Abstract
Background
The toxicity of anticancer agents and the difficulty in delivering drugs selectively to tumor cells pose a challenge in overcoming multidrug resistance (MDR). Recently, nanotechnology has emerged as a powerful tool in addressing some of the barriers to drug delivery, including MDR in cancer, by utilizing alternate routes of cellular entry and targeted delivery of drugs and genes. However, it is unclear whether doxorubicin (Dox) can be delivered by nanotechnologic approaches.
Questions/Purposes
We asked whether (1) Dox-loaded lipid-functionalized dextran-based biocompatible nanoparticles (Dox/NP) can reverse MDR, (2) Dox/NP has more potent cytotoxic effect on MDR tumors than poly(ethylene glycol)-modified liposomal Dox (PLD), and (3) multidrug resistance protein 1 (MDR1) small interfering RNA loaded in these nanoparticles (siMDR1/NP) can modulate MDR.
Methods
To create stable Dox/NP and siMDR1/NP, we used two different lipid-modified dextran derivatives. The effect of Dox or Dox/NP was tested on drug-sensitive osteosarcoma (KHOS) and ovarian cancer (SKOV-3) cell cultures in triplicate and their respective MDR counterparts KHOSR2 and SKOV-3TR in triplicate. We determined the effects on drug retention, transfection efficacy of siMDR1/NP, and P-glycoprotein expression and the antiproliferative effect between Dox/NP and PLD in MDR tumor cells.
Results
Fluorescence microscopy revealed efficient uptake of the Dox/NP and fluorescently tagged siMDR1/NP. Dox/NP showed five- to 10-fold higher antiproliferative activity at the 50% inhibitory concentration than free Dox in tumor cells. Dox/NP showed twofold higher activity than PLD in MDR tumor cells. siMDR1/NP (100 nM) suppressed P-glycoprotein expression in KHOSR2.
Conclusions
Dextran-lipid nanoparticles are a promising platform for delivering Dox and siRNAs.
Clinical Relevance
Biocompatible dextran-based nanoparticles that are directly translatable to clinical medicine may lead to new potential therapeutics for reversing MDR in patients with cancer.
Introduction
The treatment of many cancers using chemotherapy still remains a great challenge. Although the cure rate of patients with diseases such as localized osteosarcoma (OS) and ovarian cancers has improved due to the introduction of combinatorial chemotherapy [6, 34], a large proportion of patients still respond poorly to drug treatment and have a high risk of local tumor relapse and metastasis to distant sites, even after multimodality treatments [2, 14, 56].
One of the major causes of the hampered efficacy of chemotherapy is the eventual development of multidrug resistance (MDR). MDR is considered one of the most important hindrances of curative therapies in humans [41]. Cancer cells almost invariably emerge to become resistant to a variety of anticancer drugs by employing a host of different mechanisms [15]. MDR in cancer is frequently associated with the overexpression of active transporters that act as drug efflux pumps. It has been well documented that P-glycoprotein (P-gp), the gene product of the multidrug resistance protein 1 gene (MDR1, ABCB1), has been a major component of the drug efflux pump that prevents intracellular accumulation of anthracycline drugs [13]. Although reversal of MDR has been reported with several agents, unfortunately, they were relatively nonspecific and had low potency and/or adverse toxicity [4, 21, 24, 43, 45]. Consequently, the development of alternative therapeutic strategies employing more efficient delivery systems is imperative to overcome MDR.
On the other hand, silencing of MDR1 mRNA expression using small interfering RNA (siRNA) for MDR1 (siMDR1) is one of the most powerful approaches for reversing MDR [32]. However, RNA interference mechanisms also confront several challenges that hinder their clinical translation. For instance, the cellular uptake of siRNA is limited due to its poor membrane permeability, and in general, siRNAs are also prone to rapid degradation by nucleases [35]. Although commercially available transfection agents effectively protect and deliver siRNA in vitro, they have limited clinical utility because of their inherent toxicities. Thus, a more safe and effective delivery system for clinical use of siRNAs is warranted.
Recently, nanotechnology has gained tremendous interest for overcoming the limitations of conventional drug and gene delivery problems [9, 54]. Among the several reported systems, polymer-conjugated drugs and polymeric nanoparticle-based advanced delivery systems are gaining more attention because of their superior and distinct advantages, including the ability to overcome MDR in cancers [31]. Furthermore, polymeric nanoparticles can not only be easily tailored to stably encapsulate drug payloads or conveniently self-assembled into nanoparticles but can also be designed with spatial and temporal control based on the nature and choice of the biodegradable polymer used for constructing self-disintegrating nanosystems [5, 10, 52, 55]. Among the commercially available natural polymers, dextran and its derivatives have been widely studied and used as polymeric drug carriers due to their biocompatibility and biodegradability [49].
We therefore explored the role of lipid-functionalized dextran-based nanosystems to overcome MDR. We asked whether (1) doxorubicin (Dox)-loaded lipid-functionalized dextran-based biocompatible nanoparticles (Dox/NP) can reverse MDR, (2) Dox/NP shows more potent cytotoxic effect on MDR tumors than poly(ethylene glycol) (PEG)-modified liposomal Dox (PLD), and (3) MDR1 siRNA-loaded nanoparticles (siMDR1/NP) can modulate MDR.
Materials and Methods
To explore whether nanoparticles can overcome MDR in cancer, we first tested whether our formulation of Dox and siMDR1 using a lipid-modified dextran derivative resulted in formation of stable nanoparticles (Dox/NP and siMDR1/NP, respectively) (Fig. 1). We analyzed subcellular distribution of Dox in OS (KHOS, KHOSR2) and ovarian cancer cells (SKOV-3, SKOV-3TR) treated with Dox alone or Dox/NP using fluorescent microscopy and Dox and Dox/NP cytotoxicity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays. Then, we compared the antiproliferative effect of Dox/NP and PLD in MDR tumor cells. Finally, we assessed the transfection efficacy of AF488-tagged siMDR1/NP on KHOSR2 cells and evaluated the effect of siMDR1/NP on P-gp expression. Each cell condition was cultured in triplicate and each experiment was repeated at least three times.
Fig. 1.
A flow diagram shows the experiments we performed using Dox/NP and siMDR1/NP.
We obtained dextran (MW ~ 40 kDa) and other reagents from Sigma-Aldrich Chemical Co (St Louis, MO, USA) and Acros Organics (Parsipanny, NJ, USA) and used them as obtained. We synthesized the clickable dextran, thiol-modified dextran, and the PEG-modified dextran according to a previously reported procedure [1]. The dextran acrylate and lipid-modified dextran were formulated based on the published procedure of Susa et al. [48].
The Dox/NP were formulated based on the procedure of Abeylath and Amiji [1]. Briefly, the drug-loaded nanoparticles were prepared by self-assembly of dextran blocks. Dextran-C6 lipid (19.5 mg), dextran-thiol (15 mg), and dextran-PEG (15 mg) were synthesized separately and then dissolved together in 4 mL water; Dox (0.5 mg) in water (1 mL) was added to it and vortexed for 5 minutes. The mixture was lyophilized and the fluffy powder was dispersed in 5 mL deionized water. The particle size and zeta potentials of the self-assembled nanoparticles were measured using a dynamic light scattering (DLS) instrument as described below.
The siMDR1/NP were formulated based on the procedure of Susa et al. [48]. Briefly, stock solutions of 5 mg/mL PEG-thiol, octylamine-modified dextran, and thiol-dextran were prepared in deionized water. A stock solution of 2 mM siRNA solution was prepared using RNase-free water. A 12-mL solution of the stock siMDR1 was taken in a tube and 40 μL thiol-dextran was first added and mixed well using a vortex shaker. It was then incubated for 5 minutes. To this mixture, 40 μL dextran-octylamine was then added and incubated for another 5 minutes. Finally, 40 μL PEG-thiol was added and incubated for another 15 minutes to form the hydrophilic shell of the nanoparticles. The nanoparticles were lyophilized to yield a fluffy powder. This method of sequential addition and lyophilization was used for the stable entrapment of the siRNA in the dextran hydrogel network. siRNAs at concentrations of 10, 30, 50, and 100 nM were prepared by adjusting the stock solution of siRNA while preparing the dextran nanoparticles. The particle size and zeta potentials of the nanosystems so formed were measured using a DLS instrument as described below.
We characterized the Dox/NP and siMDR1/NP for size and size distribution using a electrophoretic DLS instrument (Zetasizer® 3000HS; Malvern Instruments Ltd, Malvern, UK) at a 90° fixed angle and at 25°C. For zeta potentials, default parameters of dielectric constant, refractive index, and viscosity of water were used based on the electrophoretic mobility of the nanoparticles. For the zeta potential measurements, a specially designed electrophoretic cell was used and the average surface charge was measured.
We obtained the human OS cell lines KHOS and the MDR (P-gp)-expressing cell line KHOSR2 from the National Hellenic Research Foundation (Athens, Greece) and the human ovarian cancer cell line SKOV-3 from the American Type Culture Collection (Manassas, VA, USA). The MDR SKOV-3TR was established as reported previously [25]. All cell lines were cultured in RPMI 1640 medium with 10% fetal bovine serum and 100 U/mL penicillin incubated at 37°C in 5% CO2-95% air atmosphere. MDR cell lines were continuously cultured in 0.1 μM Dox (KHOSR2) or 0.1 μM taxol (SKOV-3TR).
We obtained Dox and PLD (Doxil®; Janssen Biotech Inc, Horsham, PA, USA) from Massachusetts General Hospital as unused residual clinical materials. The anti-P-gp1 monoclonal antibody C219 was purchased from Signet (Dedham, MA, USA). The human β-actin monoclonal antibody and MTT reagents were purchased from Sigma-Aldrich. The siRNA sequence targeting ABCB1 (Genebank Accession Number NM_00927) corresponded to coding regions of these genes. siMDR1 (sense strand, 5′-GACCAUAAAUGUAAGGUUU-3′ and 5′-AAACCUUACAUUUAUGGUC-3′) was purchased from Sigma-Aldrich. Negative control siRNA (sense strand, 5′-GGGUAUCGACGAUUACAAAUU-3′) was obtained from Qiagen (Valencia, CA, USA). Fluorescently tagged siRNA (sense strand, 5′-GCUUAACACCCGACUUACAUUTT-3′) for MDR1 with a 3′ AF488 modification was synthesized and provided by Qiagen. Although sequences were different between tagged and untagged siMDR1 purchased from different companies, they functioned well.
For cellular Dox uptake studies, we seeded KHOS, KHOSR2, SKOV-3, and SKOV-3TR cells (n = 3 wells/cell line; 1 × 105 cells/well) in 12-well plates and incubated them for 24 hours. After the incubation, either Dox alone or Dox/NP was added and the cells were incubated for another 3 hours. To stain nuclei, 1 μg/mL Hoechst 33342 (Life Technologies Corp, Grand Island, NY, USA) was used. After incubation, the cells were visualized using a Nikon Eclipse Ti-U fluorescence microscope (Nikon Instruments, Inc, Melville, NY, USA) equipped with a SPOT RT™ digital camera (Diagnostic Instrument, Inc, Sterling Heights, MI, USA).
To assess the cellular uptake of siMDR1, we seeded KHOSR2 cells (n = 3 wells/condition; 1 × 105 cells/well) in 12-well plates and incubated them with 100 nM AF488-tagged siMDR1/NP over 24 hours. AF488-tagged siMDR1 plus Lipofectamine™ RNAiMAX Transfection Reagent (Life Technologies Corp) and AF488-tagged siMDR1 alone were used as positive and negative controls, respectively. After 2-, 4-, and 24-hour incubation, images were acquired by fluorescence microscopy.
We assayed in vitro cytotoxicity using the MTT assay. Briefly, we seeded KHOS, KHOSR2, SKOV-3, and SKOV-3TR cells (n = 3 wells/concentration; 1500 cells/well) (Dox versus Dox/NP) or KHOSR2 and SKOV-3TR cells (n = 3 wells/concentration; 1500 cells/well) (Dox/NP versus PLD) in 96-well plates. Cells were cultured in medium with 0.001, 0.01, 0.03, 0.06, 0.1, 0.3, 0.6, 1, 3, 6, and 10 μM concentrations of Dox alone, Dox/NP, or PLD. After culturing for 5 days, 10 μL MTT (5 mg/mL in phosphate-buffered saline) was added to each well and the cells were incubated for 4 hours. After dissolving the resulting formazan product with acid isopropanol, the absorbance was analyzed on a SpectraMax® Microplate Spectrophotometer (Molecular Devices LLC, Sunnyvale, CA, USA) at a wavelength of 490 nm [47, 49, 59].
To evaluate the effect of siMDR1/NP on P-gp expression, we seeded KHOSR2 (n = 2 wells/condition; 1 × 105 cells/well) in 12-well plates and incubated them with 100 nM AF488-tagged siMDR1/NP over 48 hours. AF488-tagged siMDR1 plus Lipofectamine™ RNAiMAX Transfection Reagent, siRNA plus Lipofectamine™ RNAiMAX Transfection Reagent, nanoparticles only, and cells only were used as controls. P-gp was analyzed in total cell lysates. Protein lysates from cells were generated through lysis with 1× radioimmunoprecipitation assay lysis buffer (Upstate Biotechnology, Charlottesville, VA, USA). The protein concentration was determined by a bovine serum albumin assay (Bio-Rad Laboratories Inc, Hercules, CA, USA), using a Beckman DU-640 spectrophotometer (Beckman Instruments Inc, Columbia, MD, USA). For Western blotting, 25 μg of total protein was processed on NuPAGE® 4–12% Bis-Tris Gel (Life Technologies Corp) and transferred to pure nitrocellulose membrane (Bio-Rad). After an overnight incubation with primary antibodies in Tris-buffered saline (pH 7.4, with 0.1% Tween® 20), the signal was generated through incubation with relevant secondary antibodies (Bio-Rad) incubated in Tris-buffered saline (pH 7.4, with 5% nonfat milk and 0.1% Tween® 20) at 1:2000 dilution for 1 hour at room temperature. Blots were detected using SuperSignal® West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc, Waltham, MA, USA).
We report continuous data as a mean ± SD. We checked the normality of the data using the Kolmogorov-Smirnov test and found the null hypothesis of homoscedasticity was rejected in some data. We therefore determined differences in the antiproliferative effect of each cell line (KHOS, KHOSR2, SKOV-3, SKOV-3TR) cultured with Dox alone and Dox/NP using a Welch t-test. Differences in the antiproliferative effect in MDR tumor cells (KHOSR2, SKOV-3TR) treated with Dox/NP and PLD were also assessed by Welch t-test.
Results
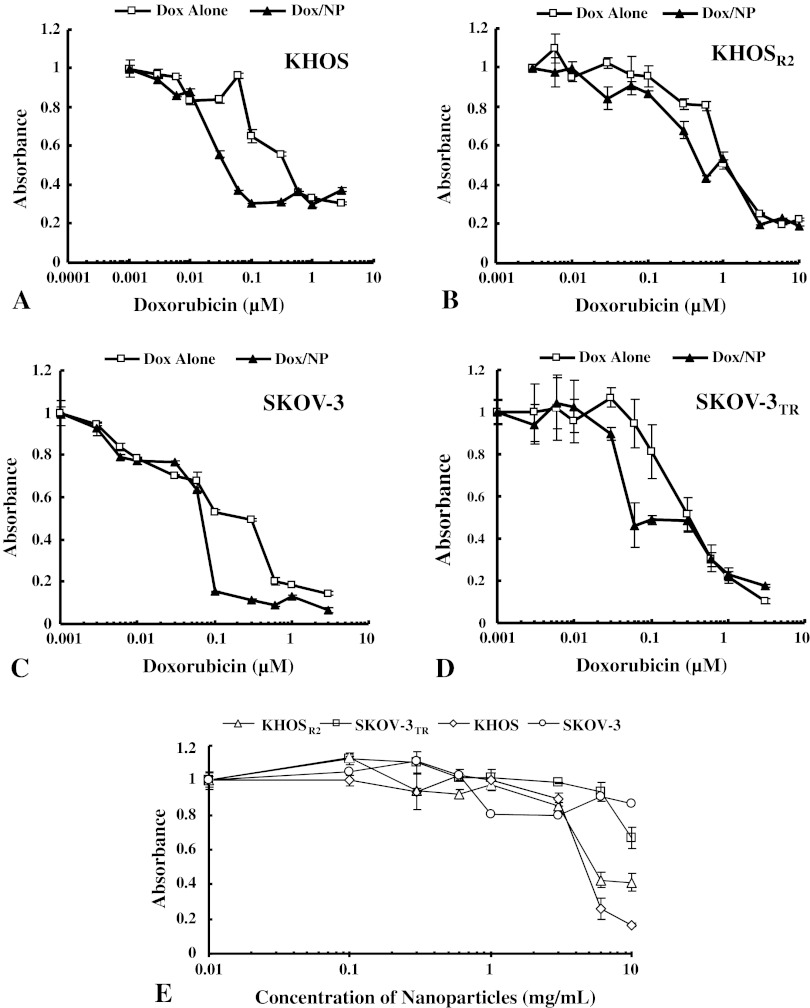
The formulation of Dox and siMDR1 using a lipid-dextran derivative resulted in formation of stable nanoparticles after lyophilization. The resuspended nanoparticles in deionized water revealed mean particle size and zeta potentials of 171 ± 2 nm and −1.21 ± 1.31 mV, respectively, for Dox/NP and 101 ± 3 nm and −0.22 ± 2.21 mV, respectively, for siMDR1/NP. Dox by itself possessed an intrinsic red fluorescence, and cell nuclei were stained with a nuclear-specific dye emitting blue fluorescence. When MDR cells were treated with Dox alone, the drug was primarily concentrated in the cytoplasm, and a very low level of fluorescence was observed in the nucleus (Fig. 2). On the other hand, MDR cells treated with Dox/NP showed increased fluorescence in the nucleus and cytoplasm. The subcellular distribution mimicked that of the drug-sensitive variants treated with Dox alone (Fig. 2). After treatment with Dox/NP, mean antiproliferative activity was increased in both OS cells (KHOS: Fig. 3A; KHOSR2: Fig. 3B) and ovarian cancer cells (SKOV-3: Fig. 3C; SKOV-3TR: Fig. 3D) in a dose-dependent manner. Dox/NP produced higher mean antiproliferative activity than Dox alone in OS cells (KHOS: p = 0.001 at 0.06 μM, p = 0.044 at 0.1 μM, p = 0.006 at 0.3 μM; KHOSR2: p = 0.001 at 0.6 μM) and ovarian cancer cells (SKOV-3: p = 0.005 at 0.1 μM, p = 0.005 at 0.3 μM). Dox/NP showed a five- to 10-fold higher activity at the 50% inhibitory concentration (IC50) compared with the free form of Dox. Dextran-lipid nanoparticles were not cytotoxic by themselves at the dose used in this study (Fig. 3E).
Fig. 2.
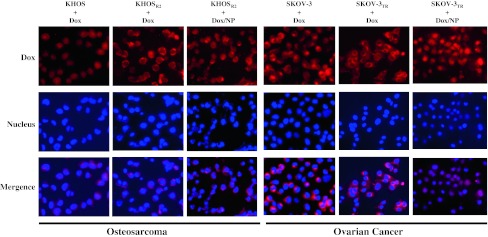
Images show subcellular distribution of Dox in drug-sensitive OS and ovarian cancer cells (KHOS and SKOV-3) and their MDR counterparts (KHOSR2 and SKOV-3TR) under fluorescent microscopy. An increase in fluorescence was observed in the MDR cancer cells treated with Dox/NP.
Fig. 3A–E.
The antiproliferative effects of Dox alone and Dox/NP as assessed by MTT assay are compared in drug-sensitive OS and ovarian cancer cells [(A) KHOS and (B) SKOV-3] and their MDR counterparts [(C) KHOSR2 and (D) SKOV-3TR]. Dox/NP showed five- to 10-fold higher antiproliferative activity at the IC50 in both the drug-sensitive and -resistant cell lines. Dox/NP showed higher activity than Dox alone in both OS cells (KHOS: p = 0.001 at 0.06 μM, p = 0.044 at 0.1 μM, p = 0.006 at 0.3 μM; KHOSR2: p = 0.001 at 0.6 μM) and ovarian cancer cells (SKOV-3: p = 0.005 at 0.1 μM, p = 0.005 at 0.3 μM). Values are given as mean ± SD. (E) The cytotoxicity of nanoparticles on KHOS, KHOSR2, SKOV-3, and SKOV-3TR was analyzed by MTT assay. The nanoparticles were not cytotoxic by themselves at the dose used in this study. The concentration of nanoparticles included in 10 μM Dox/NP was equivalent to 0.03 mg/mL nanoparticles.
The MDR cells in both cell lines showed resistance to both PLD and free Dox. Dox/NP showed an almost twofold higher mean antiproliferative activity at the IC50 than PLD in both KHOSR2 cells (IC50: 0.43–0.94 μM) (Fig. 4A) and SKOV-3TR cells (IC50: 0.48–0.82 μM) (Fig. 4B). Dox/NP showed higher mean antiproliferative activity than PLD in both KHOSR2 cells (p = 0.011 at 0.6 μM, p < 0.001 at 1 μM, p = 0.014 at 3 μM) and SKOV-3TR cells (p = 0.023 at 0.6 μM).
Fig. 4A–B.

The antiproliferative effects of Dox/NP and PLD as assessed by MTT assay are compared in MDR OS [(A) KHOSR2] and ovarian [(B) SKOV-3TR] cancer cells. Dox/NP showed an increased antiproliferative effect compared with PLD in both MDR cells. The cytotoxicity of Dox/NP was almost two times higher than that of PLD (IC50 in KHOSR2 and SKOV-3TR with Dox/NP: 0.43 and 0.48 μM, respectively; IC50 in KHOSR2 and SKOV-3TR with PLD: 0.94 and 0.82 μM, respectively). Dox/NP showed higher activity than PLD in both KHOSR2 cells (p = 0.011 at 0.6 μM, p < 0.001 at 1 μM, p = 0.014 at 3 μM) and SKOV-3TR cells (p = 0.023 at 0.6 μM). Values are given as mean ± SD. Dotted line represents the 50% decrease of absorbance.
In KHOSR2 cells incubated with AF488-tagged siMDR1/NP, fluorescence microscopy revealed bright green fluorescence in most cells within 2 hours after transfection (Fig. 5). The green fluorescence was more intense after 4 hours than at 2 hours but slightly attenuated after 24 hours. P-gp expression was confirmed in the KHOSR2 and SKOV-3TR cell lines (Fig. 6A). After 48 hours of transfection, a 100-nM concentration of siMDR1/NP suppressed the expression of P-gp in KHOSR2 at levels comparable to those of the commercial transfection reagent (Fig. 6B).
Fig. 5.

Images show the effect of AF488-tagged siMDR1/NP on KHOSR2 cells under fluorescence microscopy after 2, 4, and 24 hours of incubation. Lipofectamine™ RNAiMAX Transfection Reagent and AF488-tagged siMDR1 alone were used as positive and negative controls, respectively. AF488-labeled siMDR1/NP were efficiently incorporated into KHOSR2 cells.
Fig. 6A–B.
Images show the effects of siMDR1/NP on the P-gp expression of drug-sensitive OS and ovarian cancer cells (KHOS and SKOV-3) and their MDR counterparts (KHOSR2 and SKOV-3TR). (A) P-gp expression was confirmed in the KHOSR2 and SKOV-3TR cell lines. Lipofectamine™ RNAiMAX Transfection Reagent and siMDR1 alone were used as positive and negative controls, respectively. (B) A 100-nM concentration of siMDR1/NP suppressed P-gp expression in KHOSR2 cells as effectively as siMDR1 + Lipofectamine™ RNAiMAX Transfection Reagent but more effectively than the negative control siRNA or nanoparticles only.
Discussion
To reverse MDR, nanoparticles have the potential for encapsulating anticancer drugs and genes with the ability to passively target solid tumor tissues by exploiting the leaky vasculature and impaired lymphatic drainage system in tumors. This phenomenon is called the enhanced permeability and retention effect, which is a conventional pathway for the preferential drug accumulation in solid tumors [19, 28]. There are reports of dramatic increase in drug accumulation at the site of tumors compared with other vital organs based on this mechanism [33]. We therefore addressed three questions in this study: (1) Can Dox/NP reverse MDR? (2) Does the antiproliferative effect of Dox/NP have a better therapeutic outcome than PLD? And (3) can siMDR1/NP modulate MDR?
We acknowledge limitations to our study. First, although the clinical application of nanoparticles should be considered, our results are based on in vitro study. In vitro toxicity may not necessarily correlate with clinical response to chemotherapy in humans. In vivo studies with animal models are needed to confirm the clinical potential of our findings. Even in patients with OS, the correlation between P-gp expression and clinical outcome is still controversial [3, 18, 40, 42, 58]. Additionally, it has been reported that P-gp immunostaining does not necessarily correlate with the level of MDR1 gene expression [20]. Second, there are obstacles in delivering siMDR1 in vivo. For instance, P-gp is also expressed in various normal tissues for cellular defense and protection, although at relatively lower levels [7, 23, 26, 44, 46, 53]. Especially in the kidney, the inhibition of P-gp can decrease the renal clearance and prolong the elimination half-life of drugs [16]. Third, the current dextran-based nanoparticles are not conjugated with active targeting ligands. Nanoparticles coupled with targeting ligands such as antibodies or peptides that can bind to specific tumor cells can thus increase the bioavailability of drugs at the tumor site and decrease the residual toxicity [51]. Currently, epidermal growth factor receptor-targeted polymeric nanoparticles that actively target MDR-associated phenotypes have been reported and several targeted nanoparticles have been investigated in preclinical studies for cancers [30, 50]. Furthermore, the development of combination therapies that inhibit MDR and deliver cytotoxic drugs has been recently investigated [8, 29, 38]. Liposome-based nanoparticles can be used as effective codelivery vehicles for Dox and siRNA and cause the simultaneous suppression of drug efflux pumps and antiapoptotic cellular defense combined with cell death induction [38]. Minicells were used to deliver siRNA or small hairpin RNA, followed by the cytotoxic drug. This strategy inhibited the growth of drug-resistant tumor in vitro and in vivo [27].
We first devised a strategy to develop a biocompatible nanoparticulate system to safely deliver Dox and siMDR1 to cancer cells. We chose low-molecular-weight dextran as our base polymer. Dextran is reportedly a neutral and biocompatible polymer for synthesis of nanoparticles [49]. Further, the lack of systemic toxicity is important for drug delivery applications, especially in diseases like cancer. However, dextran in itself cannot self-assemble in aqueous solution and hence it must be hydrophobically modified to facilitate self-assembly. We have previously observed that the end group functionality of the lipid tail, its carbon chain length, and the type of chemistry used to conjugate the lipid to dextran polymer can dictate the type of (hydrophilic or hydrophobic) payload/drug that could be encapsulated/loaded in such delivery systems [1, 48, 49]. In this regard, we observed short lipid chains conjugated to linear dextran polymers using click chemical ligations were suitable for encapsulating low-molecular-weight anthracycline drugs [1]. Thus, for the purpose of stably encapsulating Dox in nanoparticles, a C8-lipid (bromooctane)-modified dextran derivative was synthesized using click chemistry [1]. It is proposed that the aromatic hydrophobic groups in Dox could form hydrophobic-hydrophobic interactions with the triazole ring present on click-based dextran-lipid derivatives [1]. On the other hand, we found a lipid-modified dextran synthesized by the Michael addition reaction facilitated the encapsulation of oligonucleotides and siRNAs [48]. We thus synthesized a C6-lipid (hexyl amine)-conjugated dextran by the Michael addition reaction to enable siMDR1 encapsulation. In this case, the amine residues on the dextran-lipid chains are proposed to electrostatically interact with the negatively charged siRNA molecules. DLS and zeta potential measurements indeed proved both the functionally variant dextran derivatives encapsulating the Dox or siMDR1 resulted in formation of stable nanosized particles with slightly negative to neutral surface charge. To evaluate the intracellular distribution of Dox/NP, we conducted in vitro cell uptake measurements using florescence microscopy. The intracellular sites of localization of the free form of the anthracycline drug is distinctly different in several MDR cancer cells compared with their drug-sensitive counterparts [17, 22, 39, 57]. Namely, anthracycline fluorescence is reportedly found mainly in the nucleus in sensitive cells, whereas it is distributed throughout the cytoplasm of resistant cells [22]. Interestingly, in our study, Dox distribution in MDR OS and ovarian cancer cells mimicked that of sensitive cells when MDR cells were treated with Dox/NP. These observations are consistent with previous reports and indicate dextran-based nanoparticles enabled the delivery of Dox to the nucleus even for the MDR variants [49]. Additionally, our study demonstrated a lower concentration of Dox/NP exhibited more potent antiproliferative activity against both drug-sensitive and -resistant cancer cells. Thus, it seems administration of lower doses of chemotherapeutic agents using biocompatible nanoparticle-based delivery systems could offer the clinical possibility of reducing systemic side effects, overcoming the issue of dose limitation, and reduction in the chances of development of the MDR tumor phenotype.
To date, liposomal delivery systems have been widely used as nanocarriers for drugs or siRNAs because of their simplicity, biocompatibility, biodegradability, and high loading for in vitro delivery of both hydrophilic drugs and siRNAs [37, 38]. PLD is a commercial liposomal Dox that prolongs the circulation of the drug in blood and potentiates intracellular drug accumulation. Clinically, PLD has been introduced in the salvage treatment of patients with recurrent ovarian cancer, while PLD does not induce objective remissions in patients with sarcoma [12, 36]. In our study, our formulation demonstrated better antiproliferative effect on both OS and ovarian cancer cells than PLD, indicating the dextran-based nanoparticulate delivery system may yield better clinical prospects than PLD, especially in sarcoma.
Reversal of drug resistance by siRNA targeted against the mRNA for MDR1 has been reported for several cancer cells [11, 32]. However, there are some obstacles for delivering siRNA in the clinical setting, as described in our Introduction. We have successfully encapsulated siMDR1 in biocompatible dextran-lipid nanoparticles [48]. In KHOSR2 cells incubated with AF488-tagged siMDR1/NP, fluorescence microscopy revealed bright green fluorescence from the intracellularly trafficked siRNA within 2 hours of incubation and the signal could be detected even 24 hours after transfection. Our results also showed siMDR1/NP efficiently suppressed the expression of P-gp in KHOSR2 cells. These observations indicate that the siMDR1 was efficiently incorporated into the cells, resulting in the modulation of P-gp expression.
Our findings indicate biocompatible dextran-lipid nanoparticles could serve as a safe and promising platform, not only for Dox delivery, but also for siRNA delivery, which can effectively modulate the downregulation of P-gp in MDR cells. The development of nanoparticles for in vivo delivery is warranted and currently underway in our laboratory.
Acknowledgments
The authors thank Dr. Michiro Susa and Dr. Henry Mankin for helpful discussions, Dr. Lingling Zhang and Dr. Sampath C. Abeylath for technical assistance, and Dr. Efstathios S. Gonos (National Hellenic Research Foundation, Athens, Greece) for providing the human OS cells line KHOS and the MDR (P-gp)-expressing cell line KHOSR2.
Footnotes
The institution of one or more of the authors certifies that they have received during the study period funding from grants from the National Cancer Institute/NIH (UO1-CA 151452) (MMA, ZD), Gattegno and Wechsler Funds (FJH, ZD), Kenneth Stanton Fund for Sarcoma (Nashua, NH, USA) (FJH, ZD), Sarcoma Foundation of America (Damascus, MD, USA) (ZD), and The Chordoma Foundation (Durham, NC, USA) (ZD). Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at Massachusetts General Hospital (Boston, MA, USA) and Northeastern University (Boston, MA, USA).
References
- 1.Abeylath SC, Amiji MM. “Click” synthesis of dextran macrostructures for combinatorial-designed self-assembled nanoparticles encapsulating diverse anticancer therapeutics. Bioorg Med Chem. 2011;19:6167–6173. doi: 10.1016/j.bmc.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 3.Baldini N, Scotlandi K, Barbanti-Brodano G, Manara MC, Maurici D, Bacci G, Bertoni F, Picci P, Sottili S, Campanacci M, et al. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med. 1995;333:1380–1385. doi: 10.1056/NEJM199511233332103. [DOI] [PubMed] [Google Scholar]
- 4.Belpomme D, Gauthier S, Pujade-Lauraine E, Facchini T, Goudier MJ, Krakowski I, Netter-Pinon G, Frenay M, Gousset C, Marie FN, Benmiloud M, Sturtz F. Verapamil increases the survival of patients with anthracycline-resistant metastatic breast carcinoma. Ann Oncol. 2000;11:1471–1476. doi: 10.1023/A:1026556119020. [DOI] [PubMed] [Google Scholar]
- 5.Bhavsar MD, Amiji MM. Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS) J Control Release. 2007;119:339–348. doi: 10.1016/j.jconrel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-K. [DOI] [PubMed] [Google Scholar]
- 8.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5:2673–2677. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devalapally H, Duan Z, Seiden MV, Amiji MM. Modulation of drug resistance in ovarian adenocarcinoma by enhancing intracellular ceramide using tamoxifen-loaded biodegradable polymeric nanoparticles. Clin Cancer Res. 2008;14:3193–3203. doi: 10.1158/1078-0432.CCR-07-4973. [DOI] [PubMed] [Google Scholar]
- 10.Dillen K, Vandervoort J, Van den Mooter G, Ludwig A. Evaluation of ciprofloxacin-loaded Eudragit RS100 or RL100/PLGA nanoparticles. Int J Pharm. 2006;314:72–82. doi: 10.1016/j.ijpharm.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3:833–838. [PubMed] [Google Scholar]
- 12.Ferrandina G, Corrado G, Licameli A, Lorusso D, Fuoco G, Pisconti S, Scambia G. Pegylated liposomal doxorubicin in the management of ovarian cancer. Ther Clin Risk Manag. 2010;6:463–483. doi: 10.2147/TCRM.S3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 14.Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- 15.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 16.Han HK. Role of transporters in drug interactions. Arch Pharm Res. 2011;34:1865–1877. doi: 10.1007/s12272-011-1107-y. [DOI] [PubMed] [Google Scholar]
- 17.Hindenburg AA, Baker MA, Gleyzer E, Stewart VJ, Case N, Taub RN. Effect of verapamil and other agents on the distribution of anthracyclines and on reversal of drug resistance. Cancer Res. 1987;47:1421–1425. [PubMed] [Google Scholar]
- 18.Hornicek FJ, Gebhardt MC, Wolfe MW, Kharrazi FD, Takeshita H, Parekh SG, Zurakowski D, Mankin HJ. P-glycoprotein levels predict poor outcome in patients with osteosarcoma. Clin Orthop Relat Res. 2000;373:11–17. doi: 10.1097/00003086-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Kandel RA, Campbell S, Noble-Topham S, Bell R, Andrulis IL. Correlation of p-glycoprotein detection by immunohistochemistry with mdr-1 mRNA levels in osteosarcomas: pilot study. Diagn Mol Pathol. 1995;4:59–65. doi: 10.1097/00019606-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Kaye SB. Reversal of drug resistance in ovarian cancer: where do we go from here? J Clin Oncol. 2008;26:2616–2618. doi: 10.1200/JCO.2008.16.2123. [DOI] [PubMed] [Google Scholar]
- 22.Keizer HG, Schuurhuis GJ, Broxterman HJ, Lankelma J, Schoonen WG, van Rijn J, Pinedo HM, Joenje H. Correlation of multidrug resistance with decreased drug accumulation, altered subcellular drug distribution, and increased P-glycoprotein expression in cultured SW-1573 human lung tumor cells. Cancer Res. 1989;49:2988–2993. [PubMed] [Google Scholar]
- 23.Klimecki WT, Futscher BW, Grogan TM, Dalton WS. P-glycoprotein expression and function in circulating blood cells from normal volunteers. Blood. 1994;83:2451–2458. [PubMed] [Google Scholar]
- 24.Kolitz JE, George SL, Dodge RK, Hurd DD, Powell BL, Allen SL, Velez-Garcia E, Moore JO, Shea TC, Hoke E, Caligiuri MA, Vardiman JW, Bloomfield CD, Larson RA. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol. 2004;22:4290–4301. doi: 10.1200/JCO.2004.11.106. [DOI] [PubMed] [Google Scholar]
- 25.Lamendola DE, Duan Z, Yusuf RZ, Seiden MV. Molecular description of evolving paclitaxel resistance in the SKOV-3 human ovarian carcinoma cell line. Cancer Res. 2003;63:2200–2205. [PubMed] [Google Scholar]
- 26.Licht T, Pastan I, Gottesman M, Herrmann F. P-glycoprotein-mediated multidrug resistance in normal and neoplastic hematopoietic cells. Ann Hematol. 1994;69:159–171. doi: 10.1007/BF02215949. [DOI] [PubMed] [Google Scholar]
- 27.MacDiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, Sedliarou I, Wetzel S, Kochar K, Brahmbhatt VN, Phillips L, Pattison ST, Petti C, Stillman B, Graham RM, Brahmbhatt H. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol. 2009;27:643–651. doi: 10.1038/nbt.1547. [DOI] [PubMed] [Google Scholar]
- 28.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/S0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 29.Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milane L, Duan Z, Amiji M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol Pharm. 2010;8:185–203. doi: 10.1021/mp1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minko T, Kopeckova P, Pozharov V, Kopecek J. HPMA copolymer bound adriamycin overcomes MDR1 gene encoded resistance in a human ovarian carcinoma cell line. J Control Release. 1998;54:223–233. doi: 10.1016/S0168-3659(98)00009-1. [DOI] [PubMed] [Google Scholar]
- 32.Nieth C, Priebsch A, Stege A, Lage H. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi) FEBS Lett. 2003;545:144–150. doi: 10.1016/S0014-5793(03)00523-4. [DOI] [PubMed] [Google Scholar]
- 33.Northfelt DW, Martin FJ, Working P, Volberding PA, Russell J, Newman M, Amantea MA, Kaplan LD. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi’s sarcoma. J Clin Pharmacol. 1996;36:55–63. doi: 10.1002/j.1552-4604.1996.tb04152.x. [DOI] [PubMed] [Google Scholar]
- 34.O’Malley DM, Richardson DL, Rheaume PS, Salani R, Eisenhauer EL, McCann GA, Fowler JM, Copeland LJ, Cohn DE, Backes FJ. Addition of bevacizumab to weekly paclitaxel significantly improves progression-free survival in heavily pretreated recurrent epithelial ovarian cancer. Gynecol Oncol. 2011;121:269–272. doi: 10.1016/j.ygyno.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poveda A, Lopez-Pousa A, Martin J, Del Muro JG, Bernabe R, Casado A, Balana C, Sanmartin O, Menendez MD, Escudero P, Cruz J, Belyakova E, Menendez D, Buesa JM. Phase II clinical trial with pegylated liposomal doxorubicin (Caelyx®/Doxil®) and quality of life evaluation (EORTC QLQ-C30) in adult patients with advanced soft tissue sarcomas: a study of the Spanish Group for Research in Sarcomas (GEIS) Sarcoma. 2005;9:127–132. doi: 10.1080/13577140500287024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riganti C, Voena C, Kopecka J, Corsetto PA, Montorfano G, Enrico E, Costamagna C, Rizzo AM, Ghigo D, Bosia A. Liposome-encapsulated doxorubicin reverses drug resistance by inhibiting P-glycoprotein in human cancer cells. Mol Pharm. 2011;8:683–700. doi: 10.1021/mp2001389. [DOI] [PubMed] [Google Scholar]
- 38.Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond). 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuurhuis GJ, Broxterman HJ, Cervantes A, van Heijningen TH, de Lange JH, Baak JP, Pinedo HM, Lankelma J. Quantitative determination of factors contributing to doxorubicin resistance in multidrug-resistant cells. J Natl Cancer Inst. 1989;81:1887–1892. doi: 10.1093/jnci/81.24.1887. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz CL, Gorlick R, Teot L, Krailo M, Chen Z, Goorin A, Grier HE, Bernstein ML, Meyers P. Multiple drug resistance in osteogenic sarcoma: INT0133 from the Children’s Oncology Group. J Clin Oncol. 2007;25:2057–2062. doi: 10.1200/JCO.2006.07.7776. [DOI] [PubMed] [Google Scholar]
- 41.Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat. 2011;14:150–163. doi: 10.1016/j.drup.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Shnyder SD, Hayes AJ, Pringle J, Archer CW. P-glycoprotein and metallothionein expression and resistance to chemotherapy in osteosarcoma. Br J Cancer. 1998;78:757–759. doi: 10.1038/bjc.1998.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sikic BI. Pharmacologic approaches to reversing multidrug resistance. Semin Hematol. 1997;34:40–47. [PubMed] [Google Scholar]
- 44.Smeets M, Raymakers R, Vierwinden G, Pennings A, van de Locht L, Wessels H, Boezeman J, de Witte T. A low but functionally significant MDR1 expression protects primitive haemopoietic progenitor cells from anthracycline toxicity. Br J Haematol. 1997;96:346–355. doi: 10.1046/j.1365-2141.1997.d01-2024.x. [DOI] [PubMed] [Google Scholar]
- 45.Sonneveld P, Suciu S, Weijermans P, Beksac M, Neuwirtova R, Solbu G, Lokhorst H, van der Lelie J, Dohner H, Gerhartz H, Segeren CM, Willemze R, Lowenberg B. Cyclosporin A combined with vincristine, doxorubicin and dexamethasone (VAD) compared with VAD alone in patients with advanced refractory multiple myeloma: an EORTC-HOVON randomized phase III study (06914) Br J Haematol. 2001;115:895–902. doi: 10.1046/j.1365-2141.2001.03171.x. [DOI] [PubMed] [Google Scholar]
- 46.Sugawara I, Kataoka I, Morishita Y, Hamada H, Tsuruo T, Itoyama S, Mori S. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res. 1988;48:1926–1929. [PubMed] [Google Scholar]
- 47.Sun HW, Wu C, Tan HY, Wang QS. Combination DLL4 with Jagged1-siRNA can enhance inhibition of the proliferation and invasiveness activity of human gastric carcinoma by Notch1/VEGF pathway. Hepatogastroenterology. 2012;59:924–929. doi: 10.5754/hge11484. [DOI] [PubMed] [Google Scholar]
- 48.Susa M, Iyer AK, Ryu K, Choy E, Hornicek FJ, Mankin H, Milane L, Amiji MM, Duan Z. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One. 2010;5:e10764. doi: 10.1371/journal.pone.0010764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Susa M, Iyer AK, Ryu K, Hornicek FJ, Mankin H, Amiji MM, Duan Z. Doxorubicin loaded polymeric nanoparticulate delivery system to overcome drug resistance in osteosarcoma. BMC Cancer. 2009;9:399. doi: 10.1186/1471-2407-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talekar M, Kendall J, Denny W, Garg S. Targeting of nanoparticles in cancer: drug delivery and diagnostics. Anticancer Drugs. 2011;22:949–962. doi: 10.1097/CAD.0b013e32834a4554. [DOI] [PubMed] [Google Scholar]
- 51.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 52.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 53.van der Valk P, van Kalken CK, Ketelaars H, Broxterman HJ, Scheffer G, Kuiper CM, Tsuruo T, Lankelma J, Meijer CJ, Pinedo HM, et al. Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues: analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein molecule. Ann Oncol. 1990;1:56–64. [PubMed] [Google Scholar]
- 54.van Vlerken LE, Duan Z, Seiden MV, Amiji MM. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67:4843–4850. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- 55.van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res. 2007;24:1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- 56.Whelan JS, Jinks RC, McTiernan A, Sydes MR, Hook JM, Trani L, Uscinska B, Bramwell V, Lewis IJ, Nooij MA, van Glabbeke M, Grimer RJ, Hogendoorn PC, Taminiau AH, Gelderblom H. Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol. 2012;23:1607–1616. doi: 10.1093/annonc/mdr491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willingham MC, Cornwell MM, Cardarelli CO, Gottesman MM, Pastan I. Single cell analysis of daunomycin uptake and efflux in multidrug-resistant and -sensitive KB cells: effects of verapamil and other drugs. Cancer Res. 1986;46:5941–5946. [PubMed] [Google Scholar]
- 58.Wunder JS, Bull SB, Aneliunas V, Lee PD, Davis AM, Beauchamp CP, Conrad EU, Grimer RJ, Healey JH, Rock MJ, Bell RS, Andrulis IL. MDR1 gene expression and outcome in osteosarcoma: a prospective, multicenter study. J Clin Oncol. 2000;18:2685–2694. doi: 10.1200/JCO.2000.18.14.2685. [DOI] [PubMed] [Google Scholar]
- 59.Zheng L, Ren JQ, Li H, Kong ZL, Zhu HG. Downregulation of wild-type p53 protein by HER-2/neu mediated PI3 K pathway activation in human breast cancer cells: its effect on cell proliferation and implication for therapy. Cell Res. 2004;14:497–506. doi: 10.1038/sj.cr.7290253. [DOI] [PubMed] [Google Scholar]