Abstract
Background
The current operative standard of care for disseminated malignant bone disease suggests stabilizing the entire bone to avoid the need for subsequent operative intervention but risks of doing so include complications related to embolic phenomena.
Questions/purposes
We questioned whether progression and reoperation occur with enough frequency to justify additional risks of longer intramedullary devices.
Methods
A retrospective chart review was done for 96 patients with metastases, myeloma, or lymphoma who had undergone stabilization or arthroplasty of impending or actual femoral or humeral pathologic fractures using an approach favoring intramedullary fixation devices and long-stem arthroplasty. Incidence of progressive bone disease, reoperation, and complications associated with fixation and arthroplasty devices in instrumented femurs or humeri was determined.
Results
At minimum 0 months followup (mean, 11 months; range, 0–72 months), 80% of patients had died. Eleven of 96 patients (12%) experienced local bony disease progression; eight had local progression at the original site, two had progression at originally recognized discretely separate lesions, and one had a new lesion develop in the bone that originally was surgically treated. Six subjects (6.3%) required repeat operative intervention for symptomatic failure. Twelve (12.5%) patients experienced physiologic nonfatal complications potentially attributable to embolic phenomena from long intramedullary implants.
Conclusions
Because most patients in this series were treated with the intent to protect the bone with long intramedullary implants when possible, the reoperation rate may be lower than if the entire bone had not been protected. However, the low incidence of disease progression apart from originally identified lesions (one of 96) was considerably lower than the physiologic complication rate (12 of 96) potentially attributable to long intramedullary implants.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Classic teaching for the treatment of metastatic carcinoma, lymphoma, and multiple myeloma in the long bones has included routine protection of the entire remaining humerus or femur when performing internal fixation or arthroplasty for a pathologic fracture or prophylactic stabilization for an impending pathologic fracture [1–8, 10, 13–15, 27, 33–35, 39].
However, the rationale for protecting the remainder of the long bone away from the area of obvious major involvement or fracture has little solid support in the literature other than recommendations in reviews and chapters [5, 11, 12, 16, 24, 29, 30, 32]. Moreover, there are potential adverse consequences to stabilizing the entire bone rather than just a limited segment, including intraoperative hypotension, O2 desaturation, and embolization, or even perioperative coma and death [18, 20, 25, 26, 28, 29, 34]. These inherent risks have been theorized to be the result of increased intramedullary vascular supply in metastatic disease to bone, allowing for a larger surface area for emboli to escape and from a further increase in surface area of exposed vasculature by reaming and instrumenting the intramedullary canal [18]. Nevertheless, in our practice, the approach to date, when possible, has been to prophylactically protect as much of an involved long bone as possible using either an intramedullary nail or long-stem cemented arthroplasty device when a patient is undergoing operative stabilization for impending or actual pathologic fracture in the setting of disseminated malignancy. In some cases, when intramedullary stabilization has not been feasible, plate and screw fixation was used, and in arthroplasty cases in which long prophylactic stems could not be inserted, standard stem lengths were used.
The main purposes of the study were to evaluate (1) the incidence of disease progression in an involved long bone (humerus or femur); and (2) the subsequent reoperation rate of operatively treated patients with metastatic carcinoma, lymphoma, or multiple myeloma for impending or actual pathologic fractures in comparison to (3) physiologic embolic-related complications attributable to protecting the entire bone with longer implants such as intramedullary nails and long-stem arthroplasty devices. To critically evaluate our beliefs and standard practices, we asked, “Is either the incidence of disease progression or the reoperation rate in patients with metastatic carcinoma, lymphoma, or multiple myeloma to the femur or humerus higher than the incidence of complications related to prophylactically stabilizing the entire bone?” We asked this direct comparison as a simplistic way of beginning to critically analyze the bigger question of, “Does the benefit of operatively protecting the entire bone outweigh the risks?”
Patients and Methods
We obtained institutional review board approval at SUNY Upstate Medical University to conduct a retrospective chart review study over a 10-year period (January 1, 1998, to December 31, 2007) for all of the patients of the principal investigator (TAD) who had multiple myeloma, lymphoma, or metastatic cancer in the humerus or femur and who had undergone operative management for their humeral or femoral disease by the principal investigator. We used the following International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes to identify potential subjects: 198.5 secondary malignancy of bone, 202.80 lymphoma extranodal unspecified, 203.00 multiple myeloma, 733.11 pathologic fracture of the humerus, 733.14 pathologic fracture of the femoral neck, and 733.15 pathologic fracture of the femur (not otherwise specified). Charts then were screened to ensure they met the inclusion criteria set forth previously and reviewed to gather data.
The preliminary ICD-9-based diagnostic search revealed 263 subjects. After excluding subjects for nonoperative management (nine subjects), absence of multiple myeloma, lymphoma, or metastatic carcinoma (43 subjects), nonpathologic fractures (eight subjects), soft tissue lesions (three subjects), osteosarcoma (two subjects), lesions not in the femur or humerus (15 subjects), and missing data (87 subjects), our final analysis yielded 96 subjects (40 males, 56 females). There were 72 femoral cases and 24 humeral cases. All 96 subjects had undergone operative treatment with most receiving either intramedullary nailing or long stem-cemented arthroplasty of the humerus or femur (Table 1). The only patients in this series treated with plate and screw fixation included two with humeral lesions not believed to be amenable to intramedullary fixation and one patient with a distal femoral replacement megaprosthesis who had a supplementary prophylactic proximal femoral hip screw and side plate. For patients undergoing arthroplasties, hemiarthroplasties were used for humeral lesions and a combination of hemiarthroplasties, THAs, and one TKA were used for the femoral lesions (Table 2).
Table 1.
Internal fixation
| Internal fixation | Number of cases |
|---|---|
| Femur | |
| Reconstruction and locked nails | 36 |
| Intramedullary nail, not distally locked | 16 |
| Dynamic hip screw | 1 |
| Humerus | |
| Intramedullary nail, locked | 8 |
| Intramedullary nail, not distally locked | 6 |
| Nonlocking plate and screws | 2 |
Table 2.
Arthroplasty cases
| Procedure | Number of cases |
|---|---|
| Shoulder | |
| Hemiarthroplasty with long cemented stem | 4 |
| Hemiarthroplasty with standard length cemented stem | 1 |
| Hemiarthroplasty with standard length press-fit stem | 1 |
| Hemiarthroplasty with proximal humeral replacement megaprosthesis | 2 |
| Femur | |
| THA with long cemented stem | 2 |
| THA with long press-fit stem | 1 |
| THA with press-fit standard length stem | 1 |
| Hemiarthroplasty with long cemented stem | 8 |
| Hemiarthroplasty with long press-fit stem | 5 |
| Hemiarthroplasty with standard length press-fit stem | 2 |
| Hemiarthroplasty with proximal femoral replacement megaprosthesis | 4 |
| TKA with distal femoral replacement megaprosthesis | 1 |
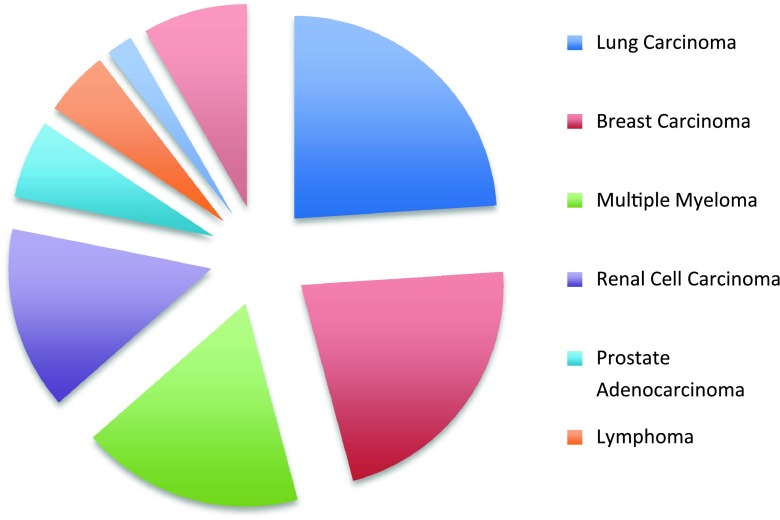
The mean age of the patients at the time of orthopaedic intervention was 60 years (range, 29–83 years). The most common primary cancers included lung, breast, multiple myeloma, renal, and prostate cancer (Fig. 1). The minimum followup was 0 months (mean, 11 months; range, 0–72 months). At latest followup, 77 patients (80.2%) had died of their disseminated disease and 13 (13.5%) were alive with disease, whereas the disease status of six (6.3%) was unknown. Preoperative evaluation of these patients consisted of plain radiographic analysis of the entire involved bone in all cases supplemented by bone scans in most, but neither CT nor MRI were used during this period on a routine basis to search for other lesions.
Fig. 1.
The pie chart shows the breakdown of primary diagnoses for the reported patient population (N = 96). The “other” category includes the following primary tumors: parotid gland carcinoma, colonic adenocarcinoma, hepatocellular carcinoma, malignant melanoma, thyroid carcinoma, bladder carcinoma, cervical squamous cell carcinoma, and metastatic chondrosarcoma.
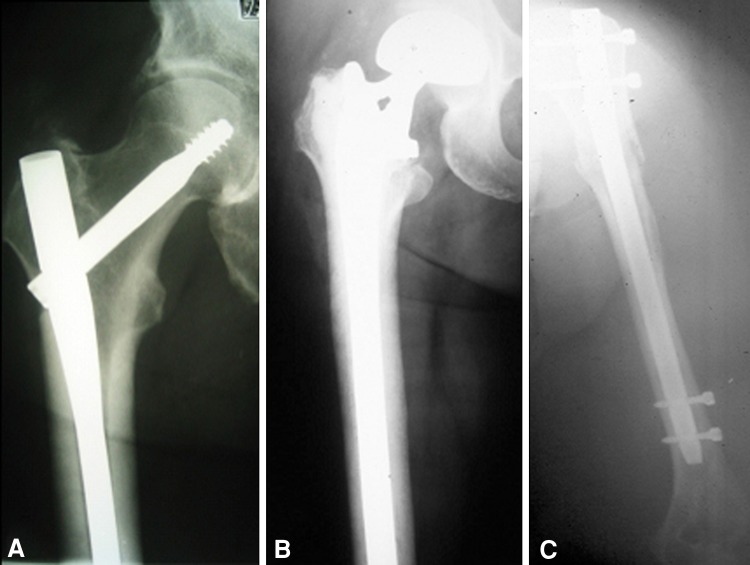
During this entire period, it was the senior author’s (TAD) routine practice to protect the entire bone with intramedullary devices when feasible and safe for the patient (Fig. 2). For prophylactic fixation of impending pathologic femoral fractures and humeral fractures, intramedullary nails generally were used. A reconstruction nail was used for impending femur fractures, most commonly a long Gamma® Nail (Stryker, Mahwah, NJ, USA). For hip and shoulder arthroplasties, long-stem devices were preferred, and these were used except when the canal did not allow their passage. For long-stem cemented femoral arthroplasties, the cement was placed in a doughy stage and no formal pressurization was done. A separate decompressive relief hole was not used in most cases. Plates and screws were used only in exceptional cases, primarily in the humerus (two) and to supplement a long-stem cemented distal femoral arthroplasty (one). To date, this is still the practice of the senior author (TAD).
Fig. 2A–C.
The most common forms of surgical instrumentation used in this series are shown here. (A) For impending proximal femoral pathologic fractures and intertrochanteric, subtrochanteric, and diaphyseal femur pathologic fractures, reconstruction intramedullary nailing was performed in most cases. A typical reconstruction intramedullary nail is shown in the right femur. (B) For femoral neck pathologic fractures, extensive periarticular destruction of the proximal femur, and selected intertrochanteric femur pathologic fractures, a long-stem cemented arthroplasty was used. A calcar-replacing long-stem cemented arthroplasty of the right femur is shown. (C) For impending humeral fractures and fractures distal to the neck region with adequate remaining proximal bone, an antegrade locked nailing of the humerus was done. A locked intramedullary nail of the humerus is shown.
All 96 subjects in our study underwent perioperative radiation therapy. All patients who had not received preoperative radiotherapy were sent to radiation oncology for postoperative external beam radiotherapy. For most patients, the goal of the surgery was to render the subjects able to progress to immediate full weightbearing on the affected extremity. For some pathologic proximal femur fractures treated with internal fixation in patients with better overall prognosis and radiosensitivity (breast and prostate cancer), a period of limited weightbearing was used to promote early healing without untoward stress on the implant.
In general, we followed subjects postoperatively with an office visit at 2 weeks and an office visit and plain radiographs at 6 weeks, 3 months, 6 months, 1 year, and then on a yearly basis afterward. This followup plan was modified depending on each patient’s circumstances. For the purposes of this study, we evaluated all radiographic studies. It was from these imaging studies that it was determined (1) if a patient had experienced disease progression; and (2) the type of progression when present. Data were collected through a combination of hospital inpatient records and outpatient charts.
We subclassified disease progression into three distinct categories: (1) local progression of the originally identified main lesion; (2) local progression of originally identified, discretely separate lesions (lesions that were not the primary source of concern); and (3) occurrence of an entirely new, previously unrecognized lesion (Table 3). The rationale for this subclassification is as follows. The potential for Type 1 progression was believed to be theoretically amenable to initial standard means of localized internal fixation (plate and screw devices or short intramedullary nails) or hip arthroplasty (with stems extending two bone diameters distal to the distal aspect of the lesion) and not believed to necessarily benefit from longer lengths of internal fixation (full-length femoral devices) or longer than standard length intramedullary stems. In other words, localized internal fixation with plate and screw or short intramedullary nail devices or standard length femoral arthroplasty implants potentially and theoretically could be sufficient despite this type of progression. The potential for Type 2 progression theoretically was believed to be handled initially by using appropriately sized longer implants (whether plate and screw or intramedullary devices) based on preoperative templating designed to cover the affected areas without necessarily protecting the entire bone on a routine basis or by routine prophylactic protection of the entire bone. The potential for Type 3 progression, however, was believed to be the only type of progression that was addressed adequately by initial routine stabilization of the entire bone on a regular basis. Therefore, it was Type 3 progression that was believed to be the target of the classic recommendations for routine stabilization of the entire bone, but Types 2 and 3 were believed to potentially benefit from the same treatment paradigm of routine whole-bone stabilization, whereas Type 1 was not believed to routinely benefit any more from routine whole-bone stabilization than from appropriate limited fixation.
Table 3.
Disease progression subclassifications
| Type | Definition |
|---|---|
| 1 | Local progression of the originally identified main lesion |
| 2 | Local progression of originally identified, discretely separate lesions (lesions that were not the primary source of concern) |
| 3 | Occurrence of an entirely new, previously unrecognized lesion |
We noted all reoperations, and details were recorded. In addition, as a result of the reported physiologic changes associated with long-stem implants, we examined specifically for perioperative oxygen desaturation, pulmonary embolism, postoperative electrocardiographic changes, postoperative hypotension, and sudden cardiac arrest.
Results
Overall, of the 96 subjects, 11 (11%) experienced disease progression of any type (1, 2, or 3) (Table 4). Among these 11 patients, there were three with metastatic renal cell carcinoma, two with metastatic breast carcinoma, two with multiple myeloma, and one each with prostate carcinoma, nonsmall-cell lung carcinoma, squamous cell carcinoma of the head and neck, and adenocarcinoma of unknown primary. All but one case of progression occurred in the femur. Eight of the 11 patients (8% of whole cohort), including all three mentioned patients with renal carcinoma, experienced Type 1 local progression of the original main lesion. Type 1 local progression occurred at a mean 10 months (range, 2–40 months) after the index procedure. Two of the 11 patients (2.08% of 96), both with myeloma, experienced Type 2 progression of originally identified lesions discretely separate from the main lesion (Fig. 3). Only one of the 11 patients (1.04% of 96) experienced Type 3 progression of an entirely new, previously unrecognized lesion. Type 2 progression of an originally identified, discretely separate lesion occurred at 4 months after the index procedure in one patient and 37 months in the other. The only patient with Type 3 progression had metastatic breast carcinoma, and the new lesion in her distal femur was identified 24 months after the index surgery. Among the 17 patients with myeloma and 14 with renal cancer, disease progression occurred in two (11.76%) patients with myeloma and three (21.43%) with renal carcinoma.
Table 4.
Summary of 11 cases with progressive disease
| Classification | Primary cancer | Site of lesion | Impending versus fracture | Initial operation | Second surgery | Time to progression (months) |
|---|---|---|---|---|---|---|
| 1 | Renal | Femur, proximal | Impending | Reconstruction nail | Proximal femoral replacement | 10 |
| 1 | Renal | Femur, proximal | Impending | Reconstruction nail | None | 5 |
| 1 | Renal | Femur, proximal | Impending | Reconstruction nail | Proximal femoral replacement | 10 |
| 1 | Breast | Femur, distal | Fracture | Reconstruction nail | Distal femoral replacement | 40 |
| 1 | Prostate | Femur, distal | Fracture | Retrograde femoral nail | None | 3 |
| 1 | SqCCa of H&N | Humerus | Impending | Humeral intramedullary nail | None | 2 |
| 1 | AdenoCa unknown primary | Femur | Impending | Reconstruction nail | None | 2 |
| 1 | Lung | Femur | Impending | Reconstruction nail | None | 8 |
| 2 | Myeloma | Femur, neck | Fracture | Long-stem cemented hemiarthroplasty | None | 4 |
| 2 | Myeloma | Femur, proximal | Impending | Reconstruction nail | Endo-recon proximal femoral replacement | 37 |
| 3 | Breast | Femur, proximal | Impending | Reconstruction nail | None | 24 |
SqCCa of H&N = squamous cell carcinoma of the head and neck; AdenoCa unknown primary = adenocarcinoma of unknown primary; Endo-recon = endoprosthetic reconstruction nail (custom device with proximal femoral arthroplasty and interlocking nail in distal stem extension).
Fig. 3A–B.

Type 2 progression was seen in this patient who initially was treated with a long-stem cemented hemiarthroplasty for a pathologic femoral neck fracture secondary to multiple myeloma. (A) An immediate postoperative radiograph after shows small lesions in the subtrochanteric region. (B) A followup radiograph taken at the 3-month office visit shows local progression of the original lesion compared with the immediate postoperative radiograph. This Type 2 progression occurred despite perioperative irradiation and protection of the entire bone by the long-stem cemented device. In this case, the patient remained asymptomatic and did not require additional operative intervention.
Six patients (6.25%) underwent reoperation during the study for symptomatic failure (four patients with Type 1 local disease progression, one patient for broken hardware, and the only patient with Type 3 progression of a discretely separate lesion) (Table 5). Therefore, half (four of eight) of the patients with Type 1 progression required reoperation despite our best effort at stabilization or arthroplasty. Neither of the two patients with Type 2 progression required reoperation. Five additional patients (5.21%) underwent second procedures for a new location of metastasis in another bone (Table 6). In total, 11 patients (11.5% of 96) required subsequent operative procedures either for the index site or for a site in another bone. Implant failure requiring reoperation occurred in five of 60 intramedullary nail cases (8.33%) compared with zero of 24 arthroplasty cases (0%). Intramedullary nail fixation failures occurred in 10.87% (five of 46) of femoral cases and none (zero of 14) of the humeral cases. Apart from the six patients described previously who required reoperation for progression or implant failure, no other patients experienced major or minor complications. Twenty-three patients (23.96%) received transfusions.
Table 5.
Patients who had reoperations secondary to hardware failure
| Patient number | Patient age at index surgery (years) | Primary cancer | Index procedure | Reason for failure | Time to failure (months) | Treatment |
|---|---|---|---|---|---|---|
| 1 | 57 | Renal cell carcinoma | Right femoral reconstruction IMN | Local disease progression | 10 | IMN removed; resection proximal femur; cemented long-stem proximal femoral megaprosthesis with bipolar head |
| 2 | 57 | Breast carcinoma | Right femoral reconstruction IMN | Local disease progression | 8 | IMN removed; resection distal femur; distal femur megaprosthesis TKA |
| 3 | 66 | Multiple myeloma | Prophylactic left femoral reconstruction IMN | First failure: nail migration with broken distal interlocking screw Second failure: local disease progression and infection |
First failure: 5 Second failure: 29 (after first failure) |
First failure: IMN removed; exchanged with Richards Endo-Recon femoral nail with distal interlocking screws and proximal hemiarthroplasty portion cemented Second failure: two-stage hardware removal of Endo-Recon device followed by cemented THA |
| 4 | 54 | Renal cell carcinoma | Right femoral reconstruction nail | Local disease progression | 10 | IMN removed; cemented, long-stem modular replacement system proximal femoral megaprosthesis with bipolar head |
| 5 | 65 | Breast carcinoma | Prophylactic right femoral reconstruction nail | Proximal migration of IMN | 2 | IMN removed; conversion to long-stem cemented hemiarthroplasty with bipolar head |
IMN = intramedullary nail; Endo-Recon = endoprosthetic reconstruction nail.
Table 6.
Patients who underwent reoperation secondary to new location of bone metastasis
| Patient number | Patient age at index procedure (years) | Primary cancer | Index procedure | Second site | Time to second site surgery (months) | Treatment of second site |
|---|---|---|---|---|---|---|
| 1 | 69 | Renal carcinoma | Right proximal humeral shoulder arthroplasty | Left femur | 0 (5 days) | Prophylactic stabilization with IMN |
| 2 | 66 | Multiple myeloma | Right proximal humeral resection and endoprosthetic shoulder arthroplasty | Right femur | 41 | Prophylactic stabilization with IMN |
| 3 | 68 | Breast carcinoma | Right humeral prophylactic IMN | Right femur | 27 | Long-stem cemented hip hemiarthroplasty |
| 4 | 59 | Breast carcinoma | Left humeral IMN ORIF for pathologic fracture | Right humerus | 12 | Prophylactic stabilization with IMN |
| 5 | 62 | Multiple myeloma | Right hip long-stem cemented calcar hemi-arthroplasty | Left femur | 2 | Prophylactic stabilization with IMN |
| 6 | 39 | Multiple myeloma | Left femur prophylactic IMN | Right humerus | 15 | Humeral IMN for pathologic fracture |
IMN = intramedullary nail; ORIF = open reduction internal fixation.
In this series, a total of 12 patients (12.5%) had complications potentially of an embolic origin that have been associated with the use of intramedullary devices. All of these complications occurred in patients with femoral instrumentation (five of 72 [7.0%]) and none in patients with humeral instrumentation (zero of 24). Five of the 96 patients (5.21%) experienced O2 desaturation, two (2.08%) had a pulmonary embolism (PE), one (1.04%) had inverted T-waves on a postoperative electrocardiogram (cardiac isoenzymes negative), and four (4.17%) had symptomatic postoperative hypotension, but none had cardiac arrest. Patients who experienced only desaturation did not require a higher level of care, specific treatment, or prolonged hospital stay, but the patients with PE required specific anticoagulation treatment and prolonged hospitalization, the patient with electrocardiographic changes required cardiology consultation and additional monitoring, and the patients with symptomatic hypotension required additional resuscitation and monitoring in the intensive care unit.
Discussion
The current operative standard of care for disseminated malignant bone disease suggests stabilizing the entire bone to avoid the need for subsequent operative intervention [1–8, 10, 15, 27, 33–35, 39]. The aim of our study was to question this standard and to determine from a retrospective chart review study how often disease progression and reoperation occurred relative to the complications typically attributed to embolic phenomenon associated with long intramedullary devices.
Our study has limitations inherent to a retrospective study. First, there was no comparative group. Although the major reason cited in the literature to support protection of the entire bone is the potential for new lesions to develop, another consideration is that even uninvolved bone may be of poorer quality than normal bone, which may compromise the stability of internal fixation devices, particularly when plate and screw constructs are used [5, 9, 19, 29, 36–38]. Therefore, although the current study addresses the former issue, it was not possible to address the latter. Furthermore, because all but two of our patients received primarily intramedullary stabilization, we cannot determine what the failure rate would have been for plate and screw or shorter intramedullary stabilization. Power to examine for differences between subsets of patients also was limited by the relatively small numbers of patients. Followup was incomplete in many of the 263 originally identified patients, and these patients had to be excluded owing to missing followup data. This weakness is inherent in this patient population of terminally ill patients. The sizes of the original lesions were not reported because, in the majority of cases, the preoperative radiographs were unavailable for review.
Disease progression did not occur at the high rate we expected given that this is the main reason cited for protecting the entire long bone. At face value, even the 11.5% overall disease progression rate in this study might be used in support of recommending prophylactic stabilization with longer than standard implants. However, on closer examination of disease progression, most patients (approximately 8% overall) simply experienced progression of their original main lesion of concern (Type 1). Only a small number (2%) experienced progression of an originally identified, discretely separate lesion (Type 2), and only one of 96 patients (1%) experienced an entirely new, previously unrecognized lesion (Type 3). Each of the patients with Type 2 progression had myeloma. Patients with renal cell carcinoma, accounting for three of the 11 with progression (all Type 1), were disproportionately represented among the patients with progression. The high percentage of progression (three of 14 [21%]) in patients with renal carcinoma agrees with previous reports [21, 22].
With appropriate preoperative planning at the time of the index procedure, at least the eight patients with Type 1 progression and possibly the two patients with Type 2 progression would have been adequately protected by appropriate initial internal fixation without resorting to prophylactic fixation of the entire bone. However, conversely, at a minimum, the one patient with Type 3 progression would have been left unprotected and the two patients with Type 2 progression potentially would have been left unprotected by standard limited fixation that does not routinely protect the entire bone.
The failure rate overall for our patients was 6.25%, which is equal to or less than in many series of patients with internal fixation and/or arthroplasty for metastatic disease [9, 13, 14, 17, 19, 23, 31, 36]. The failure rates of approximately 8% for intramedullary nails overall and approximately 11% for femoral intramedullary nails are similar to those reported by others [9, 13, 31, 36–38]. The failure rate for arthroplasty in this series (0%) is lower than that reported in other series [23, 32, 36, 37].
Fortunately, although an occasional patient was documented to have experienced perioperative hypotension and desaturation, only two of our patients had PEs and none had cardiac arrest, coma, or death. Although the overall rate of these complications was 12.5%, the severity of these complications was low, and some may not have been directly attributable to embolic phenomena from the longer intramedullary devices [5, 18, 20, 25, 26, 28, 29, 34].
In a review of the relevant literature, the overall consensus has been that patients undergoing surgery to address pathologic fractures or impending pathologic fractures of the long bones resulting from metastatic disease, myeloma, or lymphoma should undergo protection of the entire bone with reconstruction-type intramedullary nails or long-stem cemented stems to avoid the need for further operative intervention resulting from progression [1–8, 10, 15, 27, 33–35, 39]. If there were no potential adverse consequence of doing so, this would be a moot point. However, there is a downside. Long-bone intramedullary instrumentation, particularly with cemented long intramedullary femoral stems, has been associated with numerous consequences, including hypotension, O2 desaturation, embolization, coma, or death [5, 18, 20, 25, 26, 28, 29, 34]. In many instances, these may be transient and minor alterations only documented on the anesthesia records, but in others, serious complications have occurred. Other means of prophylaxis to avoid local or distant disease progression are available and should be considered in patients with metastatic disease. Oncology consultation is important to consider chemotherapy, hormonal treatment, antiangiogenic agents, and immunotherapy where appropriate [5, 11, 12, 16, 24, 29, 30, 32]. Postoperative radiotherapy has been shown to reduce the incidence of hardware failure and reoperation, likely as a result of its effects in limiting disease progression [5]. Bisphosphonates also should be considered for appropriate patients [5].
We have documented a low but concerning rate of disease progression among operatively treated patients with femoral or humeral metastatic carcinoma, myeloma, or lymphoma. Based on only the 11.5% overall incidence of progression, one still could recommend routine stabilization of the entire bone. However, even in our series in which all operatively treated patients underwent treatment according to the principle of routine stabilization of the entire bone, 6.25% of patients experienced failure requiring revision surgery. Furthermore, disease progression occurred in our patients despite perioperative radiotherapy to the implanted region. Therefore, instrumentation of a lesser anatomic extent of the affected long bone would be hard to support based on these findings. However, subsequent development of new lesions was rare (1%), so the reason for routine protection would be more properly to protect from the more common occurrence of progression of the main treated lesion or other smaller lesions identified in the proximity. Furthermore, based on the disproportionate number of cases with renal carcinoma that showed local progression of the original lesion (three of eight Type 1 progressions, three of 14 or 21% of renal carcinomas in our series) and the more frequent progression of known smaller lesions in myeloma (two of 17, 12% in our series), protection of the entire bone is strongly warranted in patients with renal cancer and myeloma. The rate of embolic complications was low and of mild severity, so in this series, the risks of longer prophylactic stabilization do not appear to outweigh the benefits. Stronger recommendations await confirmation in a larger study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Upstate Medical University, East Syracuse, NY, USA.
References
- 1.Ampil FL, Sadasivan KK. Prophylactic and therapeutic fixation of weight-bearing long bones with metastatic cancer. South Med J. 2001;94:394–396. [PubMed] [Google Scholar]
- 2.Berman AT, Hermantin FU, Horowitz SM. Metastatic disease of the hip: evaluation and treatment. J Am Acad Orthop Surg. 1997;5:79–86. doi: 10.5435/00124635-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503–1516. doi: 10.2106/JBJS.H.00175. [DOI] [PubMed] [Google Scholar]
- 4.Cheng EY. Prospective quality of life research in bony metastatic disease. Clin Orthop Relat Res. 2003;415(suppl):S289–S297. doi: 10.1097/01.blo.0000093054.96273.20. [DOI] [PubMed] [Google Scholar]
- 5.Damron TA. Metastatic disease. In: Damron TA, editor. Orthopaedic Essentials: Oncology and Basic Science. Baltimore, MD, USA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 6.Damron TA, Palomino K, Roach S. Long Gamma nail stabilization of pathologic and impending pathologic femur fractures. The University of Pennsylvania Orthopaedic Journal. 1999;12:13–20. [Google Scholar]
- 7.Damron TA, Rock MG, Choudhury SN, Grabowski JJ, An KN. Biomechanical analysis of prophylactic fixation for middle third humeral impending pathologic fractures. Clin Orthop Relat Res. 1999;363:240–248. doi: 10.1097/00003086-199906000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Damron TA, Sim FH, Shives TC, An KN, Rock MG, Pritchard DJ. Intercalary spacers in the treatment of segmentally destructive diaphyseal humeral lesions in disseminated malignancies. Clin Orthop Relat Res. 1996;324:233–243. doi: 10.1097/00003086-199603000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Dijkstra S, Stapert J, Boxma H, Wiggers T. Treatment of pathological fractures of the humeral shaft due to bone metastases: a comparison of intramedullary locking nail and plate osteosynthesis with adjunctive bone cement. Eur J Surg Oncol. 1996;22:621–626. doi: 10.1016/S0748-7983(96)92450-6. [DOI] [PubMed] [Google Scholar]
- 10.Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones: a retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160:535–542. [PubMed] [Google Scholar]
- 11.Frassica FJ, Frassica DA. Evaluation and treatment of metastases to the humerus. Clin Orthop Relat Res. 2003;415(suppl):S212–S218. doi: 10.1097/01.blo.0000093052.96273.a7. [DOI] [PubMed] [Google Scholar]
- 12.Frassica FJ, Frassica DA. Metastatic bone disease of the humerus. J Am Acad Orthop Surg. 2003;11:282–288. doi: 10.5435/00124635-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons CE, Pope SJ, Murphy JP, Hall AJ. Femoral metastatic fractures treated with intramedullary nailing. Int Orthop. 2000;24:101–103. doi: 10.1007/s002640000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habermann ET, Lopez RA. Metastatic disease of bone and treatment of pathological fractures. Orthop Clin North Am. 1989;20:469–486. [PubMed] [Google Scholar]
- 15.Haentjens P, Casteleyn PP, Opdecam P. Evaluation of impending fractures and indications for prophylactic fixation of metastases in long bones: review of the literature. Acta Orthop Belg. 1993;59(suppl 1):6–11. [PubMed] [Google Scholar]
- 16.Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614–1627. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1614::AID-CNCR12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Healey JH, Brown HK. Complications of bone metastases: surgical management. Cancer. 2000;88(12 suppl):2940–2951. doi: 10.1002/1097-0142(20000615)88:12+<2940::AID-CNCR10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Herrenbruck T, Erickson EW, Damron TA, Heiner J. Adverse clinical events during cemented long-stem femoral arthroplasty. Clin Orthop Relat Res. 2002;395:154–163. doi: 10.1097/00003086-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Król R, Radomski S. Fixation of pathological fractures and impending long bone fractures in the course of neoplastic disease with the use of polymethylmethacrylate with added methotrexate. Ortop Traumatol Rehabil. 2003;5:319–326. [PubMed] [Google Scholar]
- 20.Leddy LR. Rationale for reduced pressure reaming when stabilizing actual or impending pathological femoral fractures: a review of the literature. Injury. 2010;41(suppl 2):S48–S50. doi: 10.1016/S0020-1383(10)70009-7. [DOI] [PubMed] [Google Scholar]
- 21.Les KA, Nicholas RW, Rougraff B, Wurtz D, Vogelzang NJ, Simon MA, Peabody TD. Local progression after operative treatment of metastatic kidney cancer. Clin Orthop Relat Res. 2001;390:206–211. doi: 10.1097/00003086-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Lin PP, Mirza AN, Lewis VO, Cannon CP, Tu SM, Tannir NM, Yasko AW. Patient survival after surgery for osseous metastases from renal cell carcinoma. J Bone Joint Surg Am. 2007;89:1794–1801. doi: 10.2106/JBJS.F.00603. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson J, Gustafson P. Surgery for metastatic lesions of the femur: good outcome after 245 operations in 216 patients. Injury. 2008;39:404–410. doi: 10.1016/j.injury.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Papagelopoulos PJ, Savvidou OD, Galanis EC, Mavrogenis AF, Jacofsky DJ, Frassica FJ, Sim FH. Advances and challenges in diagnosis and management of skeletal metastases. Orthopedics. 2006;29:609–620; quiz 621–622. [DOI] [PubMed]
- 25.Patterson BM, Healey JH, Cornell CN, Sharrock NE. Cardiac arrest during hip arthroplasty with a cemented long-stem component. J Bone Joint Surg Am. 1991;73:271–277. [PubMed] [Google Scholar]
- 26.Pitto RP, Koessler M, Kuehle JW. Comparison of fixation of the femoral component without cement and fixation with use of a bone-vacuum cementing technique for the prevention of fat embolism during total hip arthroplasty: a prospective randomized clinical trial. J Bone Joint Surg Am. 1999;81:831–843. doi: 10.2106/00004623-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard DJ. Metastatic bone disease: pathologic fractures of the humerus. Orthopedics. 1992;15:557–562. doi: 10.3928/0147-7447-19920501-07. [DOI] [PubMed] [Google Scholar]
- 28.Randall RL, Aoki SK, Olson PR, Bott SI. Complications of cemented long-stem hip arthroplasties in metastatic bone disease. Clin Orthop Relat Res. 2006;443:287–295. doi: 10.1097/01.blo.0000191270.50033.3a. [DOI] [PubMed] [Google Scholar]
- 29.Randall RL, Hoang BH. Musculoskeletal oncology. In: Skinner HB, editor. Current Diagnosis and Treatment in Orthopedics. 4. New York, NY, USA: McGraw-Hill; 2006. pp. 298–380. [Google Scholar]
- 30.Rock MG. Metastatic lesions of the humerus and the upper extremity. Instr Course Lect. 1992;41:329–333. [PubMed] [Google Scholar]
- 31.Sharma H, Bhagat S, McCaul J, Macdonald D, Rana B, Naik M. Intramedullary nailing for pathological femoral fractures. J Orthop Surg (Hong Kong). 2007;15:291–294. doi: 10.1177/230949900701500309. [DOI] [PubMed] [Google Scholar]
- 32.Swanson KC, Pritchard DJ, Sim FH. Surgical treatment of metastatic disease of the femur. J Am Acad Orthop Surg. 2000;8:56–65. doi: 10.5435/00124635-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Vandeweyer E, Gebhart M. Treatment of humeral pathological fractures by internal fixation and methylmetacrylate injection. Eur J Surg Oncol. 1997;23:238–242. doi: 10.1016/S0748-7983(97)92460-4. [DOI] [PubMed] [Google Scholar]
- 34.Ward WG, Spang J, Howe D. Metastatic disease of the femur: surgical management. Orthop Clin North Am. 2000;31:633–645. doi: 10.1016/S0030-5898(05)70181-4. [DOI] [PubMed] [Google Scholar]
- 35.Weber KL, Lewis VO, Randall RL, Lee AK, Springfield D. An approach to the management of the patient with metastatic bone disease. Instr Course Lect. 2004;53:663–676. [PubMed] [Google Scholar]
- 36.Wedin R. Surgical treatment for pathologic fracture. Acta Orthop Scand Suppl. 2001;72:1–29. [PubMed] [Google Scholar]
- 37.Wedin R, Bauer HC. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br. 2005;87:1653–1657. doi: 10.1302/0301-620X.87B12.16629. [DOI] [PubMed] [Google Scholar]
- 38.Wedin R, Bauer HC, Wersäll P. Failures after operation for skeletal metastatic lesions of long bones. Clin Orthop Relat Res. 1999;358:128–139. doi: 10.1097/00003086-199901000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Weikert DR, Schwartz HS. Intramedullary nailing for impending pathological subtrochanteric fractures. J Bone Joint Surg Br. 1991;73:668–670. doi: 10.1302/0301-620X.73B4.2071657. [DOI] [PubMed] [Google Scholar]