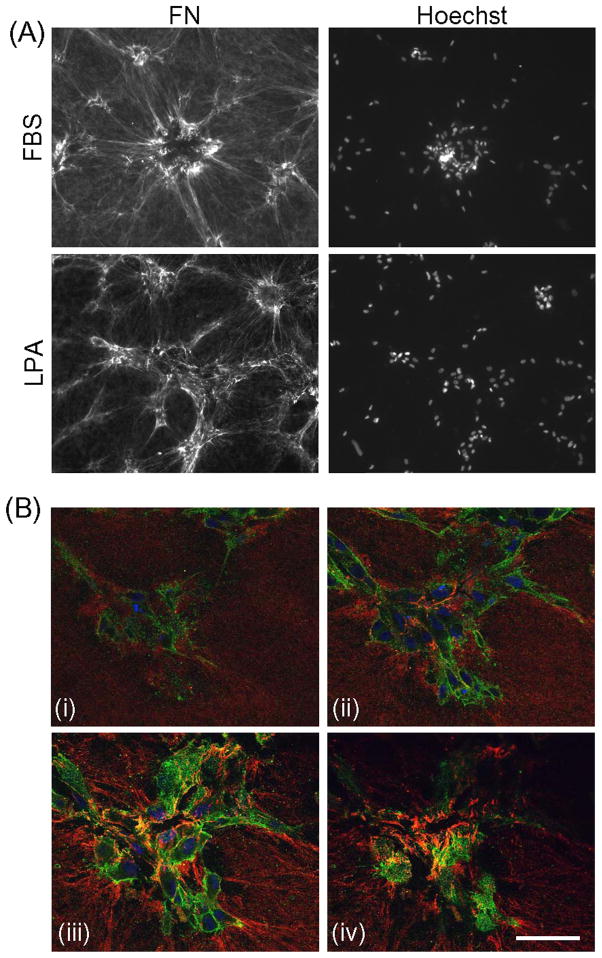
DMEM/10% FBS medium contains ~20 μg/ml plasma FN [42]. Since LPA can substitute for FBS to promote cell clustering in the absence of added plasma fibronectin, we examined whether cells also organized a FN matrix during LPA-dependent cell clustering in the absence of plasma fibronectin. Figure 4A shows that organization of cellular FN in the LPA samples was similar to organization of plasma FN in the FBS samples although the extent of matrix formation and size of cell clusters typically were smaller in LPA.
Figure 4. FN organization and cell cluster formation in LPA-containing medium.
(A) Fibroblasts were cultured for 18h on collagen matrices in DMEM/FBS and DMEM/LPA as indicated. At the end of the incubations, samples were fixed and stained for FN and Hoechst 33342. Bar = 100 μm. (B) Z-plane distribution of FN in a fibroblast cluster formed during 18h in DMEM/LPA fixed and stained for FN (red), α5 integrin (green), and nuclei (blue). (i) top area of the cluster; (ii) upper part of the cluster; (iii) lower part of the cluster; (iv) bottom of the cluster. Supplemental Movie 3 shows the full set of confocal microscopy-generated 0.5 μm Z stacks. Bar = 50 μm.
We used confocal microscopy to critically examine the localization of FN fibrils that formed during cell clustering in LPA-containing medium. Figure 4B presents images from a confocal Z series from the top to the bottom of a cell cluster (blue nuclei) (see Supplemental Movie 3). FN fibrils (red) were prominent beneath the cells (iv) and towards the bottom of the clusters (iii) but less evident higher in the clusters (ii) and essentially absent from the top (i). Integrin α5 staining (green), on the other hand, appeared to be uniformly distributed around cell peripheries. In general, FN was not found in between the clustered fibroblasts.
Dependence of cell clustering on FN matrix organization
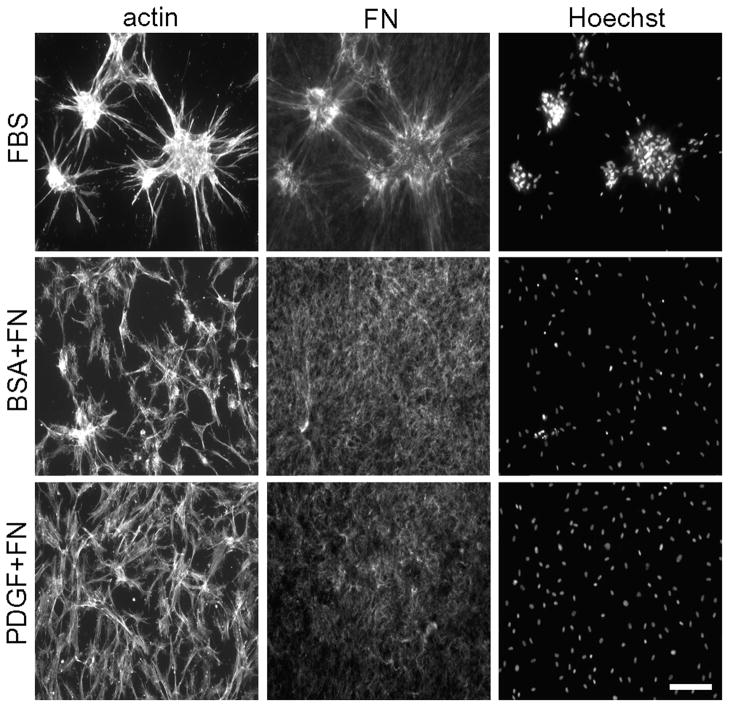
The dynamics of FN fibril organization and distribution during cell clustering suggested that the mechanism of clustering depended on multiple cells binding to a shared FN matrix organized by contracting cells. As mentioned in the Introduction, fibroblasts incubated on the surfaces of collagen matrices do not form clusters in basal medium (no growth factor added) and PDGF-containing medium [26]. Figure 5 compares the distribution of FN after fibroblasts were cultured in FBS vs. basal and PDGF media. Twenty μg/ml plasma FN was added to basal and PDGF conditions. FN was seen to become associated diffusely with the collagen matrix but not to form a fibrillar matrix, and cells did not form clusters.
Figure 5. Effect of varying the growth factor environment on FN distribution and cell cluster formation.
Cells were cultured for 18h on collagen matrices in DMEM containing FBS, PDGF and no growth factor (basal) as indicated. The PDGF and basal incubations contained 20 μg/ml plasma FN. At the end of the incubations, samples were fixed and stained for actin, FN and nuclei (Hoechst) as indicated. Bar = 100 μm.
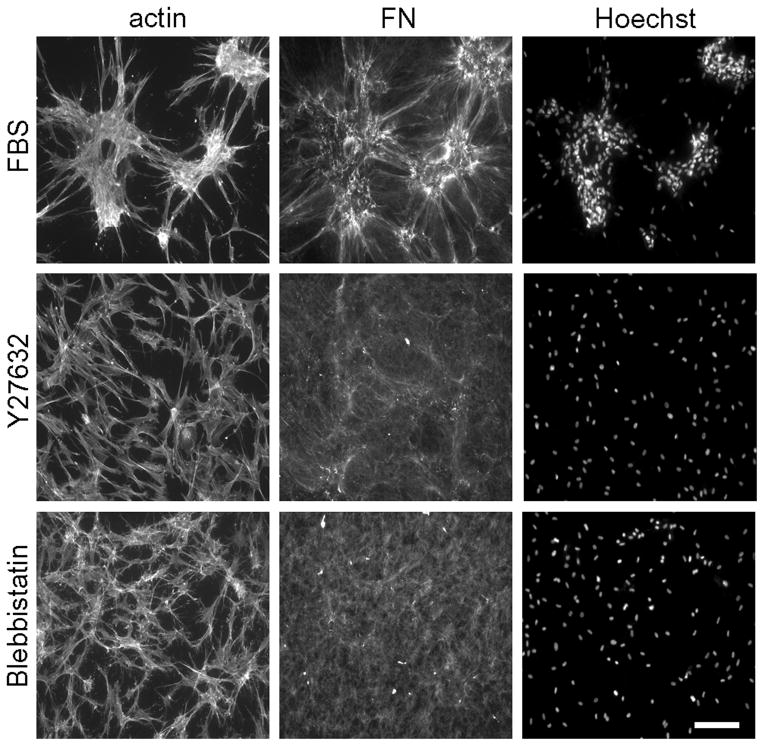
Rho kinase and myosin II-dependent cell contraction is known to play a role in FN matrix organization by fibroblasts in routine 2D culture [35, 36] and also required for cell clustering on collagen matrices [26]. Figure 6 demonstrates that conditions under which blocking Rho kinase (Y27632) and myosin II activity (blebbistatin) prevented cell clustering, also inhibited organization of FN fibrillar matrix but not cell spreading.
Figure 6. Blocking Rho kinase and myosin II contraction inhibits FN matrix assembly and cell cluster formation.
Fluorescence microscopy images of fibroblasts on collagen matrices during 18h in DMEM/FBS containing 5 μM Y27632 and 20 μM blebbistatin as indicated. At the end of the incubations, samples were fixed and stained for actin, FN and nuclei (Hoechst). Bar = 100 μm.
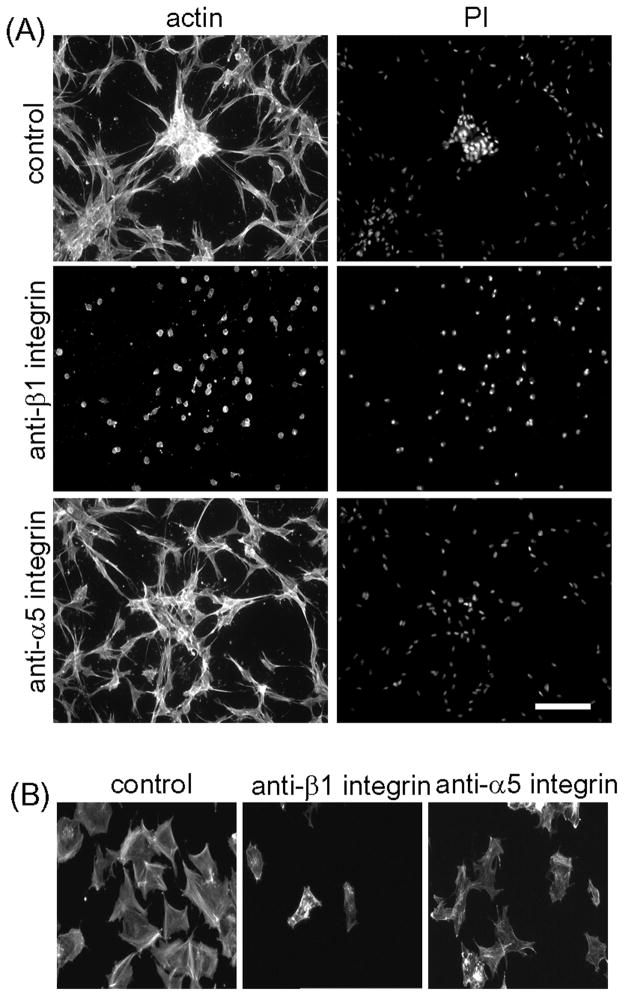
Formation of FN fibrillar matrix depends on integrin fibronectin receptors [36, 43]. Therefore, we tested the consequences for cell clustering of interfering with fibronectin receptors with function-blocking anti-integrin α5 (MAb16) and β1 (MAb13) antibodies [43]. Figure 7A shows that antibody against integrin β1 completely blocked both cell clustering and cell spreading on collagen matrices. Antibody against integrin α5 subunit also decreased clustering -- clusters that did form were smaller and less tightly packed -- but had no effect on cell spreading on the collagen matrix. Both antibodies reduced cell adhesion to FN-coated 2D surfaces (Figure 7B).
Figure 7. Effect of function blocking antibodies against α5 and β1 integrin subunits on fibroblast cluster formation.
(A) Cells were cultured for 18h on collagen matrices in DMEM/FBS containing 100 μg/ml monoclonal antibodies against β1 (MAb13) or α5 (MAb16) as indicated. At the end of the incubations, samples were fixed and stained for actin and nuclei (PI). (B) Samples were incubated on FN-coated coverslips for 1h and then fixed and stained for actin. Bar =100 μm.
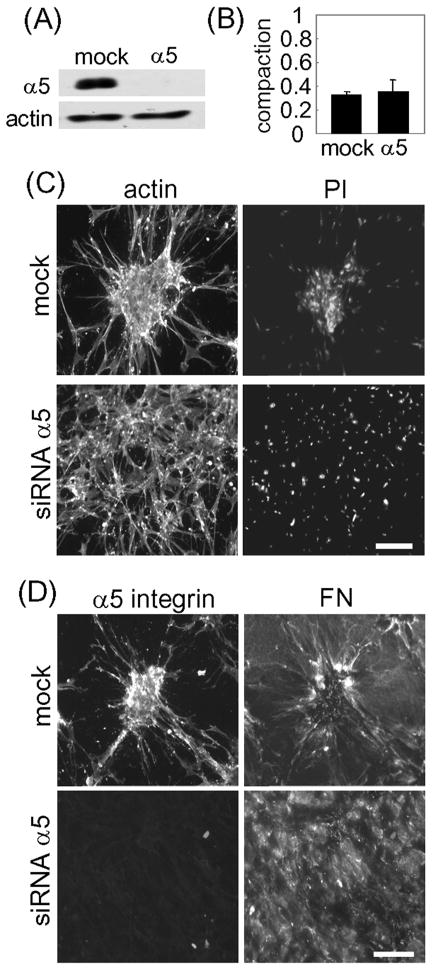
In further experiments, we tested the effect of silencing α5 integrin expression on cell clustering and fibronectin matrix formation. All four different oligonucleotide pairs in the Dharmacon ON-TARGET plus siRNA set inhibited integrin α5 expression. Figure 8A shows an example by Western blotting that α5 integrin levels were markedly reduced in cells transfected with integrin α5 specific siRNA but not with mock (scrambled) siRNA. Figure 8B demonstrates that silencing α5 had no effect on the ability of fibroblasts to interact with collagen matrices and to cause their compaction as measured by reduction of matrix height compared to starting matrices (~70% reduction).
Figure 8. Silencing α5 integrin inhibits FN matrix organization and cell cluster formation.
(A) Western blotting results showing α5 integrin and actin detection for cells after transfection with specific α5 or mock siRNA. (B) Extent of matrix compaction by mock and α5-silenced cells cultured 18h on collagen matrices in DMEM/FBS. Compaction was calculated as final matrix height/starting matrix height. (C) Mock and α5 integrin silenced cells were cultured for 18h on collagen matrices in DMEM/FBS. At the end of the incubations, samples were fixed and stained for actin and PI. (D) same as “C” except the cells were stained for α5 integrin and FN. Bar = 100 μm.
The results in Figure 8C show that silencing α5 integrin inhibited cell clustering although cells were able to spread normally on collagen as shown by actin staining. That the spread cells remained dispersed was evident from nuclear staining (PI). Figure 8D shows that unlike the fibrillar organization of FN in mock-silenced cells, the distribution of FN in α5-silenced cells was mostly diffuse. Consistent with the immunoblotting, α5 integrin was undetectable in α5 integrin-silenced cells.
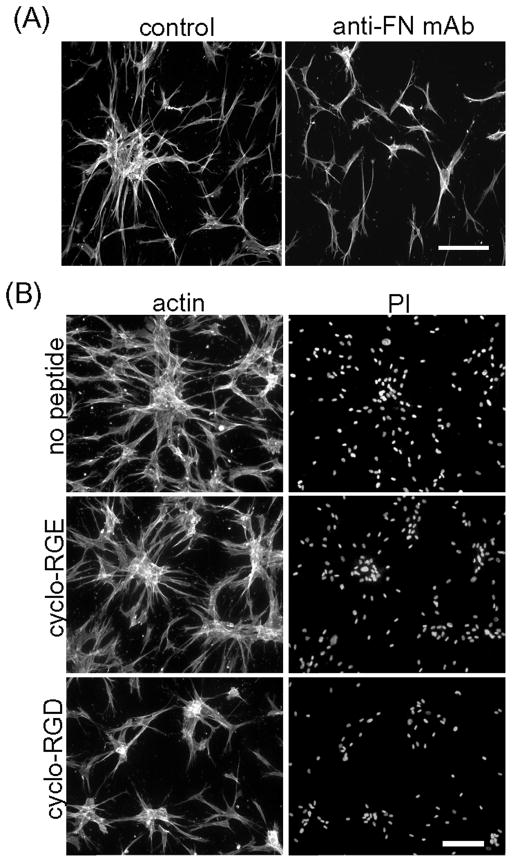
Finally, the requirement for FN in cell clustering was tested directly by adding function-inhibiting anti-FN monoclonal antibody HFN 7.1 [44] to the incubations. Figure 9A demonstrates that addition of this antibody inhibited cell clustering without blocking cell spreading on the collagen matrices. In control experiments, anti-FN inhibited cell adhesion to FN but not to collagen-coated surfaces (not shown). Figure 9B shows related experiments using a cyclic FN adhesion domain peptide that inhibits fibronectin-integrin interactions [45]. Addition of 200 μg/ml cyclo-RGD reduced cell clustering compared to control cyclo-RGE and no peptide controls. Clusters that formed in the presence of cyclo-RGD contained fewer cells and were less tightly packed.
Figure 9. Blocking FN inhibits cell cluster formation.
(A) Fibroblasts were cultured for 18h on collagen matrices in DMEM/FBS containing 50 μg/ml of monoclonal antibody against FN (HFN 7.1) as indicated. At the end of the incubations, samples were fixed and stained for actin. (B) Fibroblasts were cultured for 18h on collagen matrices in DMEM/FBS containing 200 μg/ml of cyclo-peptides as indicated. At the end of the incubations, samples were fixed and stained for actin and PI. Bar = 100 μm.
DISCUSSION
Fibroblasts interacting with 3D collagen matrices exhibit markedly different behaviors depending on the growth factor environment [3]. Under conditions that we have referred to as a procontractile -- medium containing FBS or LPA -- cells retract dendritic extensions and contract stressed collagen matrices but fail to migrate in nested collagen matrices. Under conditions that we have referred to as promigratory – medium containing PDGF – cells protrude dendritic extensions and migrate well in nested collagen matrices but are unable to contract stressed collagen matrices. The current work focused on another feature of procontractile vs. promigratory growth factor environments. For fibroblasts on the surfaces of collagen matrices, procontractile growth factor conditions (FBS or LPA), resulted in formation of cell clusters; whereas promigratory growth factor conditions (PDGF) resulted in translocation of cells as individuals and dispersion of previously formed cell clusters [26].
In the current work we studied the possible role of integrin FN receptors and FN in cell clustering. We found that fibroblasts in serum or LPA but not in PDGF or basal (no growth factor) medium organized FN into a fibrillar matrix, consistent with the known requirement for activation of Rho kinase and cell contraction in the assembly of FN dimers into a fibrillar matrix [35, 36]. The FN matrix formed during cell clustering was deoxycholate insoluble as has been reported for the pericellular fibrillar matrix of fibroblasts in routine cell culture [40]. Cellular FN was sufficient for matrix formation and cell clustering as shown by the experiments in LPA-containing medium. However, plasma fibronectin also could be incorporated into the matrix, and clusters formed in serum-containing medium were larger than with LPA. Others have shown that plasma fibronectin can be incorporated into FN matrix by fibronectin-null fibroblasts grown on collagen matrices [34].
Pharmacologically blocking Rho kinase and myosin II activity blocked cell clustering and also inhibited FN matrix organization consistent with the known role of Rho kinase and myosin II in FN matrix formation in routine 2D cell culture [35, 36]. Previous work demonstrated that fibroblasts in collagen matrices under tension become well spread with prominent stress fibers and organized FN into fibrillar structures [46]. However, the relatively round morphology of fibroblasts on collagen matrices during initial spreading on collagen matrices concomitant with FN fibril organization and initial cell clustering suggested that FN fibrillar matrix organization can occur locally in regions of cell-matrix tension.
A requirement for FN in cell clustering was shown because clustering was inhibited in the presence of antibodies that block the cell-FN binding domain (Schoen et al., 1982) and by cyclo-RGD but not cyclo-RGE peptides [45]. Fibronectin receptors have been implicated in formation of FN fibrillar matrix [36, 43]. We found that cell clustering and FN fibrillar matrix organization were inhibited by siRNA silencing of α5 integrin and by function-blocking antibodies directed against integrin subunits α5 and β1. Our findings confirm and extend previous work that demonstrated directly a role for FN, i.e., addition of exogenous FN promotes compaction of cell spheroids [30] and is required for cell clustering of FN-null fibroblasts growing on collagen matrices [34].
Except for blocking β1 integrin, none of the treatments that inhibited cell clustering prevented cell spreading on collagen or the ability of cells to exert contractile force and compact the collagen matrix. Therefore, cell contraction was not sufficient for cell clustering. Integrin α2β1 rather than α5β1 has been implicated in fibroblast adhesion to collagen [47], which explains why blocking β1 integrin had a more profound effect that then other interventions.
Fibroblast clusters have been observed in normal tissue stroma [48], in wounded, fibrotic and scar tissue [49–53], and in the dermal papilla of hair follicles [54]. Interestingly, an analogy has been suggested between tissue repair and embryogenesis [55]. From this perspective, formation of fibroblast clusters possibly relates to the general process of mesenchymal condensation [56–59]. In the case of hypertrophic scar [49] and during mesenchymal condensation [60] fibronectin has been found in association with cell clusters.
Given the physiological relevance of fibroblast clusters, understanding the regulation and mechanism of clustering is an important goal to accomplish. Our findings suggest that the procontractile growth factor environment functions in cell clustering by stimulating fibroblasts to organize FN fibrillar matrix. The dynamics of FN fibrillar matrix organization and distribution during clustering suggested that the newly organized matrix serves as an organizing center for the clusters to localize. These observations provide new insights into how procontractile (serum/LPA) and promigratory (PDGF) growth factor environments can differentially regulate fibroblast morphogenetic behavior under physiologic circumstances such as wound repair, morphogenesis and malignancy.
Supplementary Material
Acknowledgments
We thank Dr. Kenneth Yamada (NIH, Bethesda, MD) for providing the monoclonal antibodies MAb16 and MAb13 and for his helpful suggestions. This work was supported by NIH grant GM 031321 (to FG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 2.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–61. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 4.Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011;3:a005033. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–19. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 7.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–79. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa R. Mechanobiology of scarring. Wound Repair Regen. 2011;19(Suppl 1):s2–9. doi: 10.1111/j.1524-475X.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarrazy V, Billet F, Micallef L, Coulomb B, Desmouliere A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10–5. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 10.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 12.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 14.Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol. 2010;20:139–45. doi: 10.1016/j.semcancer.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 17.Brown RA, Phillips JB. Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int Rev Cytol. 2007;262:75–150. doi: 10.1016/S0074-7696(07)62002-6. [DOI] [PubMed] [Google Scholar]
- 18.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–9. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 21.Ehrlich HP. Wound closure: evidence of cooperation between fibroblasts and collagen matrix. Eye (Lond) 1988;2(Pt 2):149–57. doi: 10.1038/eye.1988.28. [DOI] [PubMed] [Google Scholar]
- 22.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- 24.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–76. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee S, Ho CH, Grinnell F. Promigratory and procontractile growth factor environments differentially regulate cell morphogenesis. Exp Cell Res. 2010;316:232–244. doi: 10.1016/j.yexcr.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–11. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 28.Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–20. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95:6044–51. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson EE, Zazzali KM, Corbett SA, Foty RA. Alpha5beta1 integrin mediates strong tissue cohesion. J Cell Sci. 2003;116:377–86. doi: 10.1242/jcs.00231. [DOI] [PubMed] [Google Scholar]
- 31.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15:973–81. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmenpera P, Kankuri E, Bizik J, Siren V, Virtanen I, Takahashi S, Leiss M, Fassler R, Vaheri A. Formation and activation of fibroblast spheroids depend on fibronectin-integrin interaction. Exp Cell Res. 2008;314:3444–52. doi: 10.1016/j.yexcr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Caicedo-Carvajal CE, Shinbrot T, Foty RA. Alpha5beta1 integrin-fibronectin interactions specify liquid to solid phase transition of 3D cellular aggregates. PLoS One. 2010;5:e11830. doi: 10.1371/journal.pone.0011830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sevilla CA, Dalecki D, Hocking DC. Extracellular matrix fibronectin stimulates the self-assembly of microtissues on native collagen gels. Tissue Eng Part A. 2010;16:3805–19. doi: 10.1089/ten.tea.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–25. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee S, Jiang H, Ho CH, Grinnell F. Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc Natl Acad Sci U S A. 2007;104:5425–30. doi: 10.1073/pnas.0608030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Grinnell F. Cell-matrix entanglement and mechanical anchorage of fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2005;16:5070–6. doi: 10.1091/mbc.E05-01-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grinnell F, Lamke CR. Reorganization of hydrated collagen lattices by human skin fibroblasts. J Cell Sci. 1984;66:51–63. doi: 10.1242/jcs.66.1.51. [DOI] [PubMed] [Google Scholar]
- 40.McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983;97:466–72. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pankov R, Momchilova A. Fluorescent labeling techniques for investigation of fibronectin fibrillogenesis (labeling fibronectin fibrillogenesis) Methods Mol Biol. 2009;522:261–74. doi: 10.1007/978-1-59745-413-1_18. [DOI] [PubMed] [Google Scholar]
- 42.Mosesson MW, Umfleet RA. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970;245:5728–36. [PubMed] [Google Scholar]
- 43.Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–75. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoen RC, Bentley KL, Klebe RJ. Monoclonal antibody against human fibronectin which inhibits cell attachment. Hybridoma. 1982;1:99–108. doi: 10.1089/hyb.1.1982.1.99. [DOI] [PubMed] [Google Scholar]
- 45.Kumagai H, Tajima M, Ueno Y, Giga-Hama Y, Ohba M. Effect of cyclic RGD peptide on cell adhesion and tumor metastasis. Biochem Biophys Res Commun. 1991;177:74–82. doi: 10.1016/0006-291x(91)91950-h. [DOI] [PubMed] [Google Scholar]
- 46.Halliday NL, Tomasek JJ. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Experimental Cell Research. 1995;217:109–17. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- 47.Schiro JA, Chan BM, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS. Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403–10. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson JE, Schor AM, Howell A, Ferguson MW. Tenascin distribution in the normal human breast is altered during the menstrual cycle and in carcinoma. Differentiation. 1990;42:199–207. doi: 10.1111/j.1432-0436.1990.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 49.Kischer CW, Hendrix MJ. Fibronectin (FN) in hypertrophic scars and keloids. Cell Tissue Res. 1983;231:29–37. doi: 10.1007/BF00215771. [DOI] [PubMed] [Google Scholar]
- 50.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991;88:6642–6. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapanci Y, Desmouliere A, Pache JC, Redard M, Gabbiani G. Cytoskeletal protein modulation in pulmonary alveolar myofibroblasts during idiopathic pulmonary fibrosis. Possible role of transforming growth factor beta and tumor necrosis factor alpha. Am J Respir Crit Care Med. 1995;152:2163–9. doi: 10.1164/ajrccm.152.6.8520791. [DOI] [PubMed] [Google Scholar]
- 52.Gatlin J, Melkus MW, Padgett A, Petroll WM, Cavanagh HD, Garcia JV, Jester JV. In vivo fluorescent labeling of corneal wound healing fibroblasts. Exp Eye Res. 2003;76:361–71. doi: 10.1016/s0014-4835(02)00302-0. [DOI] [PubMed] [Google Scholar]
- 53.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–9. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenn KS, Cotsarelis G. Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr Opin Biotechnol. 2005;16:493–7. doi: 10.1016/j.copbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–34. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 56.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–75. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 57.Tickle C. Making digit patterns in the vertebrate limb. Nat Rev Mol Cell Biol. 2006;7:45–53. doi: 10.1038/nrm1830. [DOI] [PubMed] [Google Scholar]
- 58.Newman SA, Bhat R. Activator-inhibitor dynamics of vertebrate limb pattern formation. Birth Defects Res C Embryo Today. 2007;81:305–19. doi: 10.1002/bdrc.20112. [DOI] [PubMed] [Google Scholar]
- 59.Mammoto T, Mammoto A, Torisawa YS, Tat T, Gibbs A, Derda R, Mannix R, de Bruijn M, Yung CW, Huh D, Ingber DE. Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell. 2011;21:758–69. doi: 10.1016/j.devcel.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dessau W, von der Mark H, von der Mark K, Fischer S. Changes in the patterns of collagens and fibronectin during limb-bud chondrogenesis. J Embryol Exp Morphol. 1980;57:51–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.