Abstract
Background
Obesity has reached epidemic proportions in the United States. Recently, obesity, especially morbid obesity, has been linked to increased rates of dislocation after THA. The reasons are unclear. Soft tissue engagement caused by increased thigh girth has been proposed as a possible mechanism for decreased joint stability.
Questions/Purposes
We asked (1) whether thigh soft tissue impingement could decrease THA stability, and if so, at what level of BMI this effect might become evident; and (2) how THA construct factors (eg, head size, neck offset, cup abduction) might affect stability in the morbidly obese.
Methods
The obesity effect was explored by augmenting a physically validated finite element model of a total hip construct previously comprising just implant hardware and periarticular (capsular) soft tissue. The model augmentation involved using anatomic and anthropometric data to include graded levels of increased thigh girth. Parametric computations were run to assess joint stability for two head sizes (28 and 36 mm), for normal versus high neck offset, and for multiple cup abduction angles.
Results
Thigh soft tissue impingement lowered the resistance to dislocation for BMIs of 40 or greater. Dislocation risk increased monotonically above this threshold as a function of cup abduction angle, independent of hardware impingement events. Increased head diameter did not substantially improve joint stability. High-offset necks decreased the dislocation risk.
Conclusions
Excessive obesity creates conditions that compromise stability of THAs. Given such conditions, our model suggests reduced cup abduction, high neck offset, and full-cup coverage would reduce the risks of dislocation events.
Introduction
Although the overall prevalence of obesity has approximately doubled during the past two decades, the percentages of Americans with BMIs greater than 40 kg/m2 and 50 kg/m2 have increased by 500% and 1000%, respectively [40]. In addition to its well-documented linkage with many chronic medical conditions, obesity places substantial burden on the musculoskeletal system. Obesity has long been identified as a factor contributing to lower-extremity osteoarthritis [1], underscoring the association between obesity and the need for THA.
Situations of obesity likely result in considerable alterations in the biomechanics of hip replacement. One obvious concern for obese patients with THAs involves the influence of increased body weight at the bearing surface, possibly resulting in accelerated bearing surface wear and implant failure. However, presumably owing to an overall decrease in activity [29], obesity has not been consistently associated with decreased implant survival [20]. Another specific complication of obesity in THA, potentially amenable to surgeon influence, is higher dislocation rates. Several large studies of primary THAs [2, 9, 12, 16, 26, 35] report an average relative risk for dislocation of 3.7 for obese versus nonobese patients with THAs, with higher relative risks occurring for patients in the higher BMI stratifications. The obesity-associated relative risk for dislocation is even greater for revision THAs [22, 25].
The cause or causes for the increased dislocation propensity in the obese remain unclear. It is well appreciated that, although highly complex and multifactorial, dislocation is a biomechanically dominated event [7]. It has been shown empirically [32] that dislocation risk increases with extreme excursions of joint angulation. Paradoxically, however, obese patients have varying degrees of structural and functional limitations that tend to reduce functional hip excursion during ambulation and activities of daily living, including reduced excursions of hip flexion, adduction, and internal rotation [20], extremes of which are associated with dislocation risk. Given these factors, from a purely biomechanical point of view, it seems intuitive that obesity, because of reduced motion utilization ranges, would tend to provide some degree of instability protection, or at least not be associated with increased dislocation risk. Yet, although not unanimous [31, 33, 42], clinical studies generally show increased dislocation risk among obese patients with THAs [2, 9, 12, 16, 17, 22, 25, 26, 35]. One possible mechanism, initially proposed by Kim et al. [22], is that thigh-to-thigh soft tissue contact during flexion and adduction leads to laterally directed external force on the hip, tending to decrease joint stability (Fig. 1). To move beyond conjecture regarding this potential cause for THA instability, we augmented an existing (and previously physically validated) finite element (FE) model of total hip dislocation [14], by including the thigh-to-thigh soft tissue contact. This FE model was used to explore two issues: (1) whether thigh-to-thigh soft tissue impingement could substantially decrease THA stability, and if so, at what level of BMI this effect would become important; and (2) how factors under the surgeon’s control (head size, neck offset, cup geometry, cup abduction) would affect stability under those conditions.
Fig. 1.
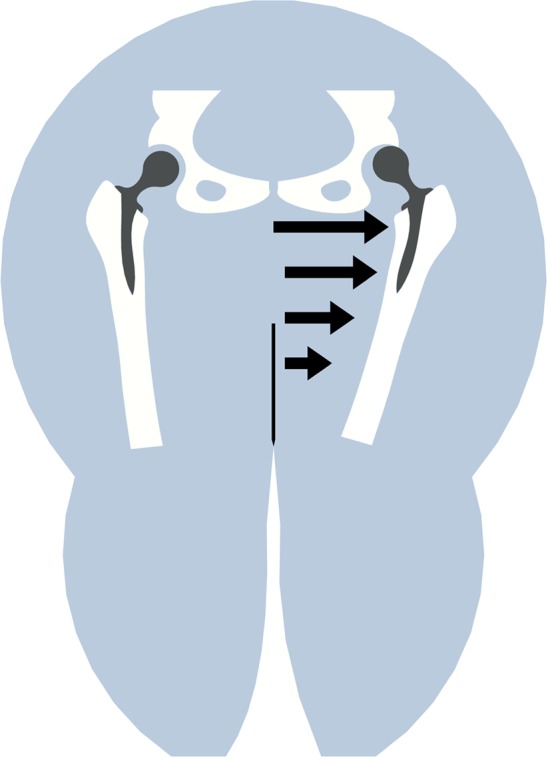
During hip adduction, a laterally directed force arises owing to thigh-to-thigh contact, acting to push the femoral head from the cup, compromising joint stability. (Modified and reprinted with permission of Lippincott Williams & Wilkins from Kim Y, Morshed S, Joseph T, Bozic K, Ries MD. Clinical impact of obesity on stability following revision total hip arthroplasty. Clin Orthop Relat Res. 2006;453:142–146.)
Materials and Methods
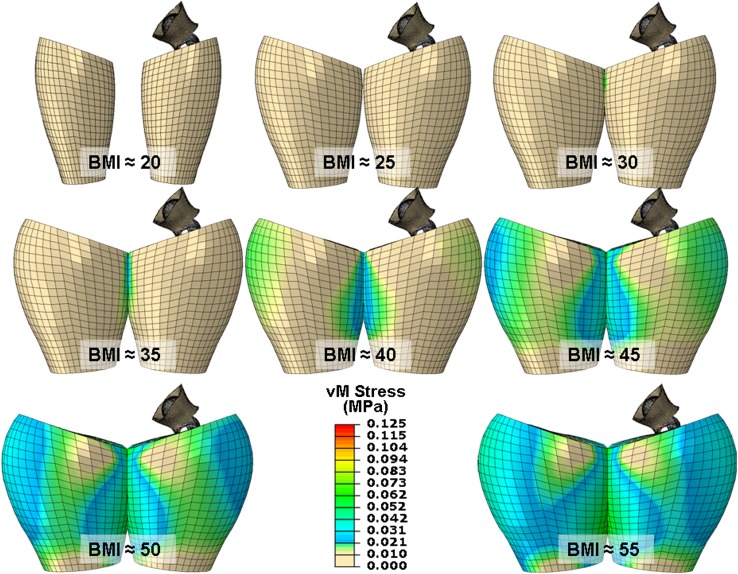
The obesity THA dislocation FE model consisted of three parts (Fig. 2): (1) THA implant hardware, (2) hip capsule, and (3) mirrored left and right thighs. The hip capsule was modeled using an anisotropic fiber-dominated constitutive model, technical details of which are described elsewhere [14]. Left and right thighs were taken as being composed of a femoral shaft (assumed rigid), muscle, adipose tissue, and skin (Fig. 2). The bulk material of the adipose tissue in compression was modeled as a neo-Hookian hyperelastic solid [37]. Using a small strain assumption, skin and (passive) muscle were modeled as linearly elastic [3, 37]. Morphologic features of the thigh were based on anatomic geometric shape functions. Using anthropometric data [30], these shape functions were scaled for eight distinct graded levels of BMI (Fig. 3). It was assumed that the skin thickness and muscle mass would remain constant across all levels of BMI, with the girth increase across the models being attributable solely to increase in adipose tissue.
Fig. 2.
The FE model of extraarticular soft tissue contact and subsequent THA instability consisted of (1) implanted THA hardware, (2) hip capsule (posterior ½ of the capsule is here rendered transparent for clarity), and (3) extraarticular soft tissue including muscle, adipose tissue, and skin, surrounding a rigid femoral canal. The model assumed right-left symmetry of the native anatomy. E = Young’s modulus; K = bulk modulus; ε = nominal strain; ν = Poisson’s ratio.
Fig. 3.
Eight graded levels of BMI were simulated. Anatomic shape parameters were used for the baseline case (BMI = 20 kg/m2), which then were scaled using anthropometric data [31] to model thigh geometry for an overweight patient (BMI = 25 kg/m2) and six grades of obesity. An initial analysis step brought the thighs into nominal apposition (as shown) for the beginning of the analysis, resulting in contact occurring between the thighs in all six obese FE models.
Three separate THA implants were modeled. The first incorporated a 28-mm head. The second and third were contemporary modular large-head (36-mm) implants with standard- and high-offset (8-mm) necks. Fifteen separate acetabular cup orientations were considered, with cup abduction angles varying from 30° to 65° in 2.5°-increments, all with 10° cup anteversion. The resulting permutations in cup orientation (15) and BMI (eight) resulted in a total of 360 distinct FE simulations, 120 for each of the three THA implant geometries considered.
In addition to head diameter and neck offset, the role of cup geometry was investigated. Recently, an FE investigation [13] documented a substantial decrease in THA construct intrinsic dislocation resistance with increased roundedness of the cup edge geometry. To further explore the role of the cup edge radius on THA stability, an FE series was conducted to investigate the relationship between thigh-to-thigh contact force required to dislocate the femoral head, versus cup lip radius. For the 28-mm cup, 10 cup lip radii (0–10 mm) were considered for each of three values of BMI (45, 50, and 55 kg/m2), resulting in an additional 30 FE simulations.
For all FE simulations, a sit-to-stand maneuver was used as the dislocation challenge. Input kinematics and kinetics were obtained from motion data previously collected for 10 subjects performing this motion [32]. Owing to a documented 23° decrement in hip flexion observed in obese patients performing sit-to-stand motions [38], the original kinematic input data (obtained from nonobese subjects) were modified by truncating the input motion curve so as to reduce peak hip flexion from 115° to 92°. This same obese sit-to-stand input sequence was used for all BMIs addressed in the current FE series to remove the potentially confounding influence of differences in the joint angulation range used. Right-left motion symmetry was assumed, ie, each leg was driven by mirrored input kinematics. Joint contact forces were scaled for each model’s simulated body weight. For all simulations, stability was indexed in terms of femoral head subluxation (computationally determined by tracking the femoral head center displacement).
To our knowledge, there are no anthropometric data currently available on adult hip-center-to-hip-center distances, an important consideration in our investigation. Therefore, radiographic analysis of 146 healthy adults (Fig. 4) and cadaveric analyses of six intact pelves were conducted to determine an appropriate interhip separation distance. From these analyses, an average hip-center-to-hip-center separation of 200 mm was inferred as representative.
Fig. 4A–B.
The graphs show the hip-center-to-hip-center distance as measured from standard AP radiographs in (A) 113 healthy women and (B) 33 healthy men. Subjects with degeneration or anomalies in the hip and pelvic anatomy were excluded. The center of the femoral head was defined as the center of the circle best fitting the bony contour of the femoral head. Interhip center distance was defined as the distance between the centers of femoral heads, corrected for radiograph magnification. Considering the radiographic data shown here, along with cadaveric measurement, a median interhip separation distance of 200 mm was inferred and used in all FE simulations.
All mesh zonings for the model were preprocessed with TrueGrid® (v. 2.3; XYZ Scientific Applications, Inc, Livermore, CA, USA). For each analysis, the (assumed rigid) metal backing was taken as being rigidly fixed in space (ie, anchored to the bony pelvis) by constraining its control point’s nodal rotation and translation. The acetabular liner was maintained in the shell through frictional interactions. The femoral stem was oriented at 5° anteversion in all instances. All rotations and loads associated with each dislocation motion sequence were prescribed at the center of the femoral head. Because of the nonlinear behavior of the capsule and adipose tissue, all analyses were done using Abaqus/Explicit (v. 6.9.3; Dassault Systèmes, Providence, RI, USA). For computational economy, the THA hardware was assumed rigid. For each FE simulation, femoral head displacement was tracked throughout the entire input sequence.
The hardware/capsule FE model had been physically validated previously using a purpose-built four-degree-of-freedom servohydraulic hip simulator, in which dislocation resistance (resisting moment) encountered during various activities of daily living could be determined. A 3% error in agreement was achieved between the computational and experimental simulations of a sit-to-stand simulation, technical details of which are reported elsewhere [14]. Physical corroboration of the thigh impingement FE model was conducted using a calibrated interface pressure mat (CONFORMat®; Tekscan, Inc, South Boston, MA, USA), allowing for real-time analysis of contact pressures and for (postprocessing) integration of the total loads registered between the thighs of obese subjects during a sit-to-stand kinematic challenge (Fig. 5). The pressure mat had a sensing area of 47.1 by 47.1-cm, with 1024 sensing elements distributed over 32 rows and 32 columns. The pressure mat was modified by attaching a series of eyelets along the longitudinal edge, allowing the mat to be securely attached to test subjects by lacing the mat along the lateral area of the leg. Elastic straps were used suspend the mat in place during data acquisition. The active sensing area of the mat began approximately 5 cm below the greater trochanter. Real-time Tekscan pressure acquisition was performed on a female subject with a BMI of 40 kg/m2 during a sit-to-stand sequence from a normal height chair. Peak thigh-to-thigh contact force was reached at the terminal phase of the sit-to-stand maneuver during hip adduction. The maximum thigh circumference of the subject fell between the simulated thigh circumference of the 35- and 40-kg/m2 BMI FE models. Registering the mat’s sensing area to the FE mesh, agreement of the physically measured versus computed thigh-thigh contact force was within 16%, for the 35-kg/m2 BMI case (Fig. 5).
Fig. 5A–D.
(A) Physical corroboration of thigh-to-thigh contact computation was performed using an interface pressure mat applied between the thighs of an obese subject (without THA) performing a sit-to-stand maneuver. (B) Spatial integration of the Tekscan mat contact stress measured during thigh impingement resulted in 146.4 N of peak lateral load. Integration of contact stresses from the corresponding portion of the FE-computed thigh-to-thigh contact region (anatomic constraints precluded positioning the mat to cover the entirety of the contact region) provided a basis for direct comparison. Physical representation of the placement of the pressure mat is shown for the (C) 35- and (D) 40-kg/m2 BMI FE models. (The peak thigh circumference of the study subject fell between these two simulated cases.) Peak contact force computed in the approximate area covered by the sensing mat (dashed boxes) was 170 N (approximately a 16% discrepancy versus experimental) for the 35-kg/m2 BMI case and 250 N for the 40-kg/m2 BMI case.
Results
Thigh-to-thigh contact in the obese patient models resulted in a mechanism for dislocation that was independent of implant hardware impingement and/or of bony or intraarticular soft tissue impingement. Peak thigh-to-thigh contact forces were developed during the terminal phase of the maneuver, when the hip adduction was greatest, reducing joint stability (Fig. 6). For all three implant designs modeled, however, instability (indexed in terms of femoral head subluxation) did not become appreciable (ie, macroscopic, and plausibly clinically consequential) until BMI reached approximately 40 kg/m2 (Fig. 7A–B). Beyond that threshold, with increasing values of BMI, there was a monotonic increase in femoral head subluxation, often resulting in frank dislocation.
Fig. 6.

Instability (quantified in terms of femoral head subluxation distance) in the simulated obese THA models occurred during hip adduction, at the terminal stage of the sit-to-stand maneuver. Contact between the thighs, the intensity of which increased during hip adduction, caused development of a laterally directed force whose magnitude was sufficient to cause joint instability, leading to frank dislocation in the highest BMI models, as illustrated for a 50-kg/m2 BMI simulation.
Fig. 7A–C.
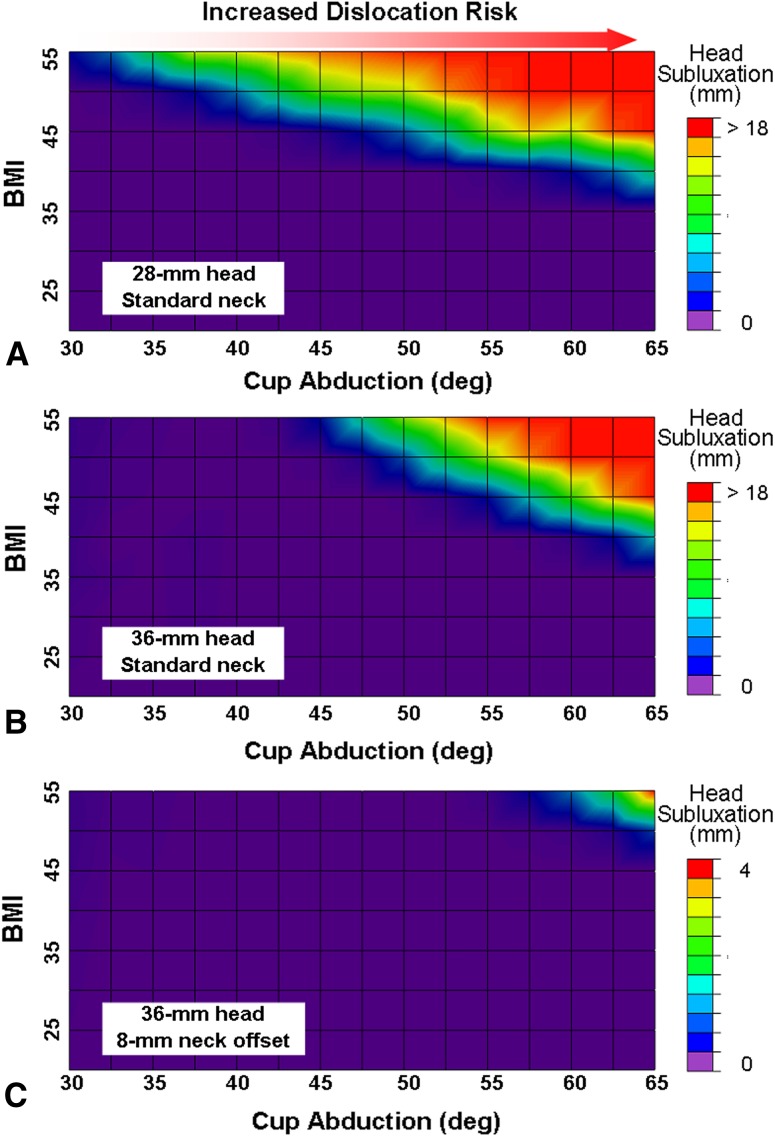
(A) Dislocation risk was sensitive to increased BMI and increased cup abduction for the 28-mm head, although substantial instability (subluxation > 1 mm) did not occur for simulated BMIs of 35 kg/m2 or less. Increased cup abduction also led to elevated dislocation risk. (B) A generally similar BMI-versus-cup-abduction relationship was seen for the standard-offset 36-mm cup, although instability was somewhat more pronounced at higher cup abduction angles. (C) However, dislocation risk was reduced substantially when a high-offset (8 mm) neck was used with the 36-mm cup. Subluxation for the high-offset neck occurred only for the most extreme values of BMI and cup abduction and even then remained far below the level required for frank dislocation. Deg = degrees.
Dislocation propensity was sensitive to cup abduction, with cups at high abduction angles showing substantial decreases in stability. Increased head diameter led to slight improvements in joint stability, especially for lower cup abduction angles (Fig. 7B). However, dislocation propensity decreased substantially with the use of the 8-mm high-offset neck, which effectively increased the problematic BMI threshold from 40 kg/m2 (for the standard-offset necks) to 50 kg/m2 for the high-offset necks. Although simulations of the highest BMI and the largest cup abduction still resulted in joint substantial subluxation (4 mm), frank dislocation (ie, subluxation numerically greater than the head radius) did not occur for any constructs incorporating the high-offset neck (Fig. 7C). Cup edge geometry influenced THA stability (Fig. 8). There was a nearly linear inverse relationship between cup edge radius and the load required to cause dislocation of the head from the cup.
Fig. 8.
A graph shows the thigh-to-thigh contact forces required to cause dislocation for a 28-mm cup positioned in 60° abduction, versus cup edge radius. Required thigh-to-thigh contact forces were sensitive to cup edge geometry. Cups with larger edge radii (and therefore less articular head coverage) required smaller laterally directed thigh-to-thigh contact force to become unstable.
Discussion
Given the dose-effect association between BMI and development of hip osteoarthritis [6] and the decade-sooner requirement for hip arthroplasty in morbidly obese versus normal-weight patients [8], it is clear the demand for orthopaedic management of the morbidly obese will continue to increase in the foreseeable future. Of particular concern to the orthopaedic community is the apparent association of obesity with instability after primary and revision THAs. Although this association often has been remarked on clinically, the specific mechanisms responsible are unclear. Toward the goal of providing quantitative data to help guide surgical management regarding hip replacement in the morbidly obese, a finite element model was developed to investigate (1) whether and by what mechanism thigh-to-thigh contact could predispose to dislocation in obese patients, and (2) how implant- and surgical-specific factors could affect joint stability.
The physical event of a THA dislocation is highly complex and dynamic. While reasonably robust by prevailing standards, the current FE model involved numerous limitations and assumptions. First, incorporating dynamic extraarticular (thigh-to-thigh) contact involved idealized bulk material behavior of skin, muscle, and adipose tissue, including assumed linear elastic behavior for skin and muscle tissue. Additionally, capsule, muscle, and skin material properties were assumed to not be altered with increased BMI. This may have tended computationally to overestimate thigh-to-thigh contact stresses: human radiographic studies of the obese thigh [21] have shown decreases in lean-tissue density. One study in a mouse model suggested obesity involves substantial deficiencies of soft tissue compositional integrity, including decreased levels of mucopolysaccharides, glycoproteins, elastin, and collagen [10]. Additionally, obesity has been associated with altered collagen organization and disrupted matrix properties [15], and diminished wound healing capacity [41]. These pathologic soft tissue changes might especially alter the role of the capsule in stabilizing the hip. Second, the kinematics used for the sit-to-stand maneuver, while modified to replicate altered locomotion strategies for obese patients, were not BMI-specific. As a first approximation, joint contact forces were scaled proportionally with BMI, rather than being based on BMI-specific limb segment inertial properties [27]. Third, as indicated by the dramatic reduction in dislocation propensity by using a high-offset neck, the physical distance between hip centers is seemingly a highly important consideration. Our current simulations reflected approximate population-median hip center separations. Presumably, the lateral dislocation forces would be greater for obese patients who have less-than-median hip center separation (and conversely), a factor addressable in preoperative planning. Fourth, owing to a paucity of published reports on the kinematics and kinetics of dislocation-prone motions in obese subjects, the sit-to-stand activity used in our study was chosen on the basis of its being the best-characterized dislocation-prone activity. The maneuvers that might lead to thigh-to-thigh contact are innumerable. Given the possibility of severe loading aberrations (in addition to thigh-to-thigh contact) imposed by conditions of obesity on the musculoskeletal system, biomechanical analysis of other activities of daily living in obese patients should be accorded a high research priority. Fifth, only cup abduction, and not cup anteversion, was parametrically investigated. Both parameters will influence dislocation mechanics. Cup anteversion influences slide-out dislocation resistance to a greater degree than cup abduction for posterior-directed dislocations [13]. However, since the dislocation mechanism in our study was directed laterally, it seemed logical that cup abduction would have greater influence. The effect of cup anteversion on obesity-effect dislocation is an inviting area for further study.
Model limitations notwithstanding, the current data corroborate earlier conjecture that thigh-to-thigh impingement in the obese patient with a THA can predispose to hip instability. The FE model showed a dose-effect relationship between BMI and degree of THA instability, with there being an apparent BMI threshold of 40 kg/m2 above which obesity influence became appreciable. Our findings are consistent with several clinical studies. An effect onset threshold in this range has been noted clinically when dislocation rates were stratified for higher grades of obesity [2, 16]. Additionally, our study suggested thigh circumference, rather than BMI per se, is the factor directly acting to decrease joint stability. Further, when normalized to BMI, THA dislocation rates in obese females are reportedly higher than in obese males [26]. Because of the gender differences in adipose tissue deposition [24] (females predominately depositing fat in the hip and thigh area compared with more abdominal distribution for males), the paradigm of elevated dislocation risk being attributable to increased thigh adiposity is given additional credence.
Our study suggested that classic ROM-increasing considerations for THA constructs (eg, increased head size or increased head-to-neck ratios) may need to be reevaluated for the morbidly obese patient. Rather than impingement/lever-out, the instability we observed was attributable to slide-out or shear-out, a phenomenon not dependent on hardware impingement or bony impingement. Although slide-out dislocation has been seen experimentally [5] and computationally [13, 23, 32], implant design strategies to afford protection against slide-out are not as well evolved as those for impingement-preceded dislocation. Only modest benefit was seen when using a 36-mm head compared with a 28-mm head, regardless of the theoretical improvements in ROM before neck-on-cup impingement. Regarding slide-out, these two bearings differ in that the 28-mm cup provides a full hemisphere (180°) of femoral head coverage, compared with only 163° for the 36-mm head construct. Additionally, the rounded chamfer of the 36-mm cup would tend to allow for easier slide-out head egress than the sharp edged and flat chamfer design of the 28-mm cup (Fig. 9). Such considerations suggest that cup shape and geometry (especially the extent of head coverage), rather than simply head diameter, more strongly influences stability in the morbidly obese patient. This was more fully shown in our study, where increased values of cup edge radius (with a concomitant decrease in cup coverage) resulted in decreased thigh-to-thigh loads required to cause dislocation (Fig. 8). Additionally, the use of an 8-mm neck offset in the current model yielded substantial improvement in joint stability. Increasing femoral offset increases periarticular soft tissue tension [4] and ROM before implant or osseous impingement [28], thereby resulting generally in improved joint stability. However, as opposed to simply the theoretical advantage of increased soft tissue tension, another contributing mechanism may have been the corresponding increase of the center-to-center distance between the two hips, effectively reducing the intensity of the thigh-to-thigh contact. Therefore, use of offset necks might be especially helpful to mitigate the propensity for THA dislocation in the morbidly obese. Furthermore, by increasing the abductor moment arm (thereby decreasing joint contact force [11]), increased neck offset reduces bearing surface wear [36], thus potentially improving overall implant survival. However, the use of larger neck offsets likely represents an example of the “choices and compromises” [19] inherent to the selection of orthopaedic implants. Excessive tension in the abductors or the iliotibial band from increased neck offset might lead to persistent thigh pain or trochanteric fracture [18]. Additionally, increasing the lateral offset of the femoral head increases the bending moment arm of the prosthesis, which may lead to implant or bone-implant-interface failure. Although the effect of increased offset on such stresses are reportedly inconsequential for the typical patient having a THA [11], the potential biomechanical repercussions of increased neck offset combined with increased body weight are unknown. This combination was implicated in fatigue fractures of the femoral neck [39]. Finally, our data suggest that, in light of the reduced functional hip angulation used by the morbidly obese patient, cups with higher abduction angles will have an elevated dislocation risk. While avoiding vertical cup abduction is potentially challenging intraoperatively in the obese owing to the difficult surgical exposure and retraction [34], our data suggest that every effort should be made toward that end.
Fig. 9A–B.
(A) The 28-mm THA hardware consisted of a standard-offset neck with a 5° anteverted stem (left), a 28-mm-diameter cup (middle) with a flat lip and chamfer (right), and 180° head articular coverage, resulting in 14 mm of jump distance being required for dislocation. (B) The 36-mm THA hardware also consisted of a standard-offset neck anteverted to 5° (left). The cup diameter was 36 mm (middle), but the rounded lip and chamfer of the cup (right) resulted in only 163° articular coverage, decreasing the required jump distance from a full head diameter (18 mm) to only 15.3 mm.
Our data corroborate the clinical observation that thigh-to-thigh contact in the obese patient with a THA can predispose to instability, with the degree of such instability being directly related to thigh girth. We observed a decrease in dislocation propensity for (1) increased neck offset; (2) decreased cup abduction; (3) increased head size, and (4) full hemispheric head coverage, with the use of higher stem offsets resulting in the greatest risk reduction.
Acknowledgments
We thank M. James Rudert PhD for assistance with collection of physical data related to model validation.
Footnotes
The institution of one or more of the authors has received funding from the National Institutes of Health (AR46601 and AR53553) (TDB, JJC), the Veterans Administration (JJC, TDB), and the National Center for Resource Resources (UL1 RR024979) (JME). One of the authors (JJC) certifies that he has or may receive payments or benefits, in any one year, in excess of $1,000,000, from DePuy Orthopaedics, Inc (Warsaw, IN, USA). One of the authors (TDB) certifies that he has or may receive payments or benefits, in any one year, an amount in excess of $10,000, from Smith & Nephew Orthopaedics, Inc (Memphis, TN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at the University of Iowa, Iowa City, IA, USA.
References
- 1.Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int J Obes. 2008;32:211–222. doi: 10.1038/sj.ijo.0803715. [DOI] [PubMed] [Google Scholar]
- 2.Andrew JG, Palan J, Kurup HV, Gibson P, Murray DW, Beard DJ. Obesity in total hip replacement. J Bone Joint Surg Br. 2008;90:424–429. doi: 10.1302/0301-620X.90B4.20522. [DOI] [PubMed] [Google Scholar]
- 3.Aritan S, Oyadiji SO, Bartlett RM. The in vivo mechanical properties of muscular bulk tissue. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:5259–5262. doi: 10.1109/IEMBS.2009.5334084. [DOI] [PubMed] [Google Scholar]
- 4.Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11:89–99. doi: 10.5435/00124635-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bartz RL, Noble PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82:1300–1307. doi: 10.2106/00004623-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bourne R, Mukhi S, Zhu N, Keresteci M, Marin M. Role of obesity on the risk for total hip or knee arthroplasty. Clin Orthop Relat Res. 2007;465:185–188. doi: 10.1097/BLO.0b013e3181576035. [DOI] [PubMed] [Google Scholar]
- 7.Brown TD, Callaghan JJ. Impingement in total hip replacement: mechanisms and consequences. Curr Orthop. 2008;22:376–391. doi: 10.1016/j.cuor.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Changulani M, Kalairajah Y, Peel T, Field RE. The relationship between obesity and the age at which hip and knee replacement is undertaken. J Bone Joint Surg Br. 2008;90:360–363. doi: 10.1302/0301-620X.90B3.19782. [DOI] [PubMed] [Google Scholar]
- 9.Chee YH, Teoh KH, Sabnis BM, Ballantyne JA, Brenkel IJ. Total hip replacement in morbidly obese patients with osteoarthritis: results of a prospectively matched study. J Bone Joint Surg Br. 2010;92:1066–1071. doi: 10.1302/0301-620X.92B8.22764. [DOI] [PubMed] [Google Scholar]
- 10.Dalferes ER, Jr, Radhakrishnamurthy B, Crouch MS, Berenson GS. A study of connective tissue macromolecules in skin of mice with gold thioglucose-induced obesity. Proc Soc Exp Biol Med. 1975;148:918–924. doi: 10.3181/00379727-148-38660. [DOI] [PubMed] [Google Scholar]
- 11.Davey JR, O’Connor DO, Burke DW, Harris WH. Femoral component offset: its effect on strain in bone-cement. J Arthroplasty. 1993;8:23–26. doi: 10.1016/S0883-5403(06)80103-8. [DOI] [PubMed] [Google Scholar]
- 12.Davis AM, Wood AM, Keenan AC, Brenkel IJ, Ballantyne JA. Does body mass index affect clinical outcome post-operatively and at five years after primary unilateral total hip replacement performed for osteoarthritis? A multivariate analysis of prospective data. J Bone Joint Surg Br. 2011;93:1178–1182. doi: 10.1302/0301-620X.93B9.26873. [DOI] [PubMed] [Google Scholar]
- 13.Elkins JM, Kruger KM, Pedersen DR, Callaghan JJ, Brown TD. Edge-loading severity as a function of cup lip radius in metal-on-metal total hips: a finite element analysis. J Orthop Res. 2012;30:169–177. doi: 10.1002/jor.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins JM, Stroud NJ, Rudert MJ, Tochigi Y, Pedersen DR, Ellis BJ, Callaghan JJ, Weiss JA, Brown TD. The capsule’s contribution to total hip construct stability: a finite element analysis. J Orthop Res. 2011;29:1642–1648. doi: 10.1002/jor.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enser M, Avery NC. Mechanical and chemical properties of the skin and its collagen from lean and obese-hyperglycaemic (ob/ob) mice. Diabetologia. 1984;27:44–49. doi: 10.1007/BF00253500. [DOI] [PubMed] [Google Scholar]
- 16.Grant JA, Viens N, Bolognesi MP, Olson SA, Cook CE. Two-year outcomes in primary THA in obese male Veterans Administration Medical Center patients. Rheumatol Int. 2008;28:1105–1109. doi: 10.1007/s00296-008-0575-y. [DOI] [PubMed] [Google Scholar]
- 17.Haverkamp D, Klinkenbijl MN, Somford MP, Albers GHR, van der Vis HM. Obesity in total hip arthroplasty: does it really matter? A meta-analysis. Acta Orthop. 2011;82:417–422. doi: 10.3109/17453674.2011.588859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Incavo SJ, Havener T, Benson E, McGrory BJ, Coughlin KM, Beynnon BD. Efforts to improve cementless femoral stems in THR: 2-to 5-year follow-up of a high-offset femoral stem with distal stem modification (Secur-Fit Plus) J Arthroplasty. 2004;19:61–67. doi: 10.1016/j.arth.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Insall JN. Presidential address to The Knee Society: Choices and compromises in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:43–48. [PubMed] [Google Scholar]
- 20.Jackson MP, Sexton SA, Yeung E, Walter WL, Walter WK, Zicat BA. The effect of obesity on the mid-term survival and clinical outcome of cementless total hip replacement. J Bone Joint Surg Br. 2009;91:1296–1300. doi: 10.1302/0301-620X.91B10.22544. [DOI] [PubMed] [Google Scholar]
- 21.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, Morshed S, Joseph T, Bozic K, Ries MD. Clinical impact of obesity on stability following revision total hip arthroplasty. Clin Orthop Relat Res. 2006;453:142–146. doi: 10.1097/01.blo.0000238874.09390.a1. [DOI] [PubMed] [Google Scholar]
- 23.Kluess D, Martin H, Mittelmeier W, Schmitz KP, Bader R. Influence of femoral head size on impingement, dislocation and stress distribution in total hip replacement. Med Eng Phys. 2007;29:465–471. doi: 10.1016/j.medengphy.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Ley CJ, Lees B, Stevenson JC. Sex-and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 25.Lubbeke A, Moons KG, Garavaglia G, Hoffmeyer P. Outcomes of obese and nonobese patients undergoing revision total hip arthroplasty. Arthritis Rheum. 2008;59:738–745. doi: 10.1002/art.23562. [DOI] [PubMed] [Google Scholar]
- 26.Lubbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57:327–334. doi: 10.1002/art.22542. [DOI] [PubMed] [Google Scholar]
- 27.Matrangola SL, Madigan ML, Nussbaum MA, Ross R, Davy KP. Changes in body segment inertial parameters of obese individuals with weight loss. J Biomech. 2008;41:3278–3281. doi: 10.1016/j.jbiomech.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushita A, Nakashima Y, Jingushi S, Yamamoto T, Kuraoka A, Iwamoto Y. Effects of the femoral offset and the head size on the safe range of motion in total hip arthroplasty. J Arthroplasty. 2009;24:646–651. doi: 10.1016/j.arth.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 29.McClung CD, Zahiri CA, Higa JK, Amstutz HC, Schmalzried TP. Relationship between body mass index and activity in hip or knee arthroplasty patients. J Orthop Res. 2000;18:35–39. doi: 10.1002/jor.1100180106. [DOI] [PubMed] [Google Scholar]
- 30.McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric reference data for children and adults: US population, 1999–2002. Adv Data. 2005;361:1–5. [PubMed] [Google Scholar]
- 31.McLaughlin JR, Lee KR. The outcome of total hip replacement in obese and non-obese patients at 10- to 18-years. J Bone Joint Surg Br. 2006;88:1286–1292. doi: 10.1302/0301-620X.88B10.17660. [DOI] [PubMed] [Google Scholar]
- 32.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, kinetics, and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J Biomech. 2003;36:577–591. doi: 10.1016/S0021-9290(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 33.Paterno SA, Lachiewicz PF, Kelley SS. The influence of patient-related factors and the position of the acetabular component on the rate of dislocation after total hip replacement. J Bone Joint Surg Am. 1997;79:1202–1210. doi: 10.2106/00004623-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Rittmeister M, Callitsis C. Factors influencing cup orientation in 500 consecutive total hip replacements. Clin Orthop Relat Res. 2006;445:192–196. doi: 10.1097/01.blo.0000194669.77849.3c. [DOI] [PubMed] [Google Scholar]
- 35.Sadr Azodi O, Bellocco R, Eriksson K, Adami J. The impact of tobacco use and body mass index on the length of stay in hospital and the risk of post-operative complications among patients undergoing total hip replacement. J Bone Joint Surg Br. 2006;88:1316–1320. doi: 10.1302/0301-620X.88B10.17957. [DOI] [PubMed] [Google Scholar]
- 36.Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH. Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res. 2001;388:125–134. doi: 10.1097/00003086-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Samani A, Bishop J, Yaffe MJ, Plewes DB. Biomechanical 3-D finite element modeling of the human breast using MRI data. IEEE Trans Med Imaging. 2001;20:271–279. doi: 10.1109/42.921476. [DOI] [PubMed] [Google Scholar]
- 38.Sibella F, Galli M, Romei M, Montesano A, Crivellini M. Biomechanical analysis of sit-to-stand movement in normal and obese subjects. Clin Biomech (Bristol, Avon). 2003;18:745–750. doi: 10.1016/S0268-0033(03)00144-X. [DOI] [PubMed] [Google Scholar]
- 39.Skendzel JG, Blaha JD, Urquhart AG. Total hip arthroplasty modular neck failure. J Arthroplasty. 2011;26:338.e1–4. [DOI] [PubMed]
- 40.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121:492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care. 2004;17:426–435. doi: 10.1097/00129334-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Woolson ST, Rahimtoola ZO. Risk factors for dislocation during the first 3 months after primary total hip replacement. J Arthroplasty. 1999;14:662–668. doi: 10.1016/S0883-5403(99)90219-X. [DOI] [PubMed] [Google Scholar]