Abstract
Background
Durability of plate fixation is important in delayed union. Although locking plates result in stronger constructs, it is not known if locking affects the fatigue life of a plate. Two locking screws on either side of the nonunion could decrease working length and increase strain in the plate. However, the reinforcing effect of the locking head on the plate may compensate, so that it is unclear whether locking reduces fatigue life.
Questions/purposes
We determined whether locking screws, compression screws, and locking buttons reduce or increase the fatigue life of a plate.
Methods
We tested fatigue life of four constructs using an eight-hole locking plate in a segmental defect model: (1) all locking screws (Locked; n = 5); (2) all compression screws (Unlocked; n = 5); (3) six compression screws with two locking buttons in the central holes (Button; n = 6); and (4) six compression screws with two open central holes (Open; n = 6).
Results
The Button group had the longest fatigue life (1.3 million cycles). There was no difference between the Locked and Unlocked groups. All of the constructs failed by fracture of the plates through a screw hole adjacent to the defect.
Conclusions
Locking screws did not improve fatigue life, however a locking button increased the fatigue life of a locking plate in a segmental bone defect model.
Clinical Relevance
Locking buttons in holes adjacent to a defect may improve durability, which is important when delayed union is a possibility.
Introduction
Examples of situations that require long-term durability of internal plate fixation include large bulk allografts used for reconstruction after tumor resection and severe traumatic injuries. Nonunion of allograft to host bone is defined as lack of healing at 1 year and occurs 10% to 20% of the time [4, 21, 29, 34, 39, 40]. When a nonunion occurs there is frequently bone resorption of several millimeters at the bone-allograft junction (Fig. 1), adding additional stress to the plate [21, 40]. In such situations, where delayed healing is anticipated, the long-term durability of the internal fixation construct becomes important. Fixation integrity must be maintained, at least until bone grafting can be performed.
Fig. 1.
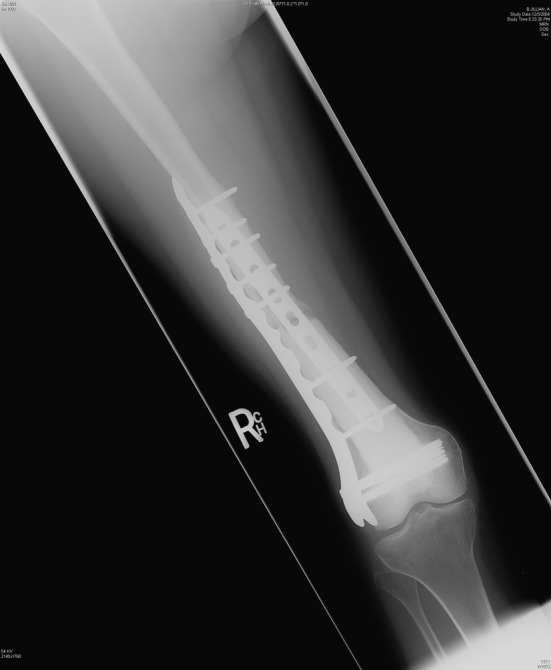
The radiograph shows bony resorption at an allograft-host bone nonunion. The long-term durability of the internal fixation is of critical importance in a nonunion.
Allografts traditionally have been fixed to the host bone with nonlocking (compression) plates. Some surgeons have begun to use locking plates, although it remains unknown if the altered biomechanics in locked plating provide any real advantage [11, 16, 19, 26, 28, 30, 33, 35]. Buecker et al. [5] compared allograft fixation with locking and conventional plating and found a higher union rate with locking plates. However, details regarding use of chemotherapy, the number of plates used, the number and combination of locking and compression screws, and whether allografts were filled with cement were not reported.
Previous in vitro biomechanical studies have compared locking plates and nonlocking plates in fracture models [13, 14, 22, 25, 31, 41]. In most of these studies the effect of locked plating on rigidity or strength was assessed, but the comparisons involved plates and/or screws of different designs, since the shapes of the locking and compression plates and screws are different in most systems. This makes interpretation of the data difficult. Studies involving fatigue testing have shown that bone is usually the weak link, with failure resulting from bone fractures or screw pullout [13, 14, 22]. This problem often is mitigated where allografts are used clinically by filling them with bone cement [17].
The data for locked plating are further complicated because locked plates can accommodate different combinations of locked screws, compression screws, locking buttons, or empty screw holes. The choice of screw configuration is characterized by competing tradeoffs. In a biomechanical analysis of axial and torsional rigidity, locking the near and far screws, relative to a comminuted fracture or osteotomy site, increased the rigidity [35]. However, having two screws on either side of the fracture could decrease the working length and increase the strain in the plate, predisposing to earlier plate failure [32]. On the other hand, the engagement of the threads on the head of a locking screw with the plate may reinforce the plate, compensating for the reduced working length, and thereby increasing fatigue life. In allograft reconstruction, there is the additional concern about placing a screw immediately adjacent the allograft-host bone junction for fear of inducing a stress riser in the allograft, which can lead to fracture. We presumed all locking screws would result in longer fatigue life compared with all compression screws, increasing the working length by leaving the holes open adjacent to the defect would increase fatigue life, and locking buttons in the two holes adjacent to the defect would result in the longest fatigue life.
We focused our attention on the holes on either side of a defect and determined: (1) the effect of locking screws, compression screws, locking buttons, and open holes on fatigue life of the plate; and (2) the mode of failure.
Materials and Methods
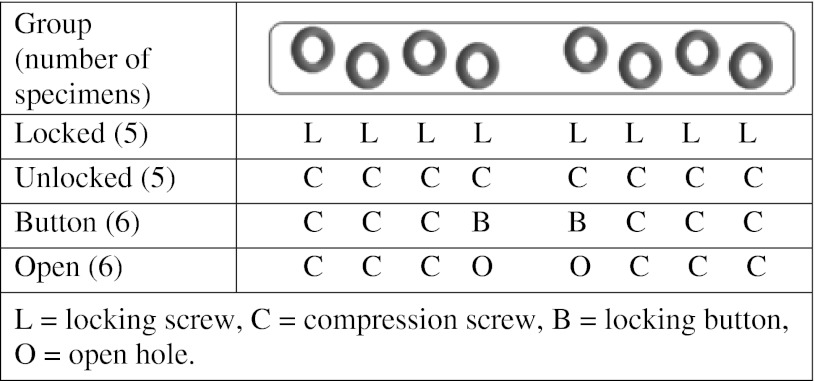
Fatigue life of eight-hole locking plates fixed with different combinations of locking screws, compression screws, locking buttons, and open holes was determined in vitro using a segmental defect model designed to simulate allograft nonunion. There were four experimental groups: (1) all locking screws (Locked; n = 5); (2) all compression screws (Unlocked; n = 5); (3) six compression screws with two locking buttons in the holes on either side of the defect (Button; n = 6); and (4) six compression screws with two open holes on either side of the defect (Open; n = 6) (Table 1).
Table 1.
Configuration of internal fixation of 4.5-mm locking plates in each group
Sample size estimates were based on pilot testing of compression screw-affixed plates, and calculated to yield 80% power of detecting a 50% difference in cycles to failure between groups. Cycles to failure in the pilot study were in the range of 500,000 to 1 million. One million cycles is equivalent to 1 year of walking [3, 27, 36–38]. Because allograft nonunions typically are not treated until 1 year postoperatively (the time to complete chemotherapy and allow a chance for healing), a 50% increase in cycles to failure beyond 1 million is a clinically relevant difference in construct durability that would allow for treatment of the nonunion before failure of the internal fixation.
We simulated a segmental bone defect model using fourth-generation Sawbones® composite femurs (Sawbones®, Vashon, WA, USA), which mimic the biomechanical properties of bone [6–9, 18, 23]. Each Sawbones® femur was cut at the level of the lesser trochanter with an electric saw. The intramedullary canal was filled with polymethylmethacrylate (PMMA) cement to maximize screw purchase as is practiced for allograft fixation in clinical practice [17]. The intramedullary canal was plugged at the intercondylar notch and cement was introduced with a cement gun, pressurized, and allowed to cure for 24 hours. The femurs then were placed in a custom-designed jig, which allowed for placement of an eight-hole, 4.5-mm by 157-mm stainless steel locking plate (PERI-LOC™ 4.5 mm Locking Plate, Smith & Nephew, Memphis, TN, USA) in a reproducible position on the anterior aspect of the femoral shaft. Each hole was drilled and tapped, and 4.5-mm screws were inserted in the prepared holes (in sequence, moving away from the center to the ends of the plate). The screws and locking buttons also were stainless steel and were tightened using a torque wrench to 35 inch-pounds. New plates, screws, and buttons were used for each test. The heads of the locking screws and the buttons were identical. The threads of the locking screws and compression screws also were identical (4.5-mm, Smith and Nephew PERI-LOC™ Screw Hole Filler; 4.5- × 40-mm, Smith & Nephew PERI-LOC™ Cortex Locking Screw; 4.5- × 40-mm, Smith & Nephew PERI-LOC™ Self-Tapping Cortex Screw).
A 10-mm gap was created directly under the middle, solid portion of the plate, midway between the innermost two holes, to simulate allograft nonunion (Fig. 2). The same jig for reproducible placement of the plates also was designed for reproducible placement of the osteotomy cuts. The 10-mm gap was designed to leave enough bone for purchase of the screws adjacent the defect, yet provide a gap sufficient to allow flexion of the plates without contact between the opposite cortices.
Fig. 2.

An open sample undergoing fatigue testing is shown.
Specimens were mounted in a custom jig that allowed unrestricted bending (flexion) of the plates when subjected to axial loading (Fig. 2) in a servohydraulic materials testing machine (MTS® model 810, MTS Corp, Eden Prairie, MN, USA). A sinusoidal fatigue loading profile that cycled the load from 20 N to 689 N compression at a rate of 5 Hz was used. As indicated in section A2.8.4 of ASTM F382-99(2008), a dynamic test frequency of 5 Hz facilitates completion of the test in a timely manner while not producing strain-sensitive affects in the bone plate’s material [1]. Pilot testing revealed the monotonic yield load of a plate affixed with compression screws was 1060 N. Specimens were cycled to a load equal to 65% of the yield load (689 N), which is equivalent to the load of a 70 kg person and because pilot data showed fatigue life was in the range of 500,000 to 1 million cycles with this load, which equates to the cycles seen in 6 to 12 months of walking [3, 27, 36–38]. One million cycles run-out also was chosen in accordance with sections A.2.3.1.6 and appendix X3.3 of ASTM F382-99 [1]. Each specimen was tested until failure or 1 million cycles. If a specimen did not fail after 1 million cycles, the load was increased to 75% of the yield load (795 N) with resumption of testing. Fatigue life was defined as the number of cycles until there was an abrupt change in displacement with an applied load. Load and displacement data were collected for 10 seconds logarithmically at 200 Hz. After fatigue testing, the screw torque was checked for all of the screws, and plates were visually inspected. All testing was performed using ASTM F382-99 as a nominal guide (Standard Specification and Test Method for Metallic Bone Plates, Reapproved 2003, ASTM International, formerly known as the American Society for Testing and Materials [ASTM]) [1, 15].
One way ANOVA was used was to evaluate fatigue life as a function of the fixation construct. Tukey’s Honestly Significant Difference post hoc tests were performed to evaluate differences between groups. The number of specimens in each group that required testing to more than 1 million cycles was compared with the chi-square test.
Results
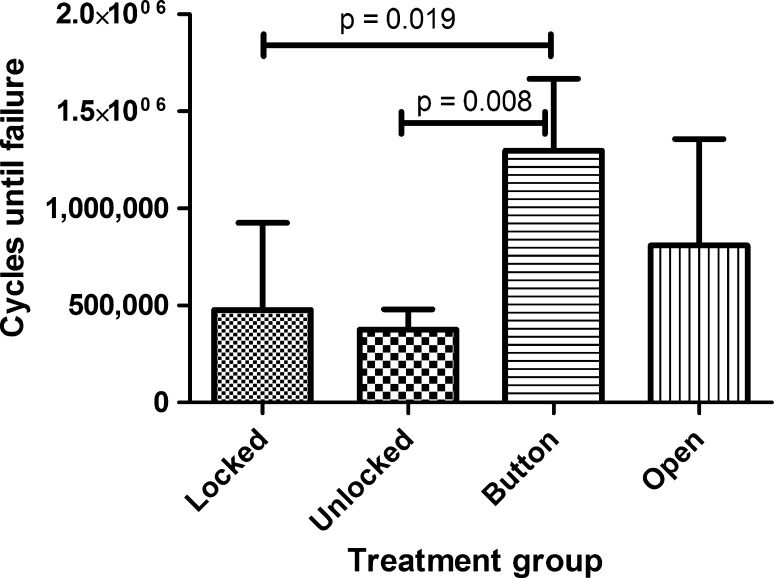
The number of cycles to failure differed (p = 0.006) among the four constructs (Fig. 3). The Button group had the longest fatigue life, 63% and 71% longer than the Locked and Unlocked groups (p = 0.019 and p = 0.008, respectively). The Locked, Unlocked, and Open groups were similar.
Fig. 3.
The fatigue life in cycles to failure is shown for each plate and screw configuration (mean ± 1 SD).
Five of the six specimens in the Button group achieved the 1-million-cycle run-out without failure, compared with three of six specimens in the Open group, one of five in the Locked group, and none of five in the Unlocked group (p = 0.029).
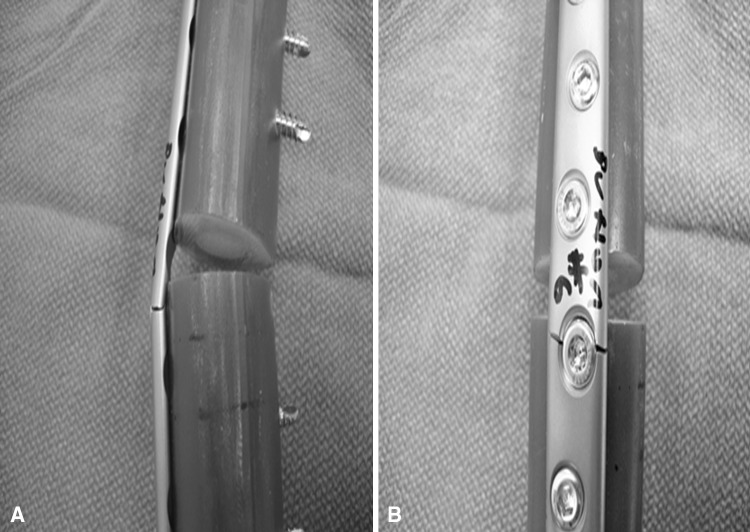
All of the constructs in every group failed by fracture of the plates through one of the two central screw holes, adjacent the defect (Fig. 4). Inspection of the failed constructs revealed no instances of screw loosening, breakage or pullout, or fractures in the Sawbones®.
Fig. 4A–B.
All plates failed through a screw hole adjacent to the defect as is seen on the (A) left and (B) right in these representative Button samples.
Discussion
In fractures where delayed union is a possibility, the durability of plate fixation is important: one would want the fracture to heal before the plate fatigued and/or failed. Although locking plates result in stronger constructs, it is unclear whether locking affects the fatigue life of a plate. Two locking screws on either side of the nonunion could decrease working length and increase strain in the plate. However, since the reinforcing effect of the locking head on the plate may compensate, it is unclear whether locking reduces or increases fatigue life. We therefore determined: (1) the effect of locking screws, compression screws, locking buttons, and open holes on fatigue life of the plate; and (2) the mode of failure.
We note the following limitations. First was the use of Sawbones® for testing, as opposed to human cadaver bone. However, we deliberately selected the PMMA-filled Sawbones® model to eliminate screw pullout and minimize weakness of the bone so we could specifically analyze the effect of screw-plate configuration on fixation fatigue life, without the confounding effect of cadaver bone failure. Sawbones® mechanical properties are comparable to those of human cadaver bones [6–9, 18, 23], although they likely would outperform human bone rendered osteopenic through aging, disease, or cancer treatment. Second, our specimens were cycled with axial loading as a first approximation to clinical loading of the femur, which in the diaphysis is primarily axial, with lesser amounts of bending and torsional forces [10]. In the presence of a segmental defect, this generated a bending moment in the plate. Third, our model was likely more severe than what typically occurs in vivo, as the 10-mm defect eliminated all contact between the bone ends. We deliberately selected a 10-mm defect to eliminate the confounding effects of contact between the far cortices of the Sawbones® during bending of the plate, yielding a rigorous model, yet one that would fail and allow meaningful comparisons in a reasonable time. Fourth, we tested a limited number of screw-plate combinations. We focused on the central holes as they are associated with the highest stresses and therefore are most at risk for failure with repeated loading. Finally, we tested single-plate constructs because single-plate constructs are commonly used clinically and are most at risk of failure. Two-plate constructs are used by some surgeons. We would expect similar results with two-plate constructs, however, with protracted fatigue lives.
We found the fatigue lives of plates fully fixed with locked or nonlocked screws to be comparable. The similar fatigue life of the locked and nonlocked constructs suggests screw type does not inherently influence plate fatigue behavior. We originally presumed locking screws would increase fatigue life by reinforcing the plate. However, the comparable fatigue life of locked and nonlocked constructs suggests working length (the distance between the innermost screws) and plate cross-sectional geometry are more important. In contrast to previous studies [13, 14, 22, 25, 31, 41], we found no difference between the fatigue life of locked and nonlocked constructs in cyclic axial loading. Previous biomechanical studies generally show locked plates have a greater fatigue life than nonlocked constructs. In these cases, however, testing typically was performed in cadaver bones and failure most often occurred owing to screw pullout. We used composite Sawbones®, which are comparable biomechanically to cortical bone [6–9, 18, 23], and then filled them with PMMA as is practiced clinically. This effectively eliminated pullout as a failure mechanism, yielding a more robust model for testing plate durability.
Leaving the centermost screw holes empty increased the working length of the plate and the threaded screw holes dominated the geometry. In a study of the effect of working length on fatigue life of locking plates, increased working length increased fatigue life of titanium plates but not stainless steel plates [20]. The plates used in this study also were stainless steel, however, testing in that study was performed using peak loads slightly greater than the elastic limit, whereas we used loads that were 65% of the elastic limit. All the plates in that study [19] and ours broke through the screw holes closest to the defect, consistent with two additional studies that showed the greatest amount of stress in the plate is at the screw holes nearest the defect [2, 24].
We found improved fatigue life when locking buttons were inserted to fill the holes adjacent to the defect. This suggests the buttons mitigated the stress riser associated with the open holes, and omission of the screws increased the working length of the plate. The combination of these reduced the local stresses at the screw holes adjacent to the defect and increased fatigue life. These results are consistent with those of a previous study using locking buttons in one-third tubular plates [2]. In another study, locking buttons did not increase fatigue life [11]. However, in that study the buttons were designed to fill the remaining portion of a combination hole designed for a locking screw or compression screw [12]. In combination holes, the remaining hole is eccentric and the button is not threaded, therefore it does not reinforce the plate effectively.
When the durability of the internal fixation is a primary concern, we suggest using a plate with locking capabilities, and filling the screw holes nearest the defect or osteotomy with locking buttons, which increases the fatigue life and minimizes the effect of screws in the bone adjacent to the defect. Ultimately, however, the best alternative may be specially designed plates with large spans between the centermost holes, which would at once have long working lengths, no hole-induced stress risers, and reduced risk of fracture through the host or allograft bone.
Acknowledgments
We thank Allison Biercevicz BS and Ryan Rich for assistance with testing the samples.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
This work was performed in the Department of Orthopaedics, The Warren Alpert Medical School of Brown University and Rhode Island Hospital; Providence, RI, USA.
References
- 1.ASTM International. ASTM Standard F382-99 Standard Specification and Test Method for Metallic Bone Plates. ASTM International, West Conshohocken, PA, 2008. DOI: 10.1520/F0382-99R08E01. Available at: http://www.astm.org/. Accessed October 11, 2012.
- 2.Bellapianta J, Dow K, Pallotta NA, Hospodar PP, Uhl RL, Ledet EH. Threaded screw head inserts improve locking plate biomechanical properties. J Orthop Trauma. 2011;25:65–71. doi: 10.1097/BOT.0b013e3181dc56b1. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34:859–871. doi: 10.1016/S0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 4.Berrey BH, Jr, Lord CF, Gebhardt MC, Mankin HJ. Fractures of allografts: frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;72:825–833. [PubMed] [Google Scholar]
- 5.Buecker PJ, Berenstein M, Gebhardt MC, Hornicek FJ, Mankin HJ. Locking versus standard plates for allograft fixation after tumor resection in children and adolescents. J Pediatr Orthop. 2006;26:680–685. doi: 10.1097/01.bpo.0000230333.73286.06. [DOI] [PubMed] [Google Scholar]
- 6.Chong AC, Friis EA, Ballard GP, Czuwala PJ, Cooke FW. Fatigue performance of composite analogue femur constructs under high activity loading. Ann Biomed Eng. 2007;35:1196–1205. doi: 10.1007/s10439-007-9284-z. [DOI] [PubMed] [Google Scholar]
- 7.Chong AC, Miller F, Buxton M, Friis EA. Fracture toughness and fatigue crack propagation rate of short fiber reinforced epoxy composites for analogue cortical bone. J Biomech Eng. 2007;129:487–493. doi: 10.1115/1.2746369. [DOI] [PubMed] [Google Scholar]
- 8.Cristofolini L, Viceconti M. Mechanical validation of whole bone composite tibia models. J Biomech. 2000;33:279–288. doi: 10.1016/S0021-9290(99)00186-4. [DOI] [PubMed] [Google Scholar]
- 9.Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29:525–535. doi: 10.1016/0021-9290(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 10.Duda GN, Schneider E, Chao EY. Internal forces and moments in the femur during walking. J Biomech. 1997;30:933–941. doi: 10.1016/S0021-9290(97)00057-2. [DOI] [PubMed] [Google Scholar]
- 11.Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18:488–493. doi: 10.1097/00005131-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Firoozabadi R, McDonald E, Nguyen TQ, Buckley JM, Kandemir U. Does plugging unused combination screw holes improve the fatigue life of fixation with locking plates in comminuted supracondylar fractures of the femur? J Bone Joint Surg Br. 2012;94:241–248. doi: 10.1302/0301-620X.94B2.27440. [DOI] [PubMed] [Google Scholar]
- 13.Fulkerson E, Egol KA, Kubiak EN, Liporace F, Kummer FJ, Koval KJ. Fixation of diaphyseal fractures with a segmental defect: a biomechanical comparison of locked and conventional plating techniques. J Trauma. 2006;60:830–835. doi: 10.1097/01.ta.0000195462.53525.0c. [DOI] [PubMed] [Google Scholar]
- 14.Gardner MJ, Brophy RH, Campbell D, Mahajan A, Wright TM, Helfet DL, Lorich DG. The mechanical behavior of locking compression plates compared with dynamic compression plates in a cadaver radius model. J Orthop Trauma. 2005;19:597–603. doi: 10.1097/01.bot.0000174033.30054.5f. [DOI] [PubMed] [Google Scholar]
- 15.Gardner MJ, Silva MJ, Krieg JC. Biomechanical testing of fracture fixation constructs: variability, validity, and clinical applicability. J Am Acad Orthop Surg. 2012;20:86–93. doi: 10.5435/JAAOS-20-02-086. [DOI] [PubMed] [Google Scholar]
- 16.Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(suppl 2):B63–B76. doi: 10.1016/j.injury.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Gerrand CH, Griffin AM, Davis AM, Gross AE, Bell RS, Wunder JS. Large segment allograft survival is improved with intramedullary cement. J Surg Oncol. 2003;84:198–208. doi: 10.1002/jso.10316. [DOI] [PubMed] [Google Scholar]
- 18.Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34:773–781. doi: 10.1016/S0021-9290(01)00015-X. [DOI] [PubMed] [Google Scholar]
- 19.Hertel R, Eijer H, Meisser A, Hauke C, Perren SM. Biomechanical and biological considerations relating to the clinical use of the Point Contact-Fixator: evaluation of the device handling test in the treatment of diaphyseal fractures of the radius and/or ulna. Injury. 2001;32(suppl 2):B10–B14. doi: 10.1016/S0020-1383(01)00121-8. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmeier KL, Hofmann GO, Muckley T. Choosing a proper working length can improve the lifespan of locked plates: a biomechanical study. Clin Biomech (Bristol, Avon). 2011;26:405–409. [DOI] [PubMed]
- 21.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98. doi: 10.1097/00003086-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Jazrawi LM, Kummer FJ, Simon JA, Bai B, Hunt SA, Egol KA, Koval KJ. New technique for treatment of unstable distal femur fractures by locked double-plating: case report and biomechanical evaluation. J Trauma. 2000;48:87–92. doi: 10.1097/00005373-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser MM, Wessel LM, Zachert G, Stratmann C, Eggert R, Gros N, Schulze-Hessing M, Kienast B, Rapp M. Biomechanical analysis of a synthetic femur spiral fracture model: influence of different materials on the stiffness in flexible intramedullary nailing. Clin Biomech (Bristol, Avon). 2011;26:592–597. [DOI] [PubMed]
- 24.Kanchanomai C, Muanjan P, Phiphobmongkol V. Stiffness and endurance of a locking compression plate fixed on fractured femur. J Appl Biomech. 2010;26:10–16. doi: 10.1123/jab.26.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Koval KJ, Hoehl JJ, Kummer FJ, Simon JA. Distal femoral fixation: a biomechanical comparison of the standard condylar buttress plate, a locked buttress plate, and the 95-degree blade plate. J Orthop Trauma. 1997;11:521–524. doi: 10.1097/00005131-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88(suppl 4):189–200. doi: 10.2106/JBJS.F.00703. [DOI] [PubMed] [Google Scholar]
- 27.Morlock M, Schneider E, Bluhm A, Vollmer M, Bergmann G, Muller V, Honl M. Duration and frequency of every day activities in total hip patients. J Biomech. 2001;34:873–881. doi: 10.1016/S0021-9290(01)00035-5. [DOI] [PubMed] [Google Scholar]
- 28.Niemeyer P, Sudkamp NP. Principles and clinical application of the locking compression plate (LCP) Acta Chir Orthop Traumatol Cech. 2006;73:221–228. [PubMed] [Google Scholar]
- 29.Ogilvie CM, Crawford EA, Hosalkar HS, King JJ, Lackman RD. Long-term results for limb salvage with osteoarticular allograft reconstruction. Clin Orthop Relat Res. 2009;467:2685–2690. doi: 10.1007/s11999-009-0726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perren SM. Evolution and rationale of locked internal fixator technology: introductory remarks. Injury. 2001;32(suppl 2):B3–B9. doi: 10.1016/S0020-1383(01)00120-6. [DOI] [PubMed] [Google Scholar]
- 31.Seide K, Triebe J, Faschingbauer M, Schulz AP, Puschel K, Mehrtens G, Jurgens Ch. Locked vs. unlocked plate osteosynthesis of the proximal humerus: a biomechanical study. Clin Biomech (Bristol, Avon). 2007;22:176–182. [DOI] [PubMed]
- 32.Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. J Bone Joint Surg Am. 2007;89:2298–2307. doi: 10.2106/JBJS.F.00615. [DOI] [PubMed] [Google Scholar]
- 33.Sommer C, Gautier E, Muller M, Helfet DL, Wagner M. First clinical results of the Locking Compression Plate (LCP) Injury. 2003;34(suppl 2):B43–B54. doi: 10.1016/j.injury.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Sorger JI, Hornicek FJ, Zavatta M, Menzner JP, Gebhardt MC, Tomford WW, Mankin HJ. Allograft fractures revisited. Clin Orthop Relat Res. 2001;382:66–74. doi: 10.1097/00003086-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Stoffel K, Dieter U, Stachowiak G, Gachter A, Kuster MS. Biomechanical testing of the LCP: how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11–B19. doi: 10.1016/j.injury.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura H, Katagiri H, Yonekawa M, Sato K, Yamamura S, Iwata H. Walking ability and activities of daily living after limb salvage operations for malignant bone and soft-tissue tumors of the lower limbs. Arch Orthop Trauma Surg. 2001;121:131–134. doi: 10.1007/s004020000192. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SJ, Walker PS, Perry JS, Cannon SR, Woledge R. The forces in the distal femur and the knee during walking and other activities measured by telemetry. J Arthroplasty. 1998;13:428–437. doi: 10.1016/S0883-5403(98)90009-2. [DOI] [PubMed] [Google Scholar]
- 38.Taylor WR, Heller MO, Bergmann G, Duda GN. Tibio-femoral loading during human gait and stair climbing. J Orthop Res. 2004;22:625–632. doi: 10.1016/j.orthres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Toy PC, White JR, Scarborough MT, Enneking WF, Gibbs CP. Distal femoral osteoarticular allografts: long-term survival, but frequent complications. Clin Orthop Relat Res. 2010;468:2914–2923. doi: 10.1007/s11999-010-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vander Griend RA. The effect of internal fixation on the healing of large allografts. J Bone Joint Surg Am. 1994;76:657–663. [DOI] [PubMed]
- 41.Walsh S, Reindl R, Harvey E, Berry G, Beckman L, Steffen T. Biomechanical comparison of a unique locking plate versus a standard plate for internal fixation of proximal humerus fractures in a cadaveric model. Clin Biomech (Bristol, Avon). 2006;21:1027–1031. [DOI] [PubMed]