Abstract
Background
Although morphometric hip parameters measured on radiographs are valuable tools guiding diagnosis and therapy in patients with hip disorders, some clinicians use MRI for such measurements, although it is unclear whether the parameters assessed on MRI differ from those assessed on radiographs.
Questions/purposes
We asked whether the lateral center-edge angle (LCE), Tönnis angle, extrusion index, and anterior center-edge angle (ACE) are similar on MRI and radiography.
Methods
We retrospectively reviewed the imaging data of 103 hips from 103 patients: 46 with femoroacetabular impingement and 57 with hip dysplasia. We manually measured the LCE, Tönnis angle, extrusion index, and ACE from radiographs and MRI in all 103 hips. Four straight coronal (Ant-10 mm, Ant-5 mm, Center, and Post-5 mm), three straight sagittal (S-Med-5 mm, S-Center, S-Lat-5 mm), and three 25º oblique sagittal (OS-Med-5 mm, OS-Center, OS-Lat-5 mm) reformats were reconstructed from a three-dimensional isotropic morphologic MRI sequence. MRI measurements were compared against the gold standard radiographic measurements.
Results
We found good agreement for the LCE angle, Tönnis angle, and extrusion index between radiographic and coronal slice MRI measurements. The mean differences between radiographic and MRI measurements were 5º or less or 5% or less (for the extrusion index) in all coronal MRI slices. However, the differences between ACE angles on sagittal MRI slices and radiographs ranged from 5° to 28º.
Conclusions
LCE, Tönnis angle, and extrusion index can be measured on MRI with comparable results to radiography. The ACE angle on radiographs cannot be estimated reliably from MRI.
Clinical Relevance
MRI provides similar morphometric measurements as radiography for most hip parameters, except for the ACE angle.
Introduction
Developmental dysplasia of the hip (DDH) and femoroacetabular impingement (FAI) are common sources of hip pain and disability [14]. FAI is defined as the abutment between the proximal femur and the acetabular rim, either as a result of reduced femoral head-neck offset (cam type) or acetabular (pincer type) overcoverage or both [14, 15]. Ganz et al. [14] and others [19] have emphasized the importance of detection of such anatomic abnormalities of the hip, which may lead to pain and osteoarthritis (OA) in young adults [14, 19]. In most cases, a combination of cam and pincer forms is present called mixed-type impingement [3].
DDH is an anatomic deformity characterized by femoral undercoverage, in which the basic mechanical abnormality is instability [34]. The process of obtaining and interpreting radiographs in an accurate and reproducible fashion is important to the diagnosis, treatment, and study of adult hip disease [2]. Lateral acetabular deficiency is the most commonly recognized abnormality in acetabular dysplasia. An abnormal lateral center-edge angle (LCE) less than 20º on an AP pelvis radiograph is considered by some as diagnostic of acetabular dysplasia [9, 35]. However, anterior undercoverage also may be associated with clinical symptoms [17] and may be more sensitive than LCE. Anterior undercoverage may be determined with the anterior center-edge (ACE or vertical center anterior margin) angle on false-profile views [21]. Other commonly used measures for hip disorders are the Tönnis angle (also known as the acetabular index) [20] and the extrusion index [7]. The Tönnis angle is a measure for steepness of the acetabular roof and the extrusion index is a measure of femoral head coverage.
Although no LCE cutoff value is defined for FAI, a high LCE (> 40º) suggests overcoverage (pincer-type impingement) [9]. Similarly, a high ACE and low Tönnis angle are signs of pincer FAI. Kappe et al. [18] emphasized that there is a challenge to differentiate the clinical presentation of FAI and DDH. They concluded that radiographic studies are needed for differentiation of the two entities and for the decision regarding treatment strategy.
Current orthopaedic practice relies on various imaging modalities including plain radiographs, MRI, and CT, which are the main imaging modalities used to assess the anatomic deformity and to quantify the extent of joint damage. Standard measures of hip deformity usually are assessed on an AP pelvic radiograph. In cases of FAI, a plain AP pelvic radiograph is often inadequate in terms of assessing morphologic features of the femoral head-neck junction and early-stage OA [25]; therefore, advanced imaging such as MRI or CT is obtained. If hip deformity measures could be assessed accurately on MRI and on a plain radiograph, it might be possible to simplify radiographic assessment. At this time, it is unknown whether standard morphometric parameters of the hip measured on MRI are comparable to measurements on radiographs.
We therefore compared morphometric measurements on radiographs and on corresponding MRI slices for: (1) LCE angle; (2) Tönnis angle; (3) extrusion index; (4) ACE angle; and (5) signs of acetabular retroversion with the acetabular version.
Materials and Methods
We performed a retrospective cross-sectional study. We identified 103 patients (103 hips) from a surgical database of patients undergoing joint preservation surgery for impingement or dysplasia who had MRI and radiographs performed between January 2007 and September 2010. The inclusion criteria were met if (1) clinical and radiographic signs of FAI (positive hip impingement sign and restriction of hip motion) or DDH had been documented by the senior author (Y-JK); and (2) there were isotropic MRI and radiographs in two planes (AP and false profile view). During the study period a total of 490 patients with FAI or DDH were eligible. FAI was diagnosed based on clinical examination findings (positive anterior impingement sign [22]) in combination with radiographic features of cam (alpha angle ≥ 55º on 45º Dunn or crosstable lateral view [10]) or pincer impingement [23]. DDH was diagnosed in patients with a LCE angle of 25º or less or Tönnis angle greater than 15º on the AP pelvic radiographs. Patients with slipped capital femoral epiphysis, Legg-Calvé-Perthes disease, chromosomal disorders, neuromuscular diseases, or prior surgery were excluded. The total cohort of 103 patients consisted of 57 patients with DDH and 46 with FAI. In the case of bilateral hip disease, the hip on which surgery was performed first was included. The patients with DDH consisted of 52 females and five males with a mean age of 27 years (range, 13–54 years). From the FAI group, 23 were males and 23 were females. Their mean age was 27 years (range, 14–54 years). This was the maximum number of patients obtainable because this specific MRI protocol was in use for only this particular period. This retrospective study was approved by the institutional review board. Informed consent was waived.
AP pelvic views were obtained as described by Clohisy et al. [5]. False-profile radiographs were taken with the patient in the standing position at an angle of 65° between the pelvis and the film as described by Lequesne and de Séze [21].
MR images were obtained with a 1.5-T system (Avanto; Siemens Healthcare, Erlangen, Germany) using a flexible eight-channel surface coil. The imaging protocol included a three-dimensional isotropic true-FISP (fast imaging with steady-state precession) sequence with a 160 mm × 160-mm field of view, base resolution of 256, and voxel size of 0.63 mm × 0.63 mm × 0.63 mm. Repetition time was 12.57 ms and echo time was 5.48 ms. The acquisition time was 7 minutes 47 seconds.
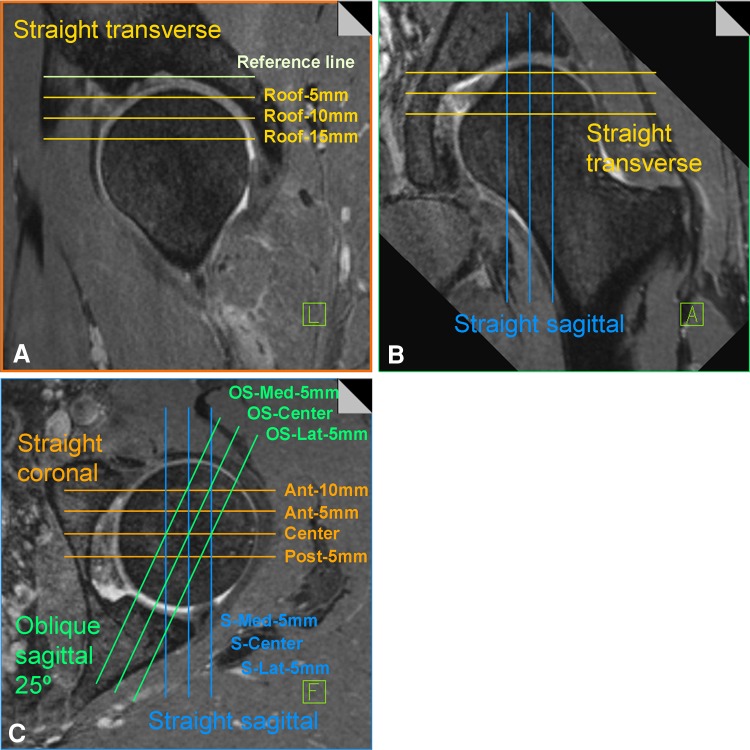
The three-dimensional true-FISP data set was used for multiplanar reconstruction on a Leonardo workstation (Siemens Healthcare). Coronal, transversal, straight sagittal, and oblique sagittal reformats with a slice thickness of 0.63 mm were created. We obtained four coronal reformats, one for the center, one posterior (Post-5 mm), and two anterior (Ant-5 and Ant-10 mm) (Fig. 1). Concerning the other reformats (in sagittal and oblique sagittal planes), we made three reformats, one from the center of the femoral head, one anterior (Ant-5 mm), and one more posterior (Post-5 mm).
Fig. 1A–C.
A schematic three-dimensional multiplanar reconstruction is shown for the (A) transverse, (B) sagittal, and (C) oblique sagittal and coronal slices. The slice name for each individual reformat is given.
To generate reformats of the oblique sagittal plane, we shifted the anterior sagittal plane by 25º in the lateral direction to get a plane comparable to the false-profile radiograph (Fig. 1). For the transversal reformats, we took the bony roof of the acetabulum as the starting point (first 0.63-mm slice superiorly where the cartilage was visible). The reformats were taken accordingly 5, 10, and 15 mm inferior to the bony roof (Roof-5 mm, Roof-10 mm, and Roof-15 mm).
Two of us (DS and AH), who were not the treating surgeons, made all imaging measurements. We determined the LCE angle, Tönnis angle, and extrusion index on AP radiographs and the ACE angle on false-profile views using OsiriX Version 3.9 (Pixmeo, Geneva, Switzerland) and the OsiriX plug-in Orthopaedic Studio Version 1.2 (Carl Siversson, Department of Medical Radiation Physics, Lund University, Lund, Sweden; http://orthostudio.spectronic.se) manually using standard technique as previously described [19]. The same observers evaluated acetabular retroversion with use of the criteria proposed by Reynolds et al. [30]. They defined a positive crossover sign as being present when the anterior aspect of the acetabular rim was more lateral than the posterior aspect in the proximal part of the acetabulum. Similarly, we evaluated the presence of the posterior wall sign, created by the outline of the posterior aspect of the acetabular rim passing medial to the center of the hip [30]. To avoid bias from abnormal pelvic tilt, only radiographs with a sacrococcygeal to pubic symphysis distance of 20 to 60 mm were included. We used the false-profile view to assess the ACE angle to evaluate anterior coverage of the acetabulum [4, 8, 21]. Similar to the LCE angle, normal values are greater than 25°.
On each MRI plane, a best-fit circle was drawn based on the three points of the bony outline of the femoral head. Two points were chosen superiorly and a third point inferomedially (inferior to fovea). On the coronal reformats, we measured the LCE angle the same way as on the radiographs [35]. Because the contralateral femoral heads were not visible on the true-FISP reformats, the localizer sequence was used to correct for pelvic obliquity. Furthermore, we measured the Tönnis angle to evaluate the steepness of the acetabular roof and superolateral coverage of the femoral head [33]. We also measured the femoral head extrusion index, which is defined as the portion of the femoral head not covered by the acetabular roof divided by the diameter of the femoral head [7]. Coverage less than 75% indicates hip dysplasia [9, 26]. On the sagittal and oblique sagittal reformats, we measured the ACE angle, which is formed by the intersection of a vertical line through the center of the femoral head and a line extending through the center of the femoral head to the anterior sourcil [8]. This angle quantifies the anterior cover of the femoral head. Angles less than 20° are considered abnormal [1]. The acetabular anteversion angle was measured in the axial plane forming the angle between the line connecting the anterior and posterior acetabular margins and a straight AP line [32]. We performed angle measurements on the localizer MR sequences to correct for pelvic obliquity in the coronal and axial planes. No acetabular roof was detectable on eight MRI slices (lateral sagittal or lateral oblique sagittal slices) of three patients. Thus, no ACE angle measurement was possible in these patients.
Interobserver reproducibility of all MRI measurements was assessed by a second trained reader (AH) in a blinded random subset of 20 MRI data sets (10 DDH and 10 FAI). The intraclass correlation coefficient (ICC) was used to determine interobserver agreement. The interobserver agreement measured with the ICC for all angles and the extrusion index ranged from 0.87 to 0.96. The lowest agreement was for the Tönnis angle with an ICC of 0.87, whereas the highest were for the ACE angle measurements (ICC, 0.96; Table 1). Interobserver agreement for radiographic measurements was not assessed in our study but reported ICCs are in the range 0.76 to 0.80 [1] and the kappa value is approximately 0.60 [6].
Table 1.
Interobserver agreement
| Measure | ICC | 95% CI | |
|---|---|---|---|
| Lower bound | Upper bound | ||
| LCE | 0.948 | 0.920 | 0.966 |
| Tönnis | 0.870 | 0.805 | 0.915 |
| Extrusion index | 0.936 | 0.901 | 0.958 |
| Sagittal ACE | 0.957 | 0.927 | 0.974 |
| Oblique sagittal ACE | 0.960 | 0.935 | 0.976 |
| Anteversion | 0.953 | 0.922 | 0.971 |
ICC = intraclass correlation coefficients; LCE = lateral center-edge; ACE = anterior center-edge.
Descriptive results for the normally distributed data are given as the mean ± SD. Normality of the data was assessed from their respective histograms. The Pearson correlation coefficient was used to assess the degree of association between radiographic and MRI measurements. The mean values of radiographic and MRI measurements were compared using paired t-tests. The mean squared error (MSE) was used as a measure of relative precision of the MRI measurements (assumed to be the estimates) in comparison to the gold standard radiograph (assumed to be the truth). The following formula was used for this measure: MSE = 1/number of patients × (SUM[radiograph − MRI]2). Because our patient groups of DDH and FAI represent a continuum of morphologic features, group effects were ignored in our evaluation. SPSS Version 19.0 (IBM, SPSS Inc, Chicago, IL, USA) was used for statistical analysis and graphic plotting.
Results
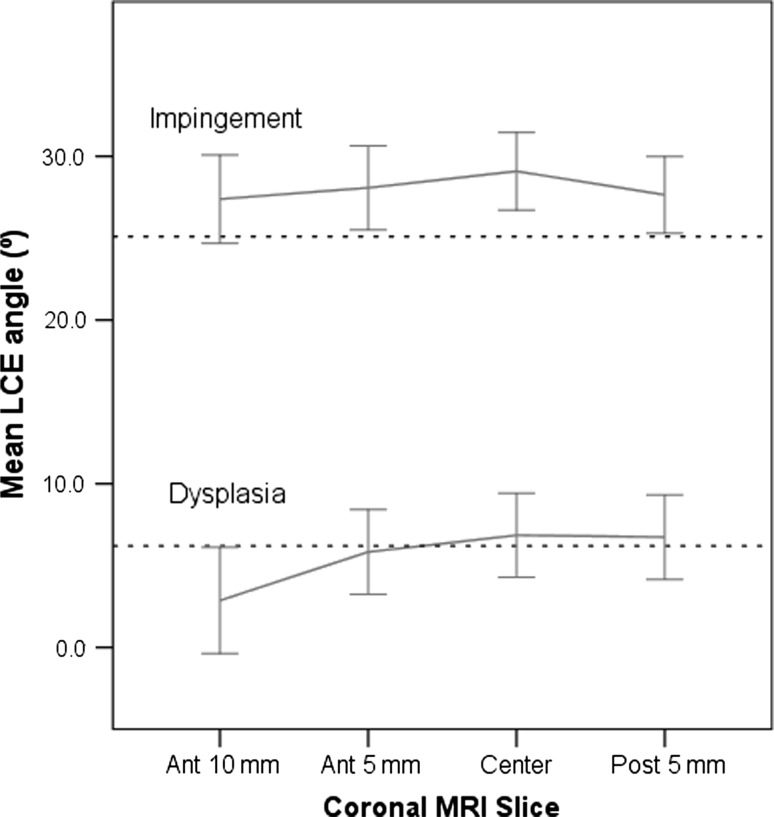
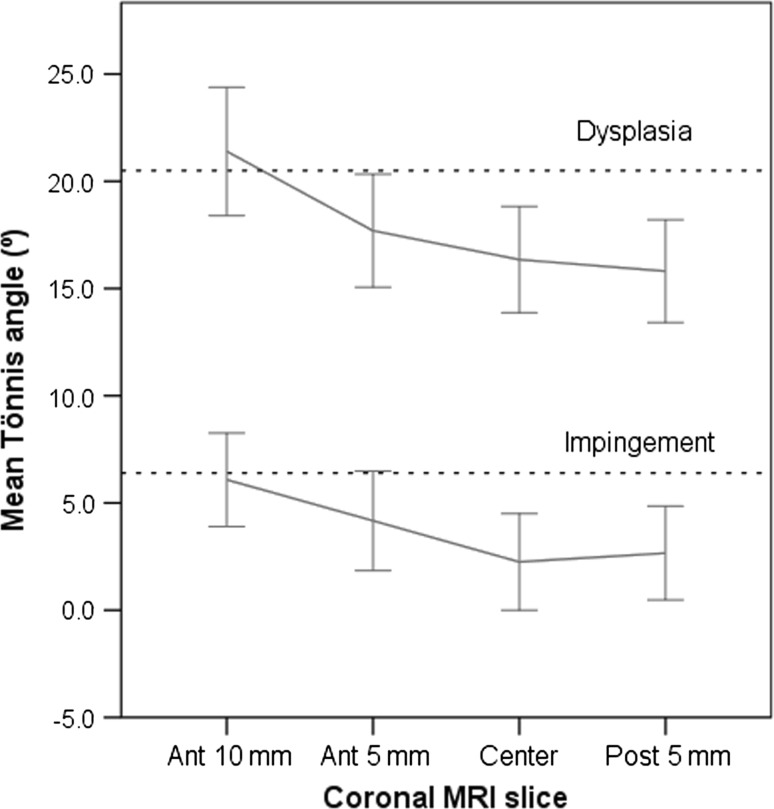
The MRI measurement on the most anterior slice (Ant-10 mm) was most similar to the radiographic LCE angle (13.8º versus 14.6º, respectively; p = 0.27) with the smallest difference (Fig. 2). All other coronal slices (Ant-5 mm, Center, and Post-5 mm) showed higher mean LCE angles on MRI than on radiographs (+1.2º, +2.2º, and +1.5º, respectively; all p < 0.05) (Table 2). The MSE between radiographs and MRI was in the range of 0.33 to 0.53, which indicates good agreement (Table 3). As expected, in the DDH hips the Ant-10 mm slice showed less coverage indicating anterior deficiency typical of DDH (Fig. 3). The correlation coefficient, r, between radiographic and MRI LCE was 0.90 for Ant +10 mm, 0.92 for Ant-5 mm, 0.90 for Center, and 0.89 for Post-5 mm (all p < 0.001). In addition, the mean Tönnis angle was most similar on the Ant-10 mm MRI slice compared with the AP radiograph (14.6º versus 14.2º; p = 0.58; Table 4; Fig. 2). The other coronal MRI slices (Ant-5 mm, Center, and Post-5 mm) showed lower Tönnis angles than on radiographs: −2.5º, −4.1º, and −4.2º, respectively (all p < 0.001) (Fig. 4). The MSE was in the range of 0.41 to 0.49 (Table 3). The correlations between radiographic and MRI Tönnis angle were 0.85, 0.84, 0.86, and 0.85 (anterior to posterior), respectively (all p < 0.001).
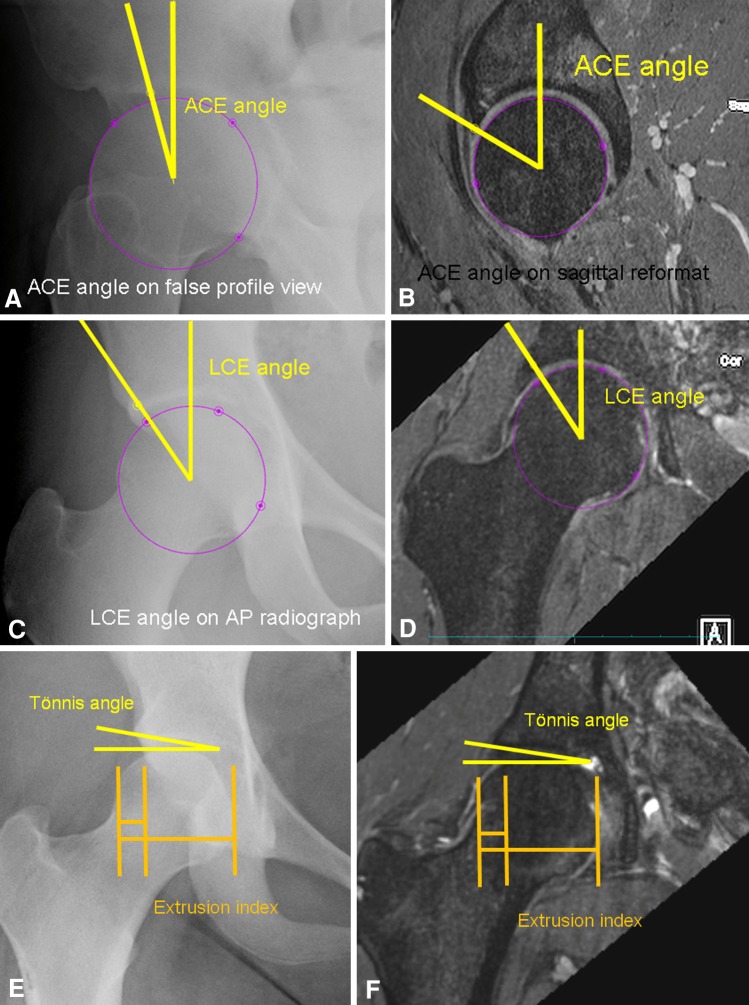
Fig. 2A–F.
Examples are shown comparing measurement of the (A) ACE angle on a false-profile view radiograph and (B) and sagittal reformatted MR image, and the LCE angle on (C) an AP radiograph and (D) MR image The ACE angle on sagittal or oblique sagittal MRI reformats is typically larger than the ACE angle measured on false-profile radiographs. The LCE measurement is comparable on MRI and radiographs. Similarly the Tönnis angle and extrusion index are comparable on (E) the radiograph and (F) the MR image.
Table 2.
Mean values of morphometric measurements
| Measurement parameter | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| LCE | ||||
| Anterior 10 mm | 13.8 | 16.4 | −41.8 | 51.5 |
| Anterior 5 mm | 15.8 | 14.5 | −18.3 | 48.4 |
| Center | 16.8 | 14.2 | −20.8 | 46.3 |
| Posterior 5 mm | 16.1 | 13.7 | −22.8 | 44.2 |
| Radiograph | 14.6 | 13.4 | −24.0 | 47.4 |
| Tönnis | ||||
| Anterior 10 mm | 14.6 | 12.3 | −13.6 | 64.1 |
| Anterior 5 mm | 11.7 | 11.3 | −21.7 | 48.4 |
| Center | 10.1 | 11.1 | −17.6 | 39.8 |
| Posterior 5 mm | 9.9 | 10.6 | −13.4 | 34.5 |
| Radiograph | 14.2 | 10.1 | −10.7 | 43.2 |
| Extrusion index | ||||
| Anterior 10 mm | 25.8% | 14.5% | 0.0% | 67.0% |
| Anterior 5 mm | 26.4% | 13.6% | 0.0% | 65.5% |
| Center | 25.3% | 12.5% | 0.0% | 53.2% |
| Posterior 5 mm | 25.6% | 12.0% | 0.0% | 57.2% |
| Radiograph | 26.7% | 12.5% | 0.0% | 55.1% |
| ACE angle sagittal | ||||
| Sagittal medial 5 mm | 47.5 | 15.8 | 0.0 | 71.4 |
| Sagittal center | 47.1 | 12.9 | 13.2 | 74.2 |
| Sagittal lateral 5 mm | 44.0 | 18.9 | −3.7 | 104.8 |
| Radiograph | 19.9 | 17.6 | −25.0 | 60.0 |
| ACE angle oblique sagittal | ||||
| Oblique sagittal medial 5 mm | 40.2 | 14.6 | −5.4 | 69.6 |
| Sagittal center | 32.9 | 16.6 | −19.5 | 63.8 |
| Oblique sagittal lateral 5 mm | 24.4 | 18.2 | −14.7 | 62.8 |
| Radiograph | 19.9 | 17.7 | −25.0 | 60.0 |
| Acetabular anteversion | ||||
| Roof 5 mm | 4.5 | 9.0 | −24.0 | 25.5 |
| Roof 10 mm | 8.2 | 8.8 | −13.8 | 27.1 |
| Roof 15 mm | 13.4 | 9.4 | −12.0 | 30.2 |
LCE = lateral center-edge; ACE = anterior center-edge.
Table 3.
Precision of measurements
| Measure | MRI slice orientation | Slice name | MSE | MSE-SD |
|---|---|---|---|---|
| LCE angle | Coronal | Ant-10 mm | 0.53 | 0.12 |
| LCE angle | Coronal | Ant-5 mm | 0.33 | 0.10 |
| LCE angle | Coronal | Center | 0.40 | 0.08 |
| LCE angle | Coronal | Post-5 mm | 0.41 | 0.11 |
| Tönnis angle | Coronal | Ant-10 mm | 0.41 | 0.14 |
| Tönnis angle | Coronal | Ant-5 mm | 0.43 | 0.16 |
| Tönnis angle | Coronal | Center | 0.48 | 0.17 |
| Tönnis angle | Coronal | Post-5 mm | 0.49 | 0.19 |
| Extrusion index | Coronal | Ant-10 mm | 0.87 | 0.24 |
| Extrusion index | Coronal | Ant-5 mm | 0.38 | 0.13 |
| Extrusion index | Coronal | Center | 0.38 | 0.11 |
| Extrusion index | Coronal | Post-5 mm | 0.47 | 0.19 |
| ACE angle | Sagittal | S-Med-5 mm | 10.34 | 6.84 |
| ACE angle | Sagittal | S-Center | 8.93 | 6.92 |
| ACE angle | Sagittal | S-Lat-5 mm | 8.02 | 5.11 |
| ACE angle | 25o oblique sagittal | OS-Med-5 mm | 5.45 | 3.71 |
| ACE angle | 25o oblique sagittal | OS-Center | 3.12 | 1.47 |
| ACE angle | 25o oblique sagittal | OS-Lat-5 mm | 1.77 | 0.41 |
MSE = mean squared error; MSE-SD = standard deviation of mean squared error; LCE = lateral center-edge; ACE = anterior center-edge; Ant = anterior; Post = posterior; Med = medial; Lat = lateral; S = sagittal; OS = oblique sagittal.
Fig. 3.
Line plots comparing mean values (and 95% confidence intervals) of the LCE angle for different slices in patients with DDH and FAI are shown. The radiograph mean value for the respective measure is shown as a dashed line.
Table 4.
Differences between radiography and MRI
| Measure | MRI slice orientation | Slice name | Mean difference | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| LCE angle | Coronal | Ant-10 mm | −0.8 | 7.4 | −29.1 | 25.1 |
| LCE angle | Coronal | Ant-5 mm | +1.1 | 5.7 | −16.3 | 19.1 |
| LCE angle | Coronal | Center | +2.1 | 6.1 | −14.3 | 25.4 |
| LCE angle | Coronal | Post-5 mm | +1.4 | 6.4 | −18.8 | 21.1 |
| Tönnis angle | Coronal | Ant-10 mm | −0.1 | 6.5 | −16.6 | 20.9 |
| Tönnis angle | Coronal | Ant-5 mm | −2.4 | 6.2 | −22.4 | 10.5 |
| Tönnis angle | Coronal | Center | −3.6 | 6.0 | −18.9 | 13.9 |
| Tönnis angle | Coronal | Post-5 mm | −3.6 | 6.1 | −17.0 | 14.2 |
| Extrusion index | Coronal | Ant-10 mm | −0.8% | 9.5% | −48.1% | 32.6% |
| Extrusion index | Coronal | Ant-5 mm | −0.3% | 6.3% | −17.5% | 17.5% |
| Extrusion index | Coronal | Center | −1.3% | 6.1% | −21.3% | 10.6% |
| Extrusion index | Coronal | Post-5 mm | −1.1% | 6.9% | −22.2% | 16.7% |
| ACE angle | Sagittal | S-Med-5 mm | +27.8 | 17.5 | −19.6 | 93.5 |
| ACE angle | Sagittal | S-Center | +27.4 | 14.1 | −17.7 | 76.7 |
| ACE angle | Sagittal | S-Lat-5 mm | +23.8 | 16.1 | −19.7 | 74.0 |
| ACE angle | 25o oblique sagittal | OS-Med-5 mm | +20.9 | 12.6 | −4.0 | 71.2 |
| ACE angle | 25o oblique sagittal | OS-Center | +13.5 | 13.0 | −24.1 | 70.7 |
| ACE angle | 25o oblique sagittal | OS-Lat-5 mm | +5.1 | 13.6 | −22.8 | 65.8 |
LCE = lateral center-edge; ACE = anterior center-edge; Ant = anterior; Post = posterior; Lat = lateral; Med = medial; S = sagittal; OS = oblique sagittal.
Fig. 4.
Line plots comparing mean values (and 95% confidence intervals) of the Tönnis angle for different slices in patients with DDH and FAI are shown. The radiograph mean value for the respective measure is shown as a dashed line.
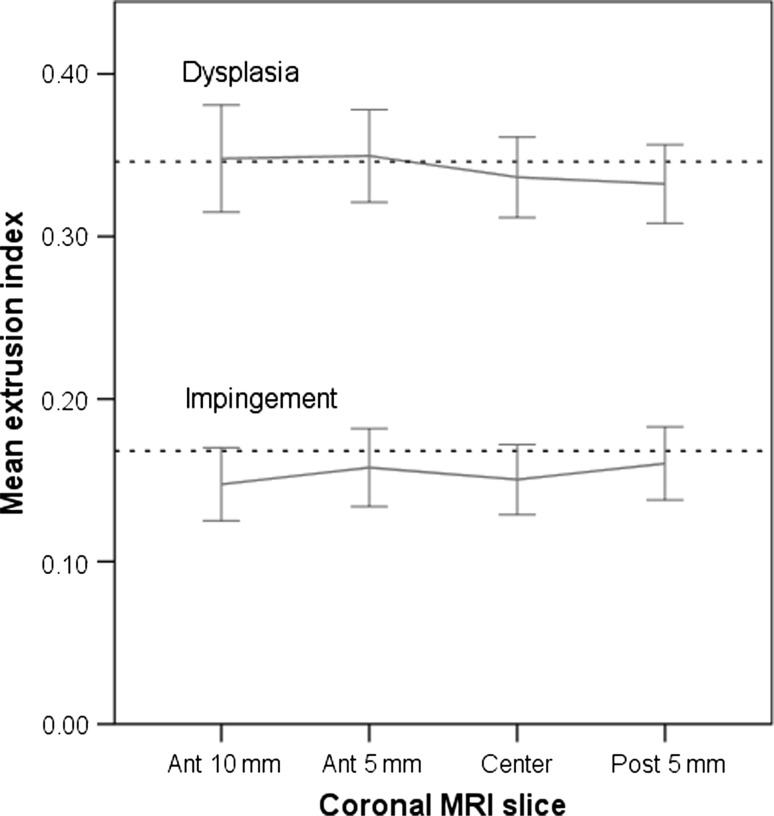
The mean MRI extrusion index was similar to that of the radiographs in all slices (p = 0.10 to 0.66) except for the center slice (p = 0.03; Fig. 5). The extrusion index showed MSE values of 0.38 to 0.87 (Table 3). The correlation between radiographic and MRI extrusion indexes was 0.76 (Ant-10 mm), 0.89 (Ant-5 mm), 0.88 (Center), and 0.84 (Post-5 mm), respectively.
Fig. 5.
Line plots comparing mean values (and 95% confidence intervals) of the femoral head extrusion index for different slices in patients with DDH and FAI are shown. The radiograph mean value for the respective measure is shown as a dashed line.
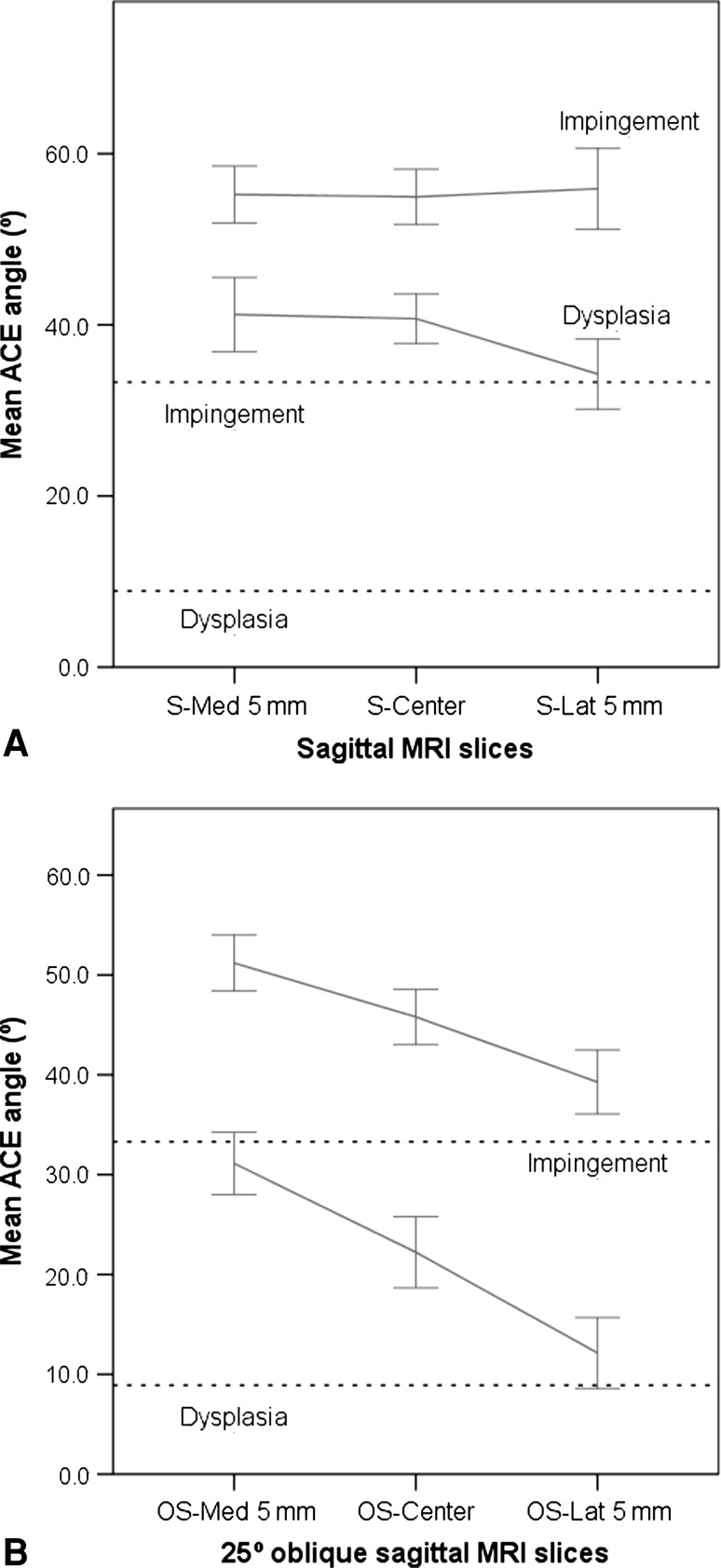
The ACE angle on false-profile view radiographs was compared with straight sagittal and 25º oblique sagittal MRI slices (Fig. 2). The straight sagittal MRI slices showed higher mean ACE values (47.5º, 47.1º, and 44.0º, medial to lateral) than the radiographs (19.9º; all p < 0.001) (Table 2). The ACE angles on oblique sagittal MRI slices (40.2º, 32.9º, and 24.4º, medial to lateral) were closer to the mean value of the radiographs (19.9º); however, they still were higher (all p < 0.001) (Fig. 6). The MSE values ranged from 8.02 to 10.34 for the straight sagittal and 1.77 to 5.45 for the oblique sagittal MRI slices (Table 3). The correlations of straight sagittal MRI ACE and radiographs were 0.47 to 0.65. The correlation with radiograph was higher on the oblique sagittal slices (0.75 to 0.76).
Fig. 6A–B.
(A) Line plots comparing mean values (and 95% confidence intervals) of the ACE angle for different straight sagittal slices in patients with DDH and FAI are shown. The radiograph mean value for the respective measure is shown as a dashed line. (B) Similarly, line plots comparing mean values (and 95% confidence intervals) of the ACE angle for different oblique sagittal slices also show disagreement between MRI and radiograph measurements, with the least difference in the OS-Lat 5 mm slice.
Hips with a positive crossover sign had lower (all p < 0.01) acetabular anteversion than those with a negative crossover sign on all three MRI slices: from superior to inferior: 3.4º, 6.9º, and 11.7º versus 7.8º, 12.6º, and 19.1º. Similar results were obtained for the posterior wall sign. When the posterior wall sign was positive, the acetabular anteversion was decreased (all p < 0.05) on all three slices: 3.1º, 7.1º, and 12.0º versus 9.1º, 12.0º, and 18.2º. These results also show an increase in acetabular anteversion from superior to inferior (Roof-5 mm to Roof-15 mm).
Discussion
Because MRI is being used more frequently to assess the hip, it could be advantageous to use MRI for morphometric assessment in patients with FAI and DDH. The measurement of established morphometric radiographic parameters on MRI could help to avoid the need for additional radiographs and thus reduce costs. We compared MRI and radiography for evaluation of standard morphometric parameters of the hip.
There are limitations to our study. First, in abnormally shaped femoral heads finding the best-fitting circle using the template method according to Mose [28] is sometimes difficult to determine. If a nonspherical head was present, the femoral head center was assumed to be the center of the circle drawn along the weightbearing surface. The same method was used on radiographs and MRI, thus we believe there was no bias in the results of our study. Second, there is limited validity of our results for hips with high-grade dysplasia since no bony acetabular roof was detectable on eight MRI slices of three patients. Third, we did not correct the MRI slices for the intrinsic radiographic projection error, which is a result of beam divergence when radiographs originate from a point-like source. This projection error is stronger in the lateral parts of an image and can be approximately 6º for the hip on an AP pelvic radiograph. This projection error on radiographs can be eliminated on MRI, thus supporting the use of MRI for morphometric assessment of the hip. Fourth, the radiographic measurement reproducibility data reported in the literature suggest only moderate to good reproducibility of measurements [1, 11, 27, 29, 32], what may be a limitation to our study. Sixth, patient positioning on MRI and radiography also might affect our results. Seventh, our findings may be valid for only preoperative evaluation of patients because we excluded patients who had prior surgery. Either acetabular or femoral surgery might cause remodeling of the joint and lead to changes [10] and susceptibility artifacts caused by metallic particles in postoperative joints can affect MRI evaluation [2]. Because MRI is used primarily for preoperative evaluation this does not limit the use of MRI to assess morphometry in the majority of patients.
Our results indicate the LCE on the MRI slice Ant-10 mm (coronal slice 10 mm anterior of the center of the femoral head) is the best estimator of the radiographic LCE angle. All other coronal slices (Ant-5 mm, Center, and Post-5 mm) showed slightly higher mean LCE angles on MRI than on radiographs, but the mean differences were still in an acceptable range (+1.1º to +2.1º). MSE values in the range of 0.33 to 0.53 suggest that MRI-based angles were in excellent agreement with the gold standard of radiographic measurements. These estimated differences were within or below the range of previously reported radiographic interobserver differences for the LCE angle [1, 11, 27, 29, 32]. This suggests MRI can be used to measure a radiograph-like LCE angle with sufficient precision.
Similarly, the excellent agreement between coronal MRI and radiographic angle measurements suggests MRI also may be used to estimate the Tönnis angle. When comparing our data with the radiographic interobserver data of Nelitz et al. [29], we show higher agreement in the comparison of MRI with radiograph measurements. This supports the use of MRI measurements of the Tönnis angle.
In addition, the extrusion index on MRI shows good agreement with radiograph measurements. The mean difference determined in our study is lower than the radiograph interobserver and intraobserver variabilities reported by Nelitz et al. [29]. This suggests that MRI can be used for assessment of the extrusion index.
The ACE angle measurements on radiographs and MRI were different, with MRI measurements showing markedly higher angles on the sagittal (+24º to +28º) and the oblique sagittal slices (+5º to +21º). The only position that was closer to the radiographic measurement was the OS-Lat MRI slice, which had a mean difference of +5.1º. This raises the question of why the projecting structures on the false-profile radiograph are so different compared with the MRI slices. Sakai et al. [31] reported that although ACE angle in normal hips is close to the true anterior coverage on CT, the ACE angle does not indicate true anterior coverage in dysplastic hips. Although CT is the gold standard for bony assessment, MRI can be used to assess bony coverage as long as care is taken to discriminate between soft tissue structures like the labrum and bone. A crucial factor for the ACE measurement on radiographs is the choice of the anterior reference point [31]. We took the radiodense shadow of the most anterior portion of the sourcil as described in the first report of the ACE angle by Lequesne and de Seze [21]. However, Sakai et al. [31] reported that this point of measurement underestimates the true anterior coverage on CT. In our opinion the evaluation on the false-profile view has limitations. First, the anterior edge on the false-profile view is a point that is more difficult to define than the lateral edge on an AP view as a result of overlay projections of anatomic structures. This leads to some inaccuracy in measuring the ACE angle. Second, the exact rotation of the pelvis when taking the false-profile view is difficult to establish and may differ among radiographs. Rotation of the pelvis around the vertical axis, as a result of inaccuracies during radiograph registration, may result in inaccurate ACE angles in dysplastic hips [24]. We found a higher correlation of ACE angles on the oblique sagittal slices than on the straight sagittal slices in comparison to the findings on the radiographs. These results are consistent because the 25º-oblique sagittal MRI slices are closer to the radiographic projection plane on false-profile views. The radiographic ACE angle on false-profile views does not appear to reflect the true anterior coverage. This might be a result of low radiographic density of the true anterior rim.
In addition, the acetabular anteversion on straight transversal MRI reformats was compared with signs of retroversion on AP radiographs. The acetabular opening is anteverted in the normal hip, but in some cases, a relative or absolute retroversion of the acetabulum can be found [30]. It is particularly important for the pathomechanics found in pincer-type FAI, in which anterolateral overcoverage leads to abutment of joint partners during flexion and internal rotation [13]. However, retroversion is common in patients with DDH [12, 30]. This retroversion may occur as a result of a hypoplastic posterior acetabular wall, a prominence of the anterior acetabular wall, or a rotational abnormality of the acetabulum, which also is associated with hip pain and osteoarthritis of the hip [30]. We found markedly lower acetabular anteversion (relative retroversion) in hips with a positive crossover sign and in hips with a positive posterior wall sign. We found a stepwise increase of acetabular anteversion from superior (Roof-5 mm) to inferior (Roof-15 mm), which is consistent with other studies [12, 16]. Because the superior transverse cuts are reportedly more sensitive for detection of retroversion [16], we chose to use superior MRI slices only. Our observations suggest radiographic signs of retroversion give an approximate estimate of retroversion found on MRI, which is consistent with reported results [12, 16].
As shown by our data the MRI slice selection may affect the results. The selection of MRI slice also depends on practical issues. In general the central coronal slice (the slice with the largest diameter of the femoral head) can be used, but small differences from radiographic measurement must be expected (Table 4). In cases where comparability of MRI and radiographic measurements is important, the Ant +10 mm slice should be used for morphometric measurements. In addition, the Ant +10 mm slice measurements are affected not only by lateral coverage, but also by anterior coverage.
Our data suggest MRI measurements of the LCE angle, Tönnis angle, and extrusion index reasonably agree with radiographic measurements and can be used to accurately quantify these parameters. However, the ACE angles measured on straight sagittal or 25º oblique sagittal MRI slices deviate from ACE angles from false-profile radiographs, and thus MRI measurements are not appropriate for quantifying these angles.
Acknowledgments
We thank Catherine Stamoulis PhD, for help with the statistics and Kerri Murray and Jeffrey Tsang for help with database research.
Footnotes
The institution of one of the authors (Y-JK) received research funding from Siemens Healthcare and the Orthopaedic Research and Education Foundation.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Anderson LA, Gililland J, Pelt C, Linford S, Stoddard GJ, Peters CL. Center edge angle measurement for hip preservation surgery: technique and caveats. Orthopedics. 2011;34:86. doi: 10.3928/01477447-20101221-17. [DOI] [PubMed] [Google Scholar]
- 2.Beaule PE, Allen DJ, Clohisy JC, Schoenecker PL, Leunig M. The young adult with hip impingement: deciding on the optimal intervention. Instr Course Lect. 2009;58:213–222. [PubMed] [Google Scholar]
- 3.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012–1018. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 4.Chosa E, Tajima N. Anterior acetabular head index of the hip on false-profile views: new index of anterior acetabular cover. J Bone Joint Surg Br. 2003;85:826–829. [PubMed] [Google Scholar]
- 5.Clohisy JC, Carlisle JC, Beaule PE, Kim YJ, Trousdale RT, Sierra RJ, Leunig M, Schoenecker PL, Millis MB. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90(suppl 4):47–66. doi: 10.2106/JBJS.H.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clohisy JC, Carlisle JC, Trousdale R, Kim YJ, Beaule PE, Morgan P, Steger-May K, Schoenecker PL, Millis M. Radiographic evaluation of the hip has limited reliability. Clin Orthop Relat Res. 2009;467:666–675. doi: 10.1007/s11999-008-0626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooperman DR, Wallensten R, Stulberg SD. Acetabular dysplasia in the adult. Clin Orthop Relat Res. 1983;175:79–85. [PubMed] [Google Scholar]
- 8.Crockarell JR, Jr, Trousdale RT, Guyton JL. The anterior centre-edge angle: a cadaver study. J Bone Joint Surg Br. 2000;82:532–534. doi: 10.1302/0301-620X.82B4.10063. [DOI] [PubMed] [Google Scholar]
- 9.Delaunay S, Dussault RG, Kaplan PA, Alford BA. Radiographic measurements of dysplastic adult hips. Skeletal Radiol. 1997;26:75–81. doi: 10.1007/s002560050197. [DOI] [PubMed] [Google Scholar]
- 10.Domayer SE, Ziebarth K, Chan J, Bixby S, Mamisch TC, Kim YJ. Femoroacetabular cam-type impingement: diagnostic sensitivity and specificity of radiographic views compared to radial MRI. Eur J Radiol. 2011;80:805–810. doi: 10.1016/j.ejrad.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Fowkes LA, Petridou E, Zagorski C, Karuppiah A, Toms AP. Defining a reference range of acetabular inclination and center-edge angle of the hip in asymptomatic individuals. Skeletal Radiol. 2011;40:1427–1434. doi: 10.1007/s00256-011-1109-3. [DOI] [PubMed] [Google Scholar]
- 12.Fujii M, Nakashima Y, Yamamoto T, Mawatari T, Motomura G, Matsushita A, Matsuda S, Jingushi S, Iwamoto Y. Acetabular retroversion in developmental dysplasia of the hip. J Bone Joint Surg Am. 2010;92:895–903. doi: 10.2106/JBJS.I.00046. [DOI] [PubMed] [Google Scholar]
- 13.Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res. 2008;466:264–272. doi: 10.1007/s11999-007-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Minka MA, 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect: a MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83:171–176. doi: 10.1302/0301-620X.83B2.11092. [DOI] [PubMed] [Google Scholar]
- 16.Jamali AA, Mladenov K, Meyer DC, Martinez A, Beck M, Ganz R, Leunig M. Anteroposterior pelvic radiographs to assess acetabular retroversion: high validity of the “cross-over-sign”. J Orthop Res. 2007;25:758–765. doi: 10.1002/jor.20380. [DOI] [PubMed] [Google Scholar]
- 17.Jessel RH, Zurakowski D, Zilkens C, Burstein D, Gray ML, Kim YJ. Radiographic and patient factors associated with pre-radiographic osteoarthritis in hip dysplasia. J Bone Joint Surg Am. 2009;91:1120–1129. doi: 10.2106/JBJS.G.00144. [DOI] [PubMed] [Google Scholar]
- 18.Kappe T, Kocak T, Reichel H, Fraitzl CR. Can femoroacetabular impingement and hip dysplasia be distinguished by clinical presentation and patient history? Knee Surg Sports Traumatol Arthrosc. 2012;20:387–392. doi: 10.1007/s00167-011-1553-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Bixby S, Mamisch TC, Clohisy JC, Carlisle JC. Imaging structural abnormalities in the hip joint: instability and impingement as a cause of osteoarthritis. Semin Musculoskelet Radiol. 2008;12:334–345. doi: 10.1055/s-0028-1100640. [DOI] [PubMed] [Google Scholar]
- 20.Lequesne M. [Coxometry: measurement of the basic angles of the adult radiographic hip by a combined protractor][in French] Rev Rhum Mal Osteoartic. 1963;30:479–485. [PubMed] [Google Scholar]
- 21.Lequesne M, de Seze S. [False profile of the pelvis: a new radiographic incidence for the study of the hip. Its use in dysplasias and different coxopathies][in French] Rev Rhum Mal Osteoartic. 1961;28:643–652. [PubMed] [Google Scholar]
- 22.Leunig M, Ganz R. [The Bernese method of periacetabular osteotomy][in German] Orthopade. 1998;27:743–750. doi: 10.1007/pl00003460. [DOI] [PubMed] [Google Scholar]
- 23.Leunig M, Huff TW, Ganz R. Femoroacetabular impingement: treatment of the acetabular side. Instr Course Lect. 2009;58:223–229. [PubMed] [Google Scholar]
- 24.Li PL, Ganz R. Morphologic features of congenital acetabular dysplasia: one in six is retroverted. Clin Orthop Relat Res. 2003;416:245–253. doi: 10.1097/01.blo.0000081934.75404.36. [DOI] [PubMed] [Google Scholar]
- 25.Locher S, Werlen S, Leunig M, Ganz R. [Inadequate detectability of early stages of coxarthrosis with conventional roentgen images][in German] Z Orthop Ihre Grenzgeb. 2001;139:70–74. doi: 10.1055/s-2001-11873. [DOI] [PubMed] [Google Scholar]
- 26.Mast JW, Brunner RL, Zebrack J. Recognizing acetabular version in the radiographic presentation of hip dysplasia. Clin Orthop Relat Res. 2004;418:48–53. doi: 10.1097/00003086-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res. 2011;469:188–199. doi: 10.1007/s11999-010-1447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mose K. Methods of measuring in Legg-Calve-Perthes disease with special regard to the prognosis. Clin Orthop Relat Res. 1980;150:103–109. [PubMed] [Google Scholar]
- 29.Nelitz M, Guenther KP, Gunkel S, Puhl W. Reliability of radiological measurements in the assessment of hip dysplasia in adults. Br J Radiol. 1999;72:331–334. doi: 10.1259/bjr.72.856.10474491. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum: a cause of hip pain. J Bone Joint Surg Br. 1999;81:281–288. doi: 10.1302/0301-620X.81B2.8291. [DOI] [PubMed] [Google Scholar]
- 31.Sakai T, Nishii T, Sugamoto K, Yoshikawa H, Sugano N. Is vertical-center-anterior angle equivalent to anterior coverage of the hip? Clin Orthop Relat Res. 2009;467:2865–2871. doi: 10.1007/s11999-009-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tannast M, Siebenrock KA, Anderson SE. [Femoroacetabular impingement: radiographic diagnosis–what the radiologist should know][in Spanish] Radiologia. 2008;50:271–284. doi: 10.1016/S0033-8338(08)71986-6. [DOI] [PubMed] [Google Scholar]
- 33.Toennis D. [On changes in the acetabular vault angle of the hip joint in rotated and tilted positions of the pelvis in children][in German] Z Orthop Ihre Grenzgeb. 1962;96:462–478. [PubMed] [Google Scholar]
- 34.Treguier C, Chapuis M, Branger B, Grellier A, Chouklati K, Bruneau B, Fraisse B, Violas P, Pladys P, Darnault P, Gandon Y. [Developmental dysplasia of the hip][in French] J Radiol. 2011;92:481–493. doi: 10.1016/j.jradio.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint. Acta Chir Scand 1939. 1939;58:5–135. [Google Scholar]