Abstract
Hyperlipidemia blunts anabolic effects of intermittent parathyroid hormone (PTH) on cortical bone, and the responsiveness to PTH are restored in part by oral administration of the antioxidant ApoA-I mimetic peptide, D-4F. To evaluate the mechanism of this rescue, mice overexpressing the high-density lipoprotein-associated antioxidant enzyme, paraoxonase 1 (Ldlr-/-PON1tg) were generated, and daily PTH injections were administered to Ldlr-/-PON1tg and to littermate Ldlr-/- mice. Expression of bone regulatory genes was determined by realtime RT-qPCR, and cortical bone parameters of the femoral bones by micro-computed tomographic analyses. PTH-treated Ldlr-/-PON1tg mice had significantly greater expression of PTH receptor (PTH1R), activating transcription factor (ATF4), and osteoprotegerin (OPG) in femoral cortical bone, as well as significantly greater bone mineral content, thickness, and area in femoral diaphyses compared with untreated Ldlr-/-PON1tg mice. In contrast, in control mice (Ldlr-/-) without PON1 overexpression, PTH treatment did not induce these markers. Calvarial bone of PTH-treated Ldlr-/-PON1tg mice also had significantly greater expression of osteoblastic differentiation marker genes as well as BMP-2-target and Wnt-target genes. Untreated Ldlr-/-PON1tg mice had significantly greater expression of PTHR1 than untreated Ldlr-/- mice, whereas sclerostin expression was reduced. In femoral cortical bones expression levels of transcription factors, FoxO1 and ATF4, were also elevated in Ldlr-/-PON1tg mice, suggesting enhancement of cellular protection against oxidants. These findings suggest that PON1 restores responsiveness to PTH through effects on oxidant stress, PTH receptor expression, and/or Wnt signaling.
Keywords: hyperlipidemia, oxidant stress, intermittent, parathyroid hormone, paraoxonase-1
Introduction
Paraoxonase-1 (PON1), a protein component of high-density lipoprotein (HDL), is synthesized mainly in the liver, with lower expression found in lung, heart, brain, kidney and small intestine [1]. It functions as an antioxidant by hydrolyzing substrates, including lipid peroxides, which are atherogenic agents [2]. In vivo studies suggest that it retards the development of atherosclerosis [3,4]. Epidemiologically, serum levels of PON1 activity correlate inversely with levels of oxidized fatty acids [5]. PON1-deficient mice are also more prone to oxidant stress [6]. We and others have previously shown that transgenic mice overexpressing human PON1 have lower levels of oxidant stress and oxidized lipoprotein particles [3,7].
Lipid oxidation products trigger a cascade of inflammatory reactions responsible for the pathogenesis of cardiovascular diseases, particularly atherosclerosis [8,9,10]. Accumulating evidence, including the finding that metabolites released from osteoblasts induce nonenzymatic oxidation of lipoproteins [11], now suggests that hyperlipidemia and oxidant stress may also contribute to similar pathological events resulting in bone loss. Levels of oxidized lipids were increased in the bone marrow of hyperlipidemic mice fed a high-fat diet [12]. Such diets also induce bone loss in hyperlipidemic mice [12,13,14]. Histochemical evidence demonstrates accumulation of lipids in the subendothelial space of Haversian canals in human osteoporotic bone [15] and in osteocytic canaliculae [16,17]. Modulation of oxidative stress in mature osteoblasts in mice also affects bone mass homeostasis [18,19]. In vitro studies have shown that oxidized lipids adversely affect osteoblastic differentiation of preosteoblasts [20,21], but promote osteoclastic potential in bone marrow-derived preosteoclasts [15,22]. These findings suggest that oxidized lipids promote bone loss both through both decreased formation and increased resorption of mineralized matrix.
Intermittent administration of PTH has robust anabolic effects on bone [23,24]. We previously found that PTH effects, especially on cortical bone, are blunted in mice with hyperlipidemia [25]. In subsequent studies, we demonstrated that administration of an ApoA-I mimetic peptide, D-4F, which reduces plasma levels of oxidized lipids, restores the bone anabolic effects of PTH in cortical bones of hyperlipidemic low-density receptor null (Ldlr-/-) mice [26]. In this report, we tested PTH anabolism in Ldlr-/- mice overexpressing human PON1. Results suggest that PON1 overexpression rescues PTH resistance in hyperlipidemic mice.
Materials and Methods
Animals
Mice overexpressing human PON1 (C57BL/6 background), generated as previously described [7], were crossed with Ldlr-/- mice (C57BL/6 background; the Jackson Laboratory, Bar Harbor, ME) to generate Ldlr-/-PON1tg mice. The pups were verified by genotyping for presence of PON1, as described previously [7] and absence of LDL receptor genes, using protocols established by the Jackson Laboratory. Three month-old male littermates of Ldlr-/- and Ldlr-/-PON1tg mice were divided into 4 groups: control Ldlr-/- (n= 7), PTH-treated Ldlr-/- (n = 8), control Ldlr-/-PON1tg (n = 5), and PTH-treated Ldlr-/-PON1tg (n = 8). The mice received either control vehicle or hPTH (1-34; 40 μg/kg) for 4 weeks, as previously described [25,26]. All the experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California at Los Angeles.
MicroCT analysis
Right femoral bones were harvested and analyzed for bone mineral content, cortical thickness, and cortical bone area by microCT (Skyscan 1172, Aartselaar, Belgium), as we previously described [12]. At mid-diaphysis, the length of each femoral bone was measured, and 40 mid-diaphyseal slices were used. The data were collected at 55 kVp and 72 μA at a resolution of 12 μm, and volumetric analysis was performed using Skyscan software.
Realtime RT-qPCR
Total RNA was isolated from the calvaria and femurs. Real-time RT-qPCR was performed using the One-Step RT-qPCR SuperMix Kit (BioChain, Inc.) and Mx3005P Real-Time PCR System (Stratagene) [27,28,29].
Serum Analyses
Serum lipid oxidation products were assayed by a fluorescence indicator-based assay [30] using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen), which is sensitive to reactive oxygen species such as lipid oxidation products. Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (ox-PAPC) was used to generate a standard curve. PON1 activity in the serum was assessed by arylesterase activity, as previously described [7].
Statistical analysis
The effects of PTH were evaluated by comparing all the groups using ANOVA, followed by Fisher's PLSD. Interaction effects were analyzed by two-way ANOVA, followed by Fisher's PLSD. Values were expressed as mean ± SEM. A value of p < 0.05 was considered statistically significant.
Results
Effects on levels of serum lipid oxidation products
Levels of lipid oxidation products were assessed in hyperlipidemic (Ldlr-/-) and hyperlipidemic mice overexpressing PON1 (Ldlr-/-PON1tg), as described previously [30]. Results showed that Ldlr-/-PON1tg mice receiving intermittent PTH had lower levels of serum lipid oxidation products compared with control Ldlr-/- mice (Table I). As expected, Ldlr-/-PON1 tg had 2-fold higher serum PON1 (arylesterase) activity compared with control Ldlr-/- mice (Table I). Interestingly, intermittent PTH treatment further increased PON1 activity in Ldlr-/-PON1tg, whereas it reduced PON1 activity in control Ldlr-/ mice (Table I).
Table I.
Serum analysis of lipid oxidation products and PON1 activity
| Parameters | Ldlr -/- | Ldlr -/- PON1tg | ||
|---|---|---|---|---|
| Control | PTH | Control | PTH | |
| Serum oxidized lipid levels (μg/ml) | 4.50 ± 0.26 | 4.38 ± 0.28 | 3.73 ± 0.43 | 3.57 ± 0.17a |
| Serum arylesterase activity (mOD/min/μl serum) | 544 ± 28 | 431 ± 12b | 1102 ± 100c | 1472 ± 75b,c |
p < 0.05 vs. Ldlr-/- control
p < 0.01 vs. respective controls
p < 0.001 vs. Ldlr-/- control
Effects on gene expression of osteoblastic markers
We previously found that induction of osteoblastic gene expression was blunted in calvarial tissues of hyperlipidemic compared with WT mice in response to intermittent PTH [25,27]. To test whether PTH induction of these genes is restored by PON1 overexpression, calvarial tissues were harvested, and realtime RT-qPCR was performed. Results showed that expression of osteoblastic differentiation markers, alkaline phosphatase (Fig. 1A) and bone sialoprotein (data not shown), were greater with PTH treatment in Ldlr-/-PON1tg but not in Ldlr-/- mice. PTH and PON1 each had a significant main effect as well as a significant interaction effect on expression of osteoblastic genes, based on two-way ANOVA.
Figure 1. Effects on osteoblastic gene expression.
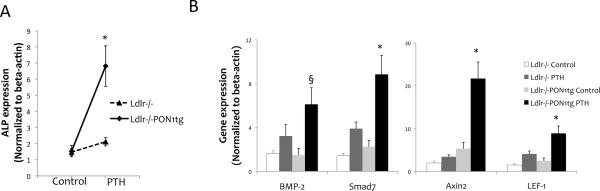
Realtime RT-qPCR analysis of RNA isolated from calvarial bone for osteoblastic differentiation markers, (A) alkaline phosphatase (ALP), (B) BMP-2 and its target gene, Smad7, and Wnt target genes, Axin-2 and LEF-1, in Ldlr-/- and Ldlr-/-PON1tg mice treated with control vehicle or intermittent PTH for 4 weeks. Values were expressed as mean ± SEM. *p < 0.005 vs. Ldlr-/- (both control and PTH) and Ldlr-/-PON1tg control; §p < 0.01 vs. control Ldlr-/- and control Ldlr-/-PON1tg.
Since PTH upregulates bone morphogenetic protein 2 (BMP-2) and Wnt signaling in osteoblasts [31,32], we further tested the effect of PON1 overexpression on these potent morphogens and their differentiation signaling pathways. Expression of BMP-2 and its downstream target Smad7, as well as the Wnt target genes, axis inhibition protein 2 (Axin2) and lymphoid enhancer-binding factor 1 (LEF-1), was significantly induced by intermittent PTH treatment in Ldlr-/-PON1tg but not in Ldlr-/- mice (Fig. 1B).
Effects on osteocytic gene expression
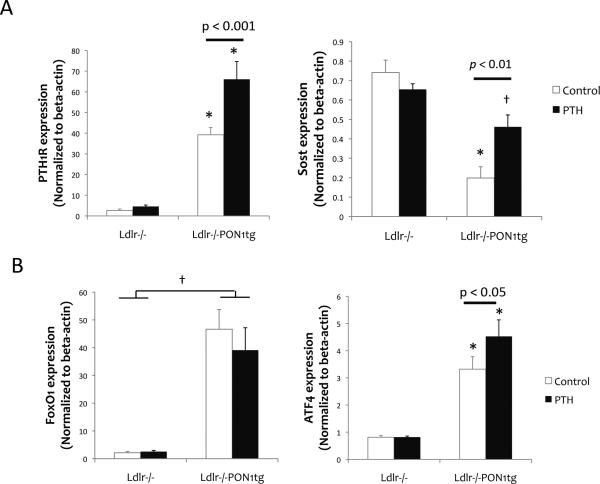
We previously found that gene expression in the osteocyte-rich cortical bones is affected by oxidant stress [12]. Therefore, we tested the effect of PON1 on expression of osteocyte-related genes, PTH receptor 1 (PTH1R) and sclerostin (Sost) using RNA isolated from femoral bones after removal of marrow. Results showed that, in mice without PTH treatment, PTH1R expression was significantly greater in Ldlr-/-PON1tg mice than in Ldlr-/- mice (Fig. 2A, left), whereas expression of Sost, a major antagonist of Wnt signaling, was significantly less in Ldlr-/-PON1tg mice than in Ldlr-/- mice (Fig. 2B). Intermittent PTH treatment significantly induced both PTH1R and Sost expression in cortical bones of Ldlr-/-PON1tg mice, but not of Ldlr-/- mice (Fig. 2A, right).
Figure 2. Effects on osteocytic gene expression.
Realtime RT-qPCR analysis of RNA isolated from femoral cortical bone, devoid of bone marrow, for (A) PTH1R and Sost, (B) FoxO1, and ATF4 expression. Values were expressed as mean ± SEM. *p < 0.001 vs. Ldlr-/- (both control and PTH); †p < 0.001 vs. Ldlr-/- (both control and PTH).
To assess effects of PON1 overexpression on cellular resistance to oxidant stress in femoral bone, expression levels of the transcription factor forkhead box O1 (FoxO1) and its binding partner, activating transcription factor 4 (ATF4), were assessed. Results showed that expression levels of both FoxO1 and ATF4 were significantly greater in Ldlr-/-PON1tg than in control Ldlr-/- mice (Fig. 2B, left). PTH treatment further induced ATF4 expression in Ldlr-/-PON1tg mice (Fig. 2B, right).
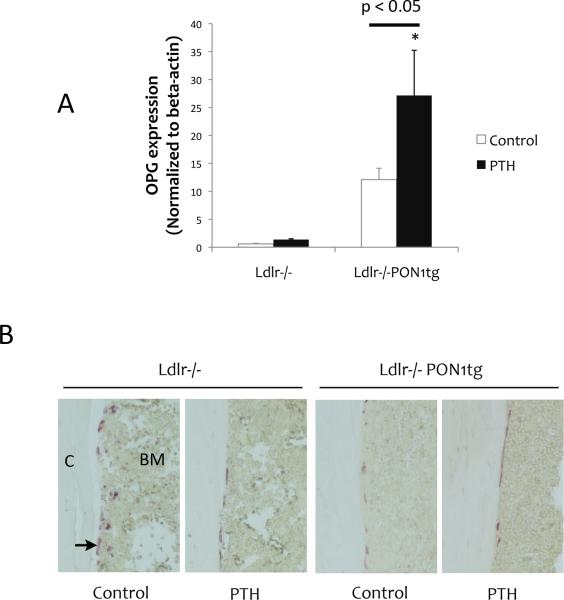
To assess whether PON1 overexpression has the potential to block bone resorptive activity, we assessed expression of the osteoclast inhibitory cytokine, osteoprotegerin (OPG), which plays a central role in osteocyte-mediated bone remodeling and bone loss [33]. Basal OPG expression trended toward higher levels in cortical bone of Ldlr-/-PON1tg mice compared with that of control Ldlr-/- mice (p = 0.07; Fig. 3A). Intermittent PTH treatment further induced OPG expression in Ldlr-/-PON1tg mice (Fig. 3A). Histochemical staining for tartrate-resistant acid phosphatase (TRAP), an osteoclast-specific enzyme, showed that, at the endocortical envelope of femoral bones, bone resorptive activity was significantly reduced by PON1 overexpression as well as by PTH treatment (Fig. 3B).
Figure 3. Effects on osteoclastic activity.
(A) Realtime RT-qPCR analysis of RNA isolated from femoral cortical bone for OPG in Ldlr-/- and Ldlr-/-PON1tg mice treated with control vehicle or intermittent PTH for 4 weeks. (B) TRAP positive osteoclasts (arrow) in the endocortical envelope of femoral bones. (C, cortex; BM, bone marrow; magnification 20x).
In vivo effects on cortical and trabecular bone parameters
We previously found that bone anabolic effects of PTH were differentially affected in cortical vs. trabecular bones in hyperlipidemic mice [25]. In cortical bone, PTH-induced bone mineral content, area, and thickness were significantly blunted, whereas its adverse effects on trabecular bone parameters were less [25]. MicroCT analysis of the femoral bones showed that intermittent PTH significantly induced bone mineral content, cortical thickness, and bone area in Ldlr-/-PON1tg mice (Table II). Consistent with our previous findings [25], PTH did not significantly increase these parameters in control Ldlr-/- mice (Table II). Trabecular bone mineral content was not significantly affected, whereas trabecular thickness was significantly induced by PTH in both groups (Table II).
Table II.
Summary of microCT analysis of femoral bone.
| Parameters | Ldlr-/- | Ldlr-/-PON1tg | ||
|---|---|---|---|---|
| Control | PTH | Control | PTH | |
| Cortical BMC (mg) | 0.529 ± 0.023 | 0.564 ± 0.015 | 0.469 ± 0.008c | 0.545 ± 0.014b |
| Cortical thickness (mm) | 0.111 ± 0.002 | 0.116 ± 0.003 | 0.105 ± 0.002 | 0.113 ± 0.001a |
| Cortical area (mm2) | 0.893 ± 0.037 | 0.954 ± 0.024 | 0.793 ± 0.010c | 0.913 ± 0.023a |
| Trabecular BMC (mg) | 0.102 ± 0.017 | 0.079 ± 0.011 | 0.059 ± 0.003 | 0.063 ± 0.011 |
| Trabecular thickness (mm) | 0.055 ± 0.001 | 0.063 ± 0.002b | 0.053 ± 0.000 | 0.061 ± 0.001b |
| Trabecular number (1/mm) | 2.573 ± 0.144 | 2.147 ± 0.104a | 2.138 ± 0.084 | 1.963 ± 0.141 |
BMC = bone mineral content; BV = bone volume; TV = tissue volume
p < 0.05 vs. respective controls
p < 0.001 vs. controls
p < 0.05 vs. Ldlr-/- control. Values are expressed as mean ± SEM.
Discussions
We previously showed that oxidized lipids blunt bone anabolic effects of intermittent PTH treatment [25,26]. We further showed that oral administration of the ApoA-I mimetic peptide, D-4F, which has antioxidant and anti-inflammatory properties, rescues at least partially the anabolic effects of intermittent PTH treatment in hyperlipidemic mice [26]. In this report, we found that responsiveness to PTH in cortical bone was restored in hyperlipidemic mice overexpressing PON1, which protects against lipoprotein oxidation through hydrolysis of lipid peroxides. Consistent with our previous findings with oral D-4F [25], cortical bone parameters, bone mineral content, thickness, and area were increased by intermittent PTH treatment in Ldlr-/-PON1tg mice. Our findings are also consistent with those of Jilka and colleagues, who showed that anabolic efficacy of intermittent PTH treatment in older mice is, in part, due to a novel effect of PTH -- antagonism of the age-associated increase in oxidative stress [34].
Our findings suggest that one possible mechanism for effects of PON1 is attenuation of oxidant stress in Ldlr-/-PON1tg mice. Redox balance in osteoblasts is regulated in large part by the transcription factor, FoxO1, which is expressed in the endosteum and periosteum, and its interaction with ATF4 [19]. In the present study, we found that basal expression levels of both FoxO1 and ATF4 were increased in cortical bones of Ldlr-/-PON1tg mice, suggesting that cellular defenses against oxidant stress may be retained in these transgenic mice. Consistent with findings of Yu and colleagues, in the present study, intermittent PTH treatment induced ATF4 expression in Ldlr-/-PON1tg but not in Ldlr-/- mice [35].
A second possible mechanism for the effects of PON1 is upregulation of the Wnt signaling pathway in Ldlr-/-PON1tg mice. Sost is a major antagonist of Wnt signaling, and the loss of Sost expression is permissive for endocortical bone formation [36]. In our study, we found that PON1 overexpression led to decreased Sost expression in cortical bone and increased expression of Wnt target genes in calvaria of Ldlr-/-PON1tg mice.
A third possible mechanism for the effects of PON1 is upregulation of PTH1R. Our previous findings showed that oxidized lipids inhibit PTH1R expression in calvarial cells [27] and that oxidant stress reduces basal expression of PTH1R in cortical bones of hyperlipidemic mice [12]. In this study, hyperlipidemic mice expressing the transgene had higher basal expression of PTH1R. Consequently, expression of osteoblastic markers was upregulated only in Ldlr-/-PON1tg mice. In addition, BMP-2 and its downstream target, Smad7, were upregulated by PTH in calvaria of Ldlr-/-PON1tg mice. This is consistent with our previous findings that PTH enhances BMP-2 in calvarial preosteoblastic MC3T3-E1 cells [37].
In summary, our findings suggest that reducing oxidant stress in hyperlipidemic mice may restore bone anabolic responsiveness to PTH, in part, through one or more of three possible mechanisms, including inhibition of oxidant stress, induction of PTH1R, and induction Wnt signaling pathways.
Highlights.
Anabolic effects of PTH were tested in hyperlipidemic mice overexpressing PON1.
Expression of antioxidant regulatory genes was induced in PON1 overexpression.
Bone resorptive activity was reduced in PON1 overexpressing hyperlipidemic mice.
PON1 restored responsiveness to intermittent PTH in bones of hyperlipidemic mice.
Acknowledgements
This research was supported by funding from National Institute of Health (DK081346, DK081346-S1).
Abbreviations
- ALP
Alkaline phosphatase
- ATF4
Activating transcription factor 4
- Axin2
Axis inhibition protein 2
- BMP-2
Bone morphogenetic protein 2
- HDL
High-density lipoprotein
- Ldlr-/-
Low-density lipoprotein receptor null
- LEF-1
Lymphoid enhancer-binding factor 1
- microCT
Micro-Computed Tomography
- Nur77
Nuclear receptor-77
- OPG
Osteoprotegerin
- PON1
Paraoxonase 1
- PTH
Parathyroid hormone
- PTH1R
PTH receptor 1
- Sost
Sclerostin
- TRAP
Tartrate-resistant acid phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/disclosure: None
References
- 1.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 2.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 3.Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- 4.Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 7.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis AJ, Shih DM. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–490. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- 8.Morgantini C, Imaizumi S, Grijalva V, Navab M, Fogelman AM, Reddy ST. Apolipoprotein A-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a murine model of diabetes. Diabetes. 2010;59:3223–3228. doi: 10.2337/db10-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, Shih DM, Van Lenten BJ, Frank JS, Demer LL, Edwards PA, Fogelman AM. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol. 1996;16:831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- 10.Towler DA. Oxidation, inflammation, and aortic valve calcification peroxide paves an osteogenic path. J Am Coll Cardiol. 2008;52:851–854. doi: 10.1016/j.jacc.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodeur MR, Brissette L, Falstrault L, Ouellet P, Moreau R. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med. 2008;44:506–517. doi: 10.1016/j.freeradbiomed.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Pirih F, Lu J, Ye F, Bezouglaia O, Atti E, Ascenzi M, Tetradis S, Demer L, Aghaloo T, Tintut Y. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012 doi: 10.1002/jbmr.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirasawa H, Tanaka S, Sakai A, Tsutsui M, Shimokawa H, Miyata H, Moriwaki S, Niida S, Ito M, Nakamura T. ApoE gene deficiency enhances the reduction of bone formation induced by a high-fat diet through the stimulation of p53-mediated apoptosis in osteoblastic cells. J Bone Miner Res. 2007;22:1020–1030. doi: 10.1359/jbmr.070330. [DOI] [PubMed] [Google Scholar]
- 14.Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, Weissleder R, Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur Heart J. 2010;31:1975–1984. doi: 10.1093/eurheartj/ehq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 16.Kawai K, Maruno H, Watanabe Y, Hirohata K. Fat necrosis of osteocytes as a causative factor in idiopathic osteonecrosis in heritable hyperlipemic rabbits. Clin Orthop Relat Res. 1980:273–282. [PubMed] [Google Scholar]
- 17.Watanabe Y, Kawai K, Hirohata K. Histopathology of femoral head osteonecrosis in rheumatoid arthritis: the relationship between steroid therapy and lipid degeneration in the osteocyte. Rheumatol Int. 1989;9:25–31. doi: 10.1007/BF00270286. [DOI] [PubMed] [Google Scholar]
- 18.Ambrogini E, Almeida M, Martin-Millan M, Paik JH, Depinho RA, Han L, Goellner J, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11:136–146. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, Kousteni S. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 11:147–160. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, Demer LL. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14:2067–2078. doi: 10.1359/jbmr.1999.14.12.2067. [DOI] [PubMed] [Google Scholar]
- 21.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 22.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–555. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 24.Iida-Klein A, Zhou H, Lu SS, Levine LR, Ducayen-Knowles M, Dempster DW, Nieves J, Lindsay R. Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. J Bone Miner Res. 2002;17:808–816. doi: 10.1359/jbmr.2002.17.5.808. [DOI] [PubMed] [Google Scholar]
- 25.Huang MS, Lu J, Ivanov Y, Sage AP, Tseng W, Demer LL, Tintut Y. Hyperlipidemia impairs osteoanabolic effects of PTH. J Bone Miner Res. 2008;23:1672–1679. doi: 10.1359/JBMR.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sage AP, Lu J, Atti E, Tetradis S, Ascenzi MG, Adams DJ, Demer LL, Tintut Y. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2011 doi: 10.1002/jbmr.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang MS, Morony S, Lu J, Zhang Z, Bezouglaia O, Tseng W, Tetradis S, Demer LL, Tintut Y. Atherogenic phospholipids attenuate osteogenic signaling by BMP-2 and parathyroid hormone in osteoblasts. J Biol Chem. 2007;282:21237–21243. doi: 10.1074/jbc.M701341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang MS, Sage AP, Lu J, Demer LL, Tintut Y. Phosphate and pyrophosphate mediate PKA-induced vascular cell calcification. Biochem Biophys Res Commun. 2008;374:553–558. doi: 10.1016/j.bbrc.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–1317. [PubMed] [Google Scholar]
- 31.Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95:1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- 32.Takase H, Yano S, Yamaguchi T, Kanazawa I, Hayashi K, Yamamoto M, Yamauchi M, Sugimoto T. Parathyroid hormone upregulates BMP-2 mRNA expression through mevalonate kinase and Rho kinase inhibition in osteoblastic MC3T3-E1 cells. Horm Metab Res. 2009;41:861–865. doi: 10.1055/s-0029-1233460. [DOI] [PubMed] [Google Scholar]
- 33.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell. 2010;9:851–867. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu S, Franceschi RT, Luo M, Fan J, Jiang D, Cao H, Kwon TG, Lai Y, Zhang J, Patrene K, Hankenson K, Roodman GD, Xiao G. Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone. PLoS One. 2009;4:e7583. doi: 10.1371/journal.pone.0007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robling AG, Kedlaya R, Ellis SN, Childress PJ, Bidwell JP, Bellido T, Turner CH. Anabolic and catabolic regimens of human parathyroid hormone 1-34 elicit bone- and envelope-specific attenuation of skeletal effects in Sost-deficient mice. Endocrinology. 152:2963–2975. doi: 10.1210/en.2011-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakao Y, Koike T, Ohta Y, Manaka T, Imai Y, Takaoka K. Parathyroid hormone enhances bone morphogenetic protein activity by increasing intracellular 3', 5'-cyclic adenosine monophosphate accumulation in osteoblastic MC3T3-E1 cells. Bone. 2009;44:872–877. doi: 10.1016/j.bone.2009.01.370. [DOI] [PubMed] [Google Scholar]