Abstract
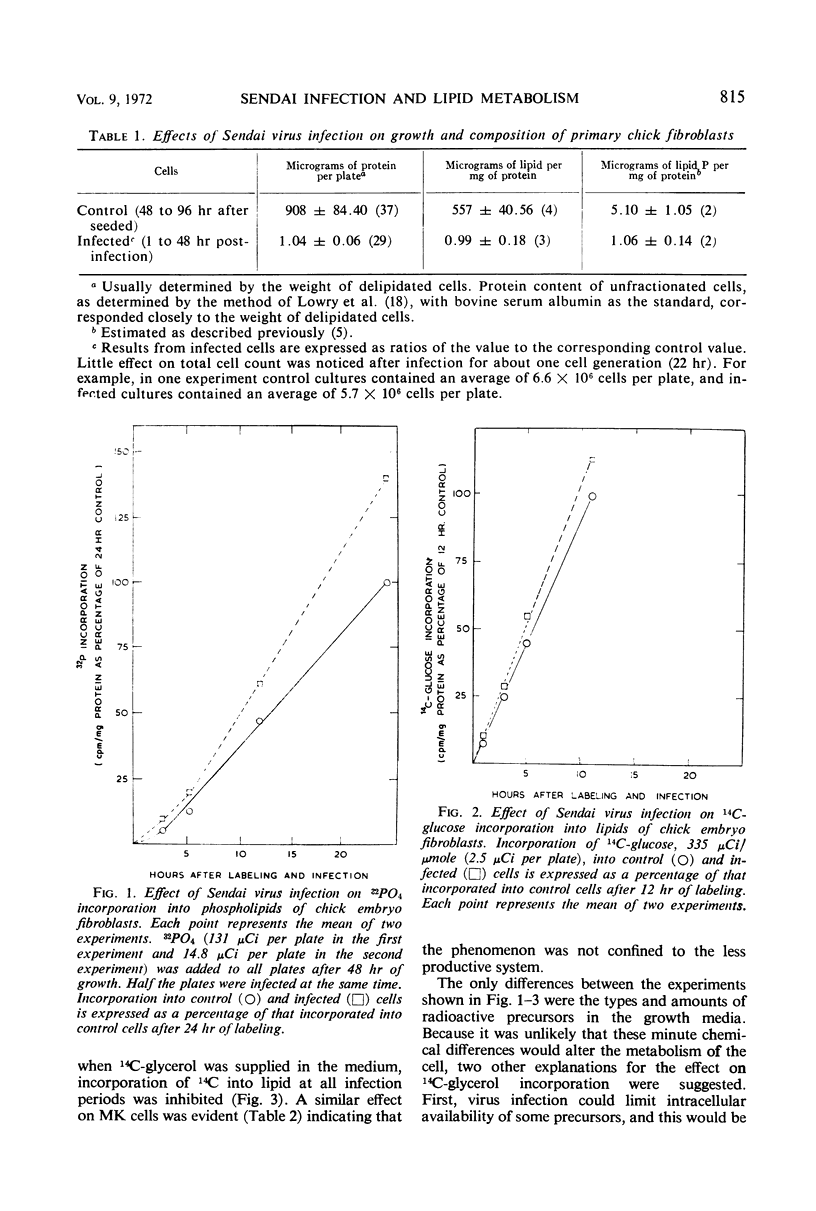
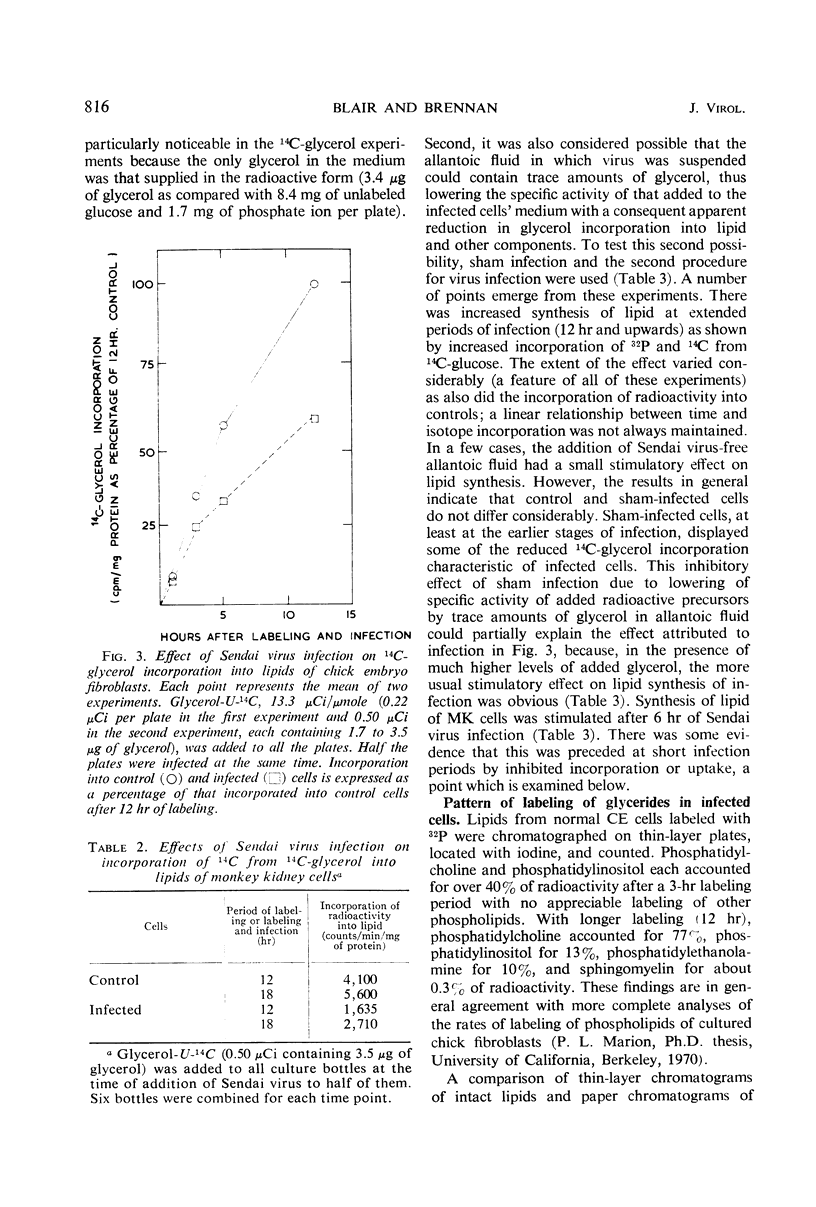
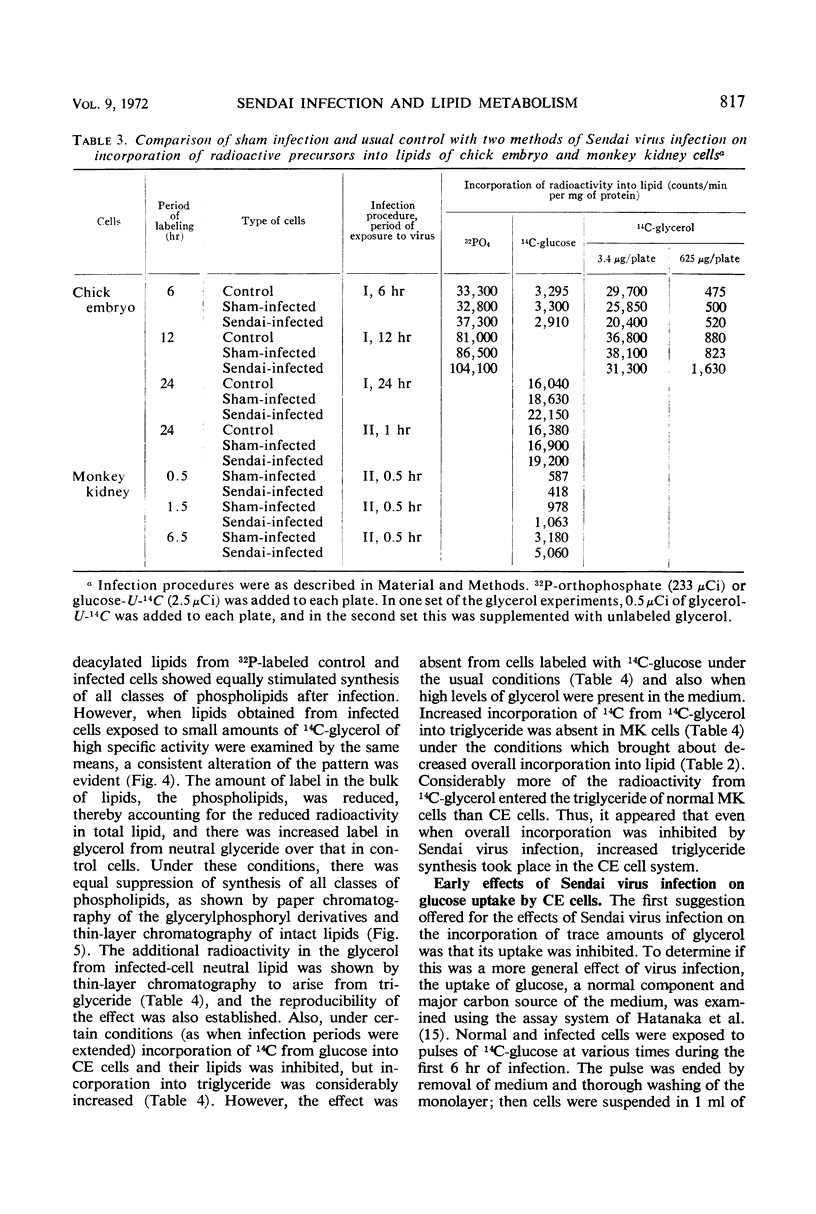
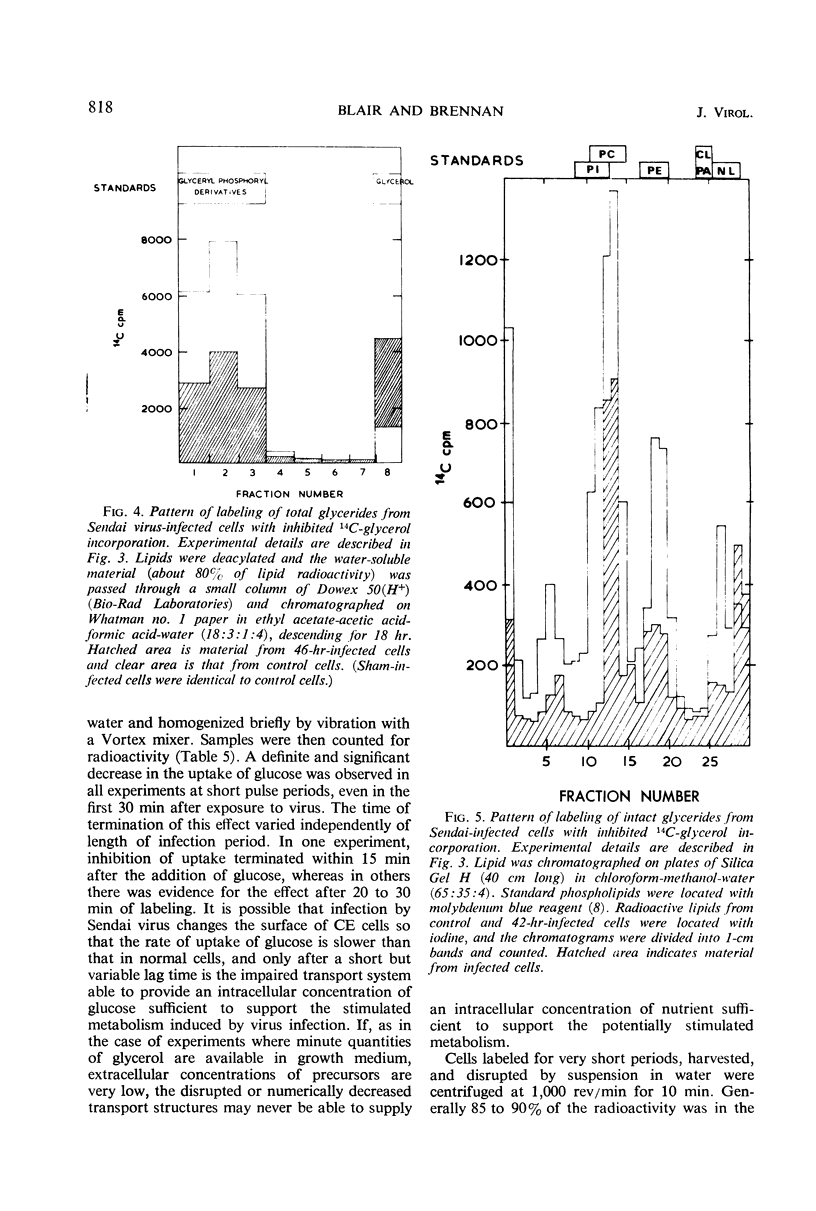
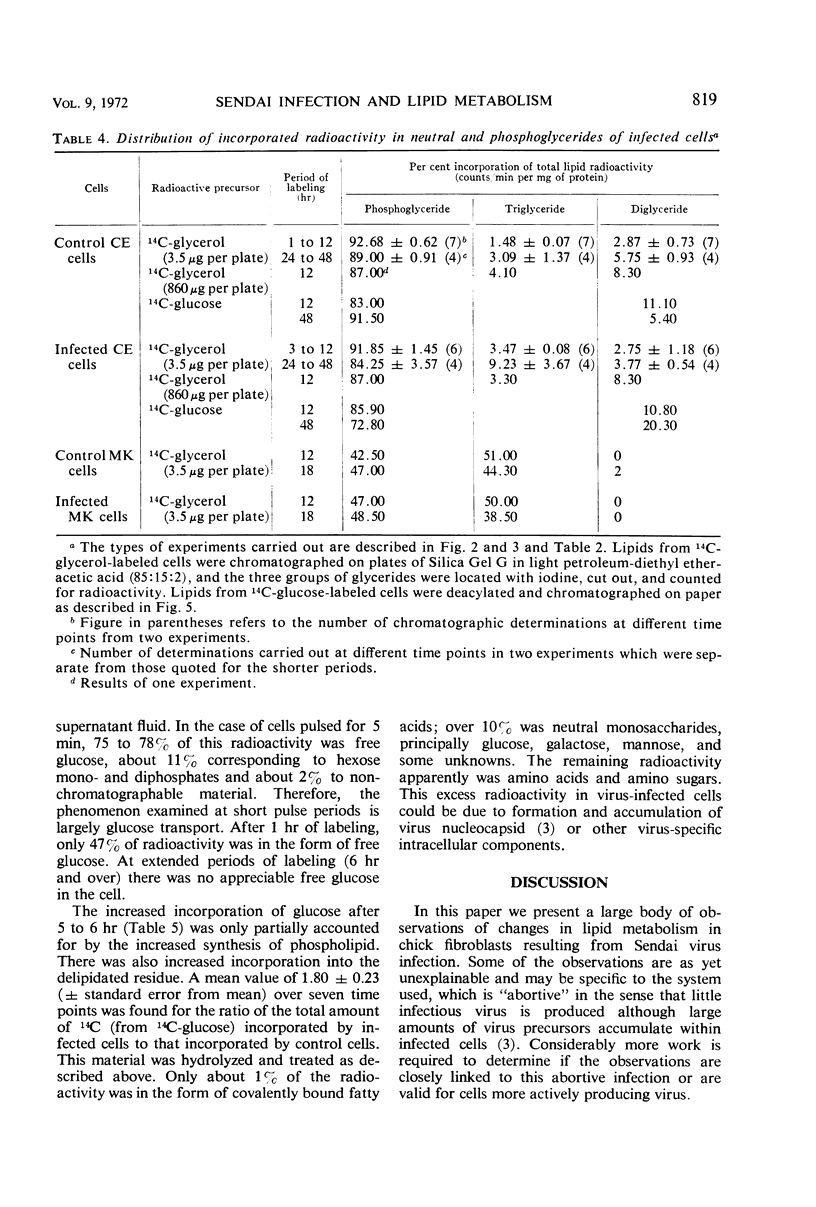
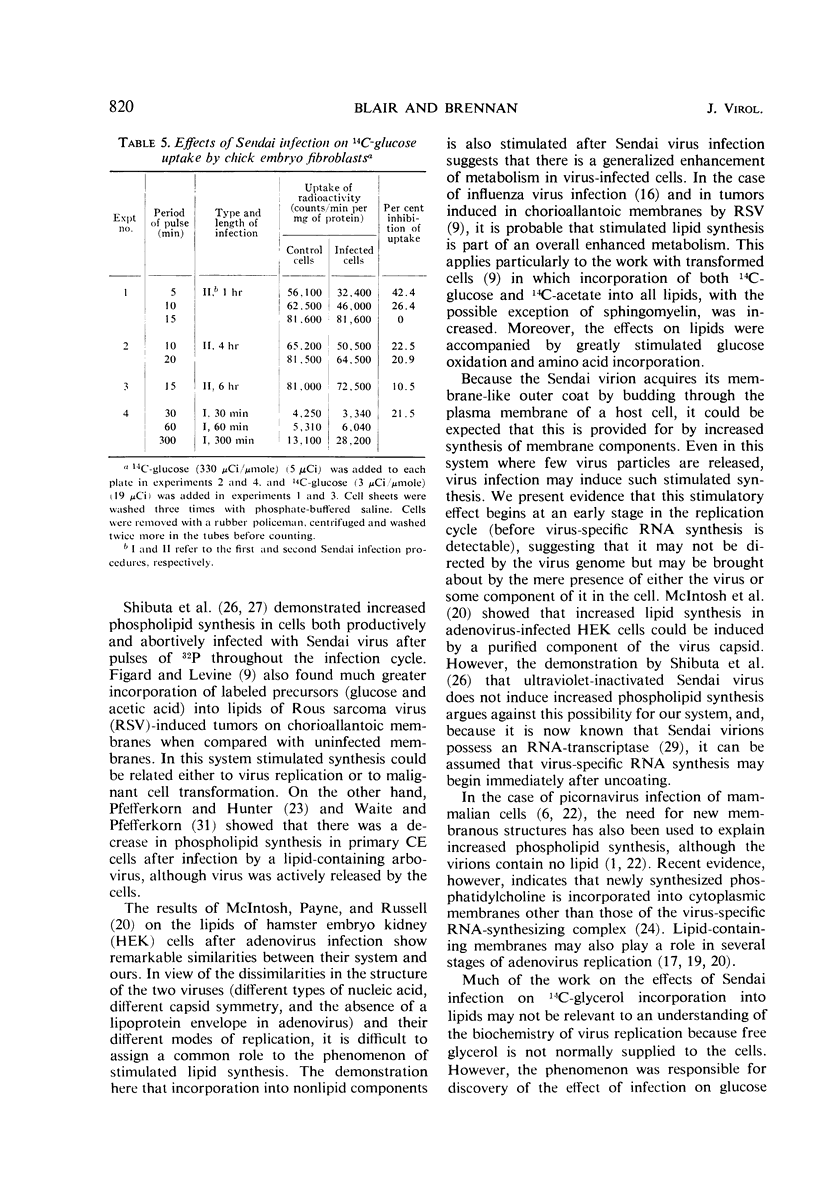
Lipid metabolism in the “abortive” system, Sendai virus-infected primary chick embryo fibroblasts, was examined by using 32P-orthophosphate, 14C-glucose, and 14C-glycerol as precursors. Incorporation of radioactivity from 32P-orthophosphate and 14C-glucose into lipid was increased in infected cells. Synthesis of all individual phospholipids was about equally stimulated. There was also evidence for increased lipid synthesis in more productively infected monkey kidney cells. Incorporation of 14C-glycerol when at a high level in the medium was also increased. However, when this precursor was supplied in minute quantities of high specific activity, incorporation was inhibited. Even though incorporation of radioactivity from 14C-glucose was stimulated during long labeling periods, the uptake of this precursor during short pulses was inhibited in infected cells. The phenomenon of increased labeling of triglyceride in infected chick cells under certain conditions is discussed, in conjunction with the other effects, in terms of related changes in other virus-infected systems.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Dales S. Cytopathology of Mengovirus infection. II. Proliferation of membranous cisternae. Virology. 1967 Jun;32(2):201–215. doi: 10.1016/0042-6822(67)90270-x. [DOI] [PubMed] [Google Scholar]
- Blair C. D., Robinson W. S. Replication of Sendai virus. I. Comparison of the viral RNA and virus-specific RNA synthesis with Newcastle disease virus. Virology. 1968 Aug;35(4):537–549. doi: 10.1016/0042-6822(68)90284-5. [DOI] [PubMed] [Google Scholar]
- Blair C. D., Robinson W. S. Replication of Sendai virus. II. Steps in virus assembly. J Virol. 1970 May;5(5):639–650. doi: 10.1128/jvi.5.5.639-650.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough H. A., Lawson D. E. The lipids of paramyxoviruses: a comparative study of Sendai and Newcastle disease viruses. Virology. 1968 Oct;36(2):286–292. doi: 10.1016/0042-6822(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Brennan P., Ballou C. E. Biosynthesis of mannophosphoinositides by Mycobacterium phlei. The family of dimannophosphoinositides. J Biol Chem. 1967 Jul 10;242(13):3046–3056. [PubMed] [Google Scholar]
- CORNATZER W. E., SANDSTROM W., FISCHER R. G. The effect of poliomyelitis virus type I (Mahoney strain) on the phospholipid metabolism of the HeLa cell. Biochim Biophys Acta. 1961 May 13;49:414–415. doi: 10.1016/0006-3002(61)90151-2. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Figard P. H., Levine A. S. Incorporation of labeled precursors into lipids of tumors induced by Rous carcoma virus. Biochim Biophys Acta. 1966 Dec 7;125(3):428–434. doi: 10.1016/0005-2760(66)90031-2. [DOI] [PubMed] [Google Scholar]
- GREEN M. Biochemical studies on adenovirus multiplication. 1. Stimulation of phosphorus incorporation into deoxyribonucleic acid and ribouncleic acid. Virology. 1959 Nov;9:343–358. doi: 10.1016/0042-6822(59)90127-8. [DOI] [PubMed] [Google Scholar]
- HARRIS H., WATKINS J. F. HYBRID CELLS DERIVED FROM MOUSE AND MAN: ARTIFICIAL HETEROKARYONS OF MAMMALIAN CELLS FROM DIFFERENT SPECIES. Nature. 1965 Feb 13;205:640–646. doi: 10.1038/205640a0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. Alterations in the characteristics of sugar uptake by mouse cells transformed by murine sarcoma viruses. J Natl Cancer Inst. 1969 Nov;43(5):1091–1096. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Payne S., Russell W. C. Studies on lipid metabolism in cells infected with adenovirus. J Gen Virol. 1971 Mar;10(3):251–265. doi: 10.1099/0022-1317-10-3-251. [DOI] [PubMed] [Google Scholar]
- PANGBORN M. C. A simplified purification of lecithin. J Biol Chem. 1951 Feb;188(2):471–476. [PubMed] [Google Scholar]
- PENMAN S. STIMULATION OF THE INCORPORATION OF CHOLINE IN POLIOVIRUS-INFECTED CELLS. Virology. 1965 Jan;25:149–152. doi: 10.1016/0042-6822(65)90263-1. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. THE SOURCE OF THE RIBONUCLEIC ACID AND PHOSPHOLIPID OF SINDBIS VIRUS. Virology. 1963 Jul;20:446–456. doi: 10.1016/0042-6822(63)90093-x. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Cleveland P. H., Shea M. A. Effect of mengovirus replication on choline metabolism and membrane formation in novikoff hepatoma cells. J Virol. 1970 Dec;6(6):800–812. doi: 10.1128/jvi.6.6.800-812.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Yamaura K., Hirano K., Matumoto M. Enhancement of 32P incorporation into phospho- lipids in cultured cells by sendai virus of parainfluenza type 1. Jpn J Microbiol. 1971 Mar;15(2):185–191. doi: 10.1111/j.1348-0421.1971.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Shibuta H., Yamaura K., Yamamoto T., Matumoto M. Incorporation of 32P into phospholipids of influenza A and parainfluenza 1 viruses. Jpn J Microbiol. 1969 Jun;13(2):212–214. doi: 10.1111/j.1348-0421.1969.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Stone H. O., Portner A., Kingsbury D. W. Ribonucleic acid transcriptases in Sendai Virions and infected cells. J Virol. 1971 Aug;8(2):174–180. doi: 10.1128/jvi.8.2.174-180.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan W. E. Removal of the sphingolipid impurity from preparations of yeast phosphatidyl inositol. J Lipid Res. 1967 May;8(3):281–282. [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Phospholipid synthesis in Sindbis virus-infected cells. J Virol. 1970 Nov;6(5):637–643. doi: 10.1128/jvi.6.5.637-643.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



