Abstract
Background
To fulfill the need for large volumes, devitalized allografts are used to treat massive bone defects despite a 60%, 10-year postimplantation fracture rate. Allograft healing is inferior to autografts where the periosteum orchestrates remodeling.
Hypothesis
By augmenting allografts with a tissue engineered periosteum consisting of tunable and degradable, poly(ethylene glycol) (PEG) hydrogels for mesenchymal stem cell (MSC) transplantation, the functions critical for periosteum-mediated healing will be identified and emulated.
Method of Study
PEG hydrogels will be designed to emulate periosteum-mediated autograft healing to revitalize allografts. We will exploit murine femoral defect models for these approaches. Critical-sized, 5-mm segmental defects will be created and filled with decellularized allograft controls or live autograft controls. Alternatively, defects will be treated with our experimental approaches: decellularized allografts coated with MSCs transplanted via degradable PEG hydrogels to mimic progenitor cell densities and persistence during autograft healing. Healing will be evaluated for 9 weeks using microcomputed tomography, mechanical testing, and histologic analysis. If promising, MSC densities, hydrogel compositions, and genetic methods will be used to isolate critical aspects of engineered periosteum that modulate healing. Finally, hydrogel biochemical characteristics will be altered to initiate MSC and/or host-material interactions to further promote remodeling of allografts.
Significance
This approach represents a novel tissue engineering strategy whereby degradable, synthetic hydrogels will be exploited to emulate the periosteum. The microenvironment, which will mediate MSC transplantation, will use tunable PEG hydrogels for isolation of critical allograft revitalization factors. In addition, hydrogels will be modified with biochemical cues to further augment allografts to reduce or eliminate revision surgeries associated with allograft failures.
Hypothesis
Three-dimensional periosteum surrogates, composed of synthetic, tunable, and degradable poly(ethylene glycol) (PEG) hydrogel microenvironments, will promote allograft healing and integration similar to that of autografts. Hydrogels will be used to encapsulate and transplant mesenchymal stem cells (MSCs) to the allograft surface at similar densities and persistence to progenitor cells in autografts. If allograft healing is confirmed, the critical aspects of this approach will be examined, namely, the role of MSCs in healing. MSCs also can be provided with biochemical cues to promote paracrine factor release, differentiation, and/or matrix synthesis to initiate healing and integration of allografts.
Background
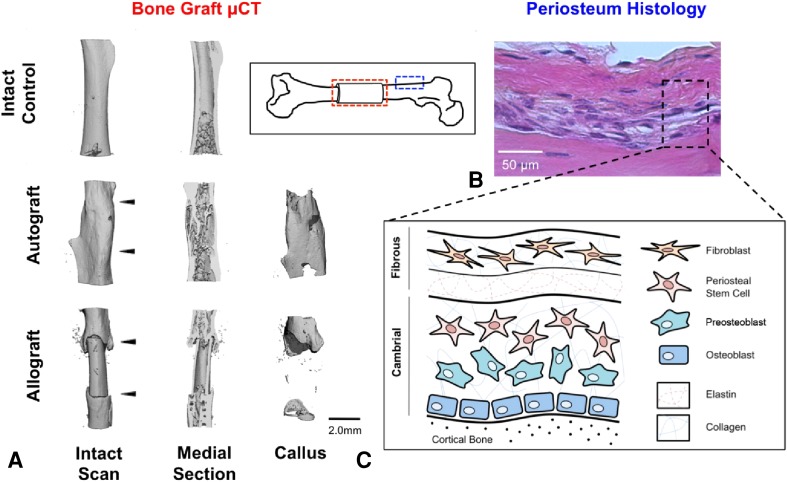
Clinically, more than 500,000 Americans require bone grafts annually [37, 48]. Allografts remain the gold standard for treating critical-sized bone defects but have slow bone formation and minimal engraftment, resulting in fibrotic nonunions and brittle fractures [44, 45, 47, 48]. In contrast, autografts completely heal, mediated by the periosteum. When the periosteum is removed from autografts used in massive defects, graft-mediated bone formation is reduced 63%, which is similar to decellularized allograft controls (Fig. 1A) [48].
Fig. 1A–C.
(A) Representative μCT images of a control autograft and decellularized allografts after 9 weeks of healing in a murine femoral defect model are shown. Briefly, a 5-mm mid-diaphyseal segment was resected from 8-week-old C56BL/6 mice and replaced with live autografts or decellularized allografts. As illustrated, the autograft control shows substantial graft-mediated callus formation, substantial host integration, and considerable graft resorption and remodeling. Conversely, the decellularized allografts show no graft-mediated callus formation, minimal host integration, and no graft resorption or remodeling as a result of lack of periosteum tissue. (B) The histologic features of mouse femoral periosteum are shown (Stain, alcian blue (red/pink, glycosaminoglycans/proteoglycans), hematoxylin (blue, cell nuclei); original magnification, × 40) (bar = 50 μm). (C) A schematic representation of hierarchical cell structure is shown. The periosteum is divided into an outer fibrous region and the inner cell-rich cambrium layer that houses a combination of mesenchymal progenitors, differentiated osteogenic progenitors, and fully matured osteoblasts.
The periosteum is thin (approximately 200 μm) and composed of extracellular matrix and periosteal stem cells, similar in differentiation and proliferation capacity to MSCs, osteogenic progenitors, and osteoblasts (Fig. 1) [14, 16, 17, 20, 36]. Combinatorial approaches using MSCs, growth factors, and biomaterials have been used to emulate factors present during periosteum-mediated autograft healing. Growth factors commonly used include bone morphogenetic protein-2 (BMP-2) [27, 34, 45, 46, 48], teriparatide (PTH1–34), the recombinant peptide of parathyroid hormone (PTH) [13, 29], vascular endothelial growth factor (VEGF) [21, 22, 46], receptor activator of nuclear factor kappa-B ligand (RANKL) [21], and the sphingosine 1-phosphate (S1P) receptor agonist, fingolimod (FTY720) [26].
The above-described approaches have resulted in variable outcomes with respect to allograft revitalization but none that match the success of autograft healing. We believe this stems from limited understanding of critical requirements of the periosteum, and subsequently, the failure to emulate those characteristics. Unlike native periosteum [16, 19, 36, 40], scaffold materials used (eg, Gelfoam® (Pfizer Inc, New York, NY) [48], acellular matrices [34, 45], poly(lactide-co-glycolide) [22]) have mismatched scaffold properties, lacking three-dimensional hierarchical tissue structure, hydration, and flexibility to incorporate biophysical and biochemical cues to promote specific host or transplanted cell functions [1–7]. These suboptimal qualities limit cell survival and graft localization [8, 10, 34, 45, 48]. Growth factor delivery also is challenging owing to diffusion and degradation, requiring delivery of supraphysiologic concentrations [12], resulting in costly clinical applications [9, 13, 39, 47].
Proposed Program
We propose development of hydrogel periosteum mimetics whose matrix properties can be controllably altered to elucidate and emulate critical factors of the periosteum to orchestrate allograft revitalization (Fig. 2). To do so, PEG hydrogels provide an especially useful platform compared with previously used scaffold materials. PEG hydrogels are resistant to nonspecific protein adsorption and are blank slates for transplanted MSCs and host cells. In addition, hydrogel degradation profiles and cell encapsulation densities can be altered, and hierarchical network architectures can be readily achieved [43]. PEG hydrogels also can be modified to controllably release small molecule drugs [3, 6, 24], or incorporate matrix cues to direct cell function via specific cell-material interactions [1, 2] (Fig. 2). Use of PEG hydrogels in bottom-up design approaches addresses the substantial need to isolate the critical healing characteristics of the periosteum and use them to improve allograft healing and integration. Specifically motivated by the native periosteum, we will emulate cell densities and persistence, using MSCs for their ease of accessibility and similar differentiation capacity to periosteal cell types [20].
Fig. 2.
A schematic representation is shown of a degradable, three-dimensional, PEG hydrogel-based periosteum mimetic for the delivery and localization of MSCs to an allograft surface. The PEG network can be easily modified to optimize cell-material interaction and use developed chemistries for the controlled release of drugs and other small soluble factors. Furthermore, MSCs encapsulated in PEG hydrogels remain greater than 95% viable as illustrated by the confocal live/dead image (Stain, calcein AM (green = live cells), ethidium homodimer (red = dead cells); bar = 200 μm; original magnification, × 10), and we observed MSC localization to cortical bone surfaces using PEG networks (Stain, hematoxylin (blue, cell nuclei), eosin (pink, collagen/extracellular matrix); original magnification, × 40) (bar = 50 μm).
Using this approach we will examine whether MSC transplantation orchestrates allograft healing and integration. Hydrolytically degradable poly(ethylene glycol)-poly(lactic acid)-dimethacrylate (PEG-PLA-DM) hydrogels will be synthesized (Fig. 2) [1–7, 23] to provide control over MSC transplantation densities and persistence. The periosteum contains approximately 90,000 cells/mm [25] and during autograft healing, progenitor cells persist in the periosteum for only approximately 21 days [48]. Therefore, hydrogels designed to degrade over approximately 21 days will be used to encapsulate and localize 500,000 MSCs to each 5-mm devitalized allograft before implantation in murine femoral segmental defect models [45, 48]. Briefly, decellularized allografts will be coated with MSC-encapsulated degradable PEG-PLA-DM hydrogels at physiologically relevant thicknesses using custom molds (Fig. 2). Healing will be followed for 9 weeks, measuring total bone volume, callus bone volume, vascular volume, matrix synthesis, and biomechanics using a combination of microcomputed tomography (μCT), histology, and torsional testing [45].
Provided this basic representation of the periosteum (eg, MSC density and persistence) shows promise for allograft revitalization, we will deconvolute the critical factors governing healing. MSC-mediated healing is likely orchestrated by transplanted MSC differentiation, matrix production, and/or paracrine factor release. However, progenitor cells have short persistence times [48] and abundant paracrine factor release [18] during autograft healing, making releasable factors and their affects on host cell infiltration and remodeling a plausible coordinator of healing. To elucidate the importance of paracrine factors, hydrogel degradation profiles and/or MSC densities will be altered to modulate the overall concentrations and durations of factor release. Moreover, effects of specific paracrine factors will be isolated using small hairpin RNA (shRNA) methods to knockdown paracrine, differentiation, and/or matrix proteins. We can further modify PEG hydrogels to influence allograft healing by promoting specific cell (MSC or host)-material interactions. Cell-material interactions can be used to control MSC and host cell functions such as migration, differentiation, vascularization, and matrix remodeling [38, 41]. Thus, we can promote specific cell-material interactions—eg, through particular integrins [1, 3]. Other cell functions such as differentiation can be modulated by incorporating releasable drug delivery mechanisms in PEG hydrogels [3, 6] to further promote specific cellular effects we posit to be important toward allograft revitalization.
Limitations
Based on careful design and thorough in vitro established cohort of degradable hydrogels capable of controlling numerous MSC functions, we expect our newly developed periosteum-mimetics to enhance the healing of allograft tissue in vivo [1–7]. Loss of osteocytes during allograft devitalization may contribute to an overall decrease in graft resorption and remodeling as compared with autograft controls [45]. Osteocytes, however, have no contribution to early osteogenesis of bone grafts [47], so we believe our approach will still enhance allograft integration and healing, even in the absence of these cells. Furthermore, by altering PEG degradation kinetics or hydrogel MSC densities we believe we can exercise temporal control over the initiation of remodeling and thereby increase early matrix production and ossification [45, 48]. Moreover, our approach may be further enhanced through chemical modification of PEG macromers to alter degradation parameters [30, 31], include controlled release of soluble factors (eg, statins, β-catenin agonists, growth factors) to increase MSC paracrine factor release, proliferation, differentiation, and/or matrix production [1–5, 7, 33, 35, 42], or use photopatterning strategies to achieve localized network modification and enable cell stratification in specific areas of hydrogels, as seen in native tissue [11, 28]. Such alterations may provide further instructional cues for cells in the periosteum mimetic and synergistic healing may result using appropriate combinatorial strategies.
Next Steps
At the culmination of this proposed program, we aim to identify critical parameters of our PEG hydrogel periosteum mimetics that promote healing and integration of allografts. These parameters include transplanted MSC density and cell persistence, as controlled by hydrogel degradation profiles, and biochemical cues to promote specific cell-material interactions. Provided promising results are obtained with murine models, the next step is translation of the best revitalization strategy to a large animal model. To show clinically relevant proof of efficacy, these experiments would use a previously established 5-cm canine segmental defect model [32]. Healing will be followed for 6 months, measuring total bone volume, callus bone volume, vascular volume, matrix synthesis, and biomechanics using a combination of cone beam (CB)-CT [15], dynamic contrast-enhanced (DCE)-MRI [15], histology [32], and torsional testing [32]. Pending successful improvements in canine allograft healing using the hydrogel periosteum mimetics, we would propose application for a phase 1 human clinical trial.
Implications and Future Directions
We propose a tissue engineered periosteum-mimetic with the goal of developing a clinically relevant treatment strategy to improve allograft healing. We presume these studies will identify important microenvironmental characteristics of the periosteum that support its critical role in allograft healing. Moreover, we believe the knowledge gained will prove advantageous in developing other MSC-based regenerative therapies, such as bone, cartilage, and other musculoskeletal tissue.
Footnotes
The institution of one of the authors (DSWB) has received, during the study period, funding from Orthopaedic Research and Education Foundation/Musculoskeletal Transplant Foundation (OREF/MTF), and the Rochester/Finger Lakes Eye & Tissue Bank (RETB/FLETB). The institution of one the authors (MH) has received, during the study period, funding from the NIH (T32 Training in Orthopaedic Research (NIH-AR053459)).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Rochester, Rochester, NY, USA
References
- 1.Benoit DS, Anseth KS. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26:5209–5220. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1:461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Benoit DS, Collins SD, Anseth KS. Multifunctional hydrogels that promote osteogenic hMSC differentiation through stimulation and sequestering of BMP2. Adv Funct Mater. 2007;17:2085–2093. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoit DS, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12:1663–1673. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 5.Benoit DS, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Benoit DS, Nuttelman CR, Collins SD, Anseth KS. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102–6110. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitbart EA, Meade S, Azad V, Yeh S, Al-Zube L, Lee YS, Benevenia J, Arinzeh TL, Lin SS. Mesenchymal stem cells accelerate bone allograft incorporation in the presence of diabetes mellitus. J Orthop Res. 2010;28:942–949. doi: 10.1002/jor.21065. [DOI] [PubMed] [Google Scholar]
- 9.Bukata SV, Puzas JE. Orthopedic uses of teriparatide. Curr Osteoporos Rep. 2010;8:28–33. doi: 10.1007/s11914-010-0006-3. [DOI] [PubMed] [Google Scholar]
- 10.Cornejo A, Sahar DE, Stephenson SM, Chang S, Nguyen S, Guda T, Wenke JC, Vazquez A, Michalek JE, Sharma R, Krishnegowda NK, Wang HT. Effect of adipose tissue-derived osteogenic and endothelial cells on bone allograft osteogenesis and vascularization in critical-sized calvarial defects. Tissue Eng Part A. 2012;18:1552–1561. doi: 10.1089/ten.tea.2011.0515. [DOI] [PubMed] [Google Scholar]
- 11.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derner R, Anderson AC. The bone morphogenic protein. Clin Podiatr Med Surg. 2005;22:607–618. doi: 10.1016/j.cpm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon RS, Schwarz EM. Teriparatide therapy as an adjuvant for tissue engineering and integration of biomaterials. J Mater Res. 2011;4:1117–1131. doi: 10.3390/ma4061117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwek JR. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol. 2010;39:319–323. doi: 10.1007/s00256-009-0849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrhart N, Kraft S, Conover D, Rosier RN, Schwarz EM. Quantification of massive allograft healing with dynamic contrast enhanced-MRI and cone beam-CT: a pilot study. Clin Orthop Relat Res. 2008;466:1897–1904. doi: 10.1007/s11999-008-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellender G, Feik SA, Carach BJ. Periosteal structure and development in a rat caudal vertebra. J Anat. 1988;158:173–187. [PMC free article] [PubMed] [Google Scholar]
- 17.Ham AW. A histological study of the early phases of bone repair. J Bone Joint Surg Am. 1930;12:827–844. [Google Scholar]
- 18.Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005;55:414–419. doi: 10.1097/01.sap.0000178809.01289.10. [DOI] [PubMed] [Google Scholar]
- 19.Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 20.Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(suppl 1):S45–S64. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 21.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O’Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–2527. doi: 10.1016/S0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 23.Nuttelman CR, Benoit DS, Tripodi MC, Anseth KS. The effect of ethylene glycol methacrylate phosphate in PEG hydrogels on mineralization and viability of encapsulated hMSCs. Biomaterials. 2006;27:1377–1386. doi: 10.1016/j.biomaterials.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Nuttelman CR, Tripodi MC, Anseth KS. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. J Biomed Mater Res Part A. 2006;76:183–195. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- 25.O’Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19:95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 26.Petrie Aronin CE, Shin SJ, Naden KB, Rios PD, Jr, Sefcik LS, Zawodny SR, Bagayoko ND, Cui Q, Khan Y, Botchwey EA. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials. 2010;31:6417–6424. doi: 10.1016/j.biomaterials.2010.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pluhar GE, Manley PA, Heiner JP, Vanderby R, Jr, Seeherman HJ, Markel MD. The effect of recombinant human bone morphogenetic protein-2 on femoral reconstruction with an intercalary allograft in a dog model. J Orthop Res. 2001;19:308–317. doi: 10.1016/S0736-0266(00)90002-0. [DOI] [PubMed] [Google Scholar]
- 28.Polizzotti BD, Fairbanks BD, Anseth KS. Three-dimensional biochemical patterning of click-based composite hydrogels via thiolene photopolymerization. Biomacromolecules. 2008;9:1084–1087. doi: 10.1021/bm7012636. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds DG, Takahata M, Lerner AL, O’Keefe RJ, Schwarz EM, Awad HA. Teriparatide therapy enhances devitalized femoral allograft osseointegration and biomechanics in a murine model. Bone. 2011;48:562–570. doi: 10.1016/j.bone.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice MA, Anseth KS. Controlling cartilaginous matrix evolution in hydrogels with degradation triggered by exogenous addition of an enzyme. Tissue Eng. 2007;13:683–691. doi: 10.1089/ten.2006.0142. [DOI] [PubMed] [Google Scholar]
- 31.Rice MA, Sanchez-Adams J, Anseth KS. Exogenously triggered, enzymatic degradation of photopolymerized hydrogels with polycaprolactone subunits: experimental observation and modeling of mass loss behavior. Biomacromolecules. 2006;7:1968–1975. doi: 10.1021/bm060086+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santoni BG, Ehrhart N, Betancourt-Benitez R, Beck CA, Schwarz EM. Quantifying massive allograft healing of the canine femur in vivo and ex vivo: a pilot study. Clin Orthop Relat Res. 2012;470:2478–2487. doi: 10.1007/s11999-012-2349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 34.Schonmeyr B, Clavin N, Avraham T, Longo V, Mehrara BJ. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells. Tissue Eng Pt A. 2009;15:1833–1841. doi: 10.1089/ten.tea.2008.0446. [DOI] [PubMed] [Google Scholar]
- 35.Sineva GS, Pospelov VA. Inhibition of GSK3beta enhances both adhesive and signalling activities of beta-catenin in mouse embryonic stem cells. Biol Cell. 2010;102:549–560. doi: 10.1042/BC20100016. [DOI] [PubMed] [Google Scholar]
- 36.Squier CA, Ghoneim S, Kremenak CR. Ultrastructure of the periosteum from membrane bone. J Anat. 1990;171:233–239. [PMC free article] [PubMed] [Google Scholar]
- 37.Stansbury LG, Lalliss SJ, Branstetter JG, Bagg MR, Holcomb JB. Amputation in U.S. military personnel in the current conflicts in Afghanistan and Iraq. J Orthop Trauma. 2008;22:43–46. doi: 10.1097/BOT.0b013e31815b35aa. [DOI] [PubMed] [Google Scholar]
- 38.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 39.Takahata M, Awad HA, O’Keefe RJ, Bukata SV, Schwarz EM. Endogenous tissue engineering: PTH therapy for skeletal repair. Cell Tissue Res. 2011;347:545–552. doi: 10.1007/s00441-011-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor JF. The periosteum and bone growth. In: Hall BK, editor. bone. Boca Raton, FL: CRC Press; 1992. pp. 21–52. [Google Scholar]
- 41.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006;13:957–963. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplant. 2008;16:1049–1057. doi: 10.3727/000000007783472336. [DOI] [PubMed] [Google Scholar]
- 44.Wheeler DL, Haynie JL, Berrey H, Scarborough M, Enneking W. Biomechanical evaluation of retrieved massive allografts: preliminary results. Biomed Sci Instrum. 2001;37:251–256. [PubMed] [Google Scholar]
- 45.Xie C, Reynolds D, Awad H, Rubery PT, Pelled G, Gazit D, Guldberg RE, Schwarz EM, O’Keefe RJ, Zhang X. Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering. Tissue Eng. 2007;13:435–445. doi: 10.1089/ten.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yazici C, Takahata M, Reynolds DG, Xie C, Samulski RJ, Samulski J, Beecham EJ, Gertzman AA, Spilker M, Zhang X, O’Keefe RJ, Awad HA, Schwarz EM. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther. 2011;19:1416–1425. doi: 10.1038/mt.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Awad HA, O’Keefe RJ, Guldberg RE, Schwarz EM. A perspective: engineering periosteum for structural bone graft healing. Clin Orthop Relat Res. 2008;466:1777–1787. doi: 10.1007/s11999-008-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O’Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20:2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]