Abstract
Background
Bony tumors of the foot account for approximately 3% of all osseous tumors. Diagnosis is frequently delayed as a result of lack of clinician familiarity and as a result of their rarity. The reasons for the delays, however, are unclear.
Questions/purposes
We therefore determined (1) how hindfoot tumors present and the specific reasons for delay in diagnosis; (2) whether the spectrum of disease varies between the talus and calcaneus; and (3) how these patients were treated.
Methods
We retrospectively reviewed the medical notes and imaging for all patients with 34 calcaneal and 23 talar tumors recorded in the Scottish Bone Tumour Registry. Demographics, presentation, investigation, histology, management, recurrence, and mortality were recorded.
Results
Hindfoot tumors present with pain and often swelling around the heel (calcaneus) or ankle (talus), most often misdiagnosed as soft tissue injury. Calcaneal lesions were more likely to be malignant than talar lesions: 13 of 34 versus three of 23.
Conclusions
Clinicians should be aware that hindfoot tumors can be initially misdiagnosed as soft tissue injuries and suspicion of a tumor should be raised in the absence of trauma or persistent symptoms. Lesions affecting the calcaneus are more likely to be malignant. Early diagnosis and adjuvant therapy are important.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bony tumors of the foot account for approximately 3% of all osseous tumors [23]. In the series of Murrari et al. [16] of 255 osseous neoplasms of the foot, the calcaneus was the second most common site after the metatarsals accounting for 31% of benign and 35% of malignant lesions. The talus was a rare location accounting for just 8% of benign lesions and none that were malignant [16]. The existing literature is limited: there have been two location-specific small case series of calcaneus and talus, respectively [4, 10], three case series of osseous neoplasms of the foot [1, 5, 16] or the hand and foot [19], and tumor-specific review articles [3, 7, 8, 11, 13, 17, 18, 21]. The majority comprise individual case reports describing specific osseous lesions and their treatment.
Delay in diagnosis or missed diagnosis of hindfoot lesions is frequently reported [2, 6, 7, 9, 10, 15, 22]. This is largely attributed to the rarity and lack of familiarity of many clinicians to tumors of the hindfoot. Surgical management poses further challenges as a result of the complex anatomy of the os calcis and talus. Early diagnosis appears essential as a result of high rates of metastasis in high-grade malignancy [2, 7, 24]. One author [2] noted four of seven patients with high-grade osteosarcoma died from metastasis (mean survival 50 months), whereas another study [7] noted nine of 14 patients died from metastasis after a mean of 2.5 years (range, 1–6 years).
We therefore determined (1) how hindfoot tumors present and the specific reasons for delay in diagnosis; (2) whether the spectrum of disease varies between the talus and calcaneus; and (3) how these patients were treated.
Patients and Methods
We retrospectively reviewed the medical records and imaging of all 63 patients with tumors or tumor-like conditions of the calcaneus and talus from the Scottish Bone Tumour Registry (SBTR) between January 1954 and July 2010. All patients presenting with primary bone tumors have been prospectively entered into the SBTR by independent audit clerks after review by histopathologists with a specialist interest in musculoskeletal oncology. The SBTR receives approximately 100 cases per year with 4800 cases recorded to date. Data include copies of all clinical and histopathology records, hard copies of original radiographic imaging as well as a typed proforma covering patient demographics, diagnosis, and management. All primary osseous tumors of the calcaneus and talus were included. We excluded six patients with lesions attributed to infection, degenerative or posttraumatic cysts, metastases, nonprimary lymphoma, and pigmented villonodular synovitis. These exclusions left 57 patients with 34 calcaneal lesions (21 benign and 13 malignant) and 23 talar tumors (20 benign and three malignant). Three of these 57 patients were lost to followup, all as a result of emigration, at 4 months, 18 months, and 8 years followup. Of the 34 calcaneal tumors, mean age at diagnosis was 25 years (range, 12–68 years) for benign lesions, 37 years (range, 17–67 years) for chondrosarcoma, and 41 years (range, 14–78 years) for high-grade sarcoma. The patients with benign talar tumors presented at a mean age of 25 years (range, 10–47 years) and those with malignant talar tumors at 49 years (range, 39–64 years). All other patients were followed for a minimum of 1 year (median, 3 years; range, 1–20 years) until discharge or death. The followup varied depending on clinician preference, histology, and patient factors. No patients were recalled specifically for this study; all data were obtained from medical records and imaging.
Patient demographics, presenting symptoms, length of symptoms, and time from tertiary referral to definitive treatment were recorded as was the biopsy modality and histological diagnosis. All diagnoses were based on histology either from biopsy or postoperative specimens. Benign lesions were subclassified as latent, active, or aggressive (Tables 2 and 3); malignant lesions were classed as either low-grade or high-grade (Tables 3 and 4). The surgical management, adjuvant therapy, local recurrence, distant metastasis, and followup were noted. Surgical treatment modality was classified as intralesional with or without bone graft, marginal excision, wide local excision, or radical involving below-knee amputation.
Table 2.
Benign calcaneal lesions
| Case number | Presented (year) | Histological diagnosis (secondary change) |
Spectrum | Sex/age (years) | Treatment |
|---|---|---|---|---|---|
| 1 | 1992 | IOL | Latent | F/44 | ILC and BG |
| 2 | 1985 | UBC | Active | M/17 | ILC |
| 3 | 1988 | UBC | Active | M/21 | ILC and BG |
| 4 | 1987 | UBC | Active | F/34 | Conservative |
| 5 | 1997 | UBC | Active | M/20 | ILC, steroid |
| 6 | 2007 | UBC | Active | M/13 | ILC and BG |
| 7 | 2009 | UBC | Active | F/23 | ILC and BG |
| 8 | 1994 | OO | Active | F/23 | ME |
| 9 | 2005 | OO | Active | F/15 | ME |
| 10 | 1979 | OC | Active | M/16 | ME |
| 11 | 1967 | OC | Active | M/57 | ME |
| 12 | 1982 | GT | Active | M/68 | ME |
| 13 | 2005 | CB | Aggressive | M/12 | ILC and BG |
| 14 | 1969 | CB | Aggressive | M/17 | ILC and BG |
| 15 | 2006 | CB (+ABC) | Aggressive | M/19 | ILC and BG |
| 16 | 2007 | ABC | Aggressive | M/23 | ILC, HSB, and BG |
| 17 | 2005 | ABC | Aggressive | F/14 | ILC, HSB, and BG |
| 18 | 1979 | CMF | Aggressive | F/13 | ILC |
| 19 | 2009 | OB | Aggressive | F/23 | RFA then ILC |
| 20 | 1954 | GCT | Aggressive | M/30 | ILC |
| 21 | 2008 | BFH | Aggressive | F/33 | ILC and BG |
IOL = intraosseous lipoma; UBC = unicameral bone cyst; OO = osteoid osteoma; GT = glomus tumor; CB = chondroblastoma; ABC = aneurysmal bone cyst; CMF = chondromyxoid fibroma; OB = osteoblastoma; BFH = benign fibrous histiocytoma; F = female; M = male; ILC = intralesional curettage; BG = bone grafting; ME = marginal excision; HSB = high-speed burr; RFA = radiofrequency ablation
Table 3.
Benign and malignant talar lesions
| Case number | Presented (year) | Histological diagnosis (secondary change) | Spectrum | Sex/age (years) | Treatment |
|---|---|---|---|---|---|
| 1 | 1985 | IOG | Latent | M/28 | ILC |
| 2 | 1997 | IOG | Latent | F/42 | ILC |
| 3 | 1964 | OO | Active | M/15 | ME |
| 4 | 1967 | OO | Active | M/16 | ME |
| 5 | 1986 | OO | Active | M/28 | ME |
| 6 | 1988 | OO | Active | F/21 | ME |
| 7 | 2007 | OO | Active | M/20 | ME |
| 8 | 2008 | OO | Active | M/27 | ME |
| 9 | 2009 | OO | Active | M/13 | RFA |
| 10 | 1968 | OC | Active | M/37 | ME |
| 11 | 1973 | OC | Active | M/35 | ME |
| 12 | 2000 | ML | Active | F/24 | ILC and BG |
| 13 | 1986 | CB | Aggressive | M/28 | ILC and BG |
| 14 | 1990 | CB | Aggressive | M/30 | ILC and BG |
| 15 | 2004 | CB | Aggressive | M/30 | ILC and BG |
| 16 | 2007 | CB | Aggressive | F/15 | ILC, HSB, and BG |
| 17 | 1977 | DF | Aggressive | M/17 | ILC and BG |
| 18 | 1962 | OB | Aggressive | F/10 | ILC and BG |
| 19 | 1962 | OB | Aggressive | M/23 | ME |
| 20 | 1967 | GCT | Aggressive | F/47 | BKA |
| 21 | 1987 | CS | Low grade | M/39 | BKA |
| 22 | 1977 | CS | High grade | M/64 | BKA |
| 23 | 2004 | OS | High grade | M/44 | BKA |
IOG = intraosseous ganglion; OO = osteoid osteoma; OC = osteochondroma; ML = membranous lipodystrophy; CB = chondroblastoma; DF = desmoplastic fibroma; OB = osteoblastoma; GCT = giant cell tumor; CS = chondrosarcoma; OS = osteosarcoma; M = male; F = female; ILC = intralesional curettage; ME = marginal excision; RFA = radiofrequency ablation; BG = bone grafting; HSB = high-speed burr; BKA = below-knee amputation
Table 4.
Malignant calcaneal lesions
| Case number | Presented (year) | Histological diagnosis (Background Disease) |
Spectrum | Sex/age (years) | Treatment | Radiotherapy/chemotherapy | Metastasis | Death resulting from disease | Survival/followup |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1974 | CS | Low Grade | M/17 | ILC then BKA | Non | Non | Uncertain | 8 years |
| 2 | 2000 | CS | Low Grade | M/41 | ILC, HSB, PC | Non | Non | No | 10 years |
| 3 | 2002 | CS (Maffucci’s) | Low Grade | F/43 | ILC, PC then BKA | Non | Non | No | 5 years |
| 4 | 2003 | CS | Low Grade | M/19 | WLE | Non | Non | No | 1 year |
| 5 | 2008 | CS | Low Grade | M/67 | BKA | Non | Non | Uncertain | 18 months |
| 6 | 1975 | ES | High Grade | M/32 | BKA | Both | Pulmonary, Spine | Yes | 1 year |
| 7 | 1978 | ES | High Grade | M/17 | BKA | Radiotherapy | Pulmonary | Yes | 6 years |
| 8 | 1987 | OS | High Grade | F/21 | ILC then BKA | Non | Non | Alive | 20 years |
| 9 | 1989 | OS | High Grade | F/14 | BKA, Thoracotomy | Chemotherapy | Pulmonary | Yes | 9 years |
| 10 | 1989 | OS | High Grade | M/75 | BKA | Non | Pulmonary | Yes | 2 years |
| 11 | 1999 | OS | High Grade | M/20 | BKA | Chemotherapy | Non | Alive | 10 years |
| 12 | 1965 | OS (Paget’s) | High Grade | M/78 | Non | Non | Pulmonary, Tibia | Yes | 6 weeks |
| 13 | 1984 | OS (Paget’s) | High Grade | F/68 | BKA | Non | Non | No | 5 years |
CS = chondrosarcoma; ES = Ewing’s sarcoma; OS = osteosarcoma; M = male; F = female; ILC = intralesional curettage; HSB = high-speed burr; PC = polymethylmethacrylate cementoplasty; WLE = wide local excision; BKA = below-knee amputation
All patients had plain radiographs taken at presentation, including AP and lateral views of the ankle, with Harris (axial) views of the calcaneus in calcaneal lesions. Depending on the decade of presentation, patients with suspected malignancy or uncertain diagnosis based on radiographs underwent further imaging with bone scans (n = 14), CT (n = 20), or MRI (n = 17).
All malignant bone tumors were followed closely. All patients attended 2 weeks postoperatively for histology results and wound inspection. Followup varied thereafter depending on grade of malignancy, adjuvant therapy, and treating surgeon. High-grade malignancies were followed up at 6 weeks, 3 months, 6 months, 9 months (four patients), 12 months, and annually thereafter unless recurrence or metastasis was noted. Patients underwent clinical examination including wound inspection; pain or tenderness around the ankle or hindfoot; ankle, subtalar, and midfoot ROM; and radiographic imaging. This included plain radiographs (AP, lateral, and Harris views in calcaneal lesions) in all patients at all visits, followup CT in three patients at 6 months (n = 1) or 1 year (n = 2), and MRI in four patients at 1 year (n = 4).
Results
Pain was the most common presenting symptom, particularly in talar tumors, and was more severe in malignancy (Table 1). Swelling was frequently present, more commonly in malignancy. Four patients presented with pathological fracture, two with large unicameral bone cysts and two with high-grade osteosarcoma. Fourteen patients with talar tumors were initially misdiagnosed with nine treated as ankle sprains, two investigated for a ganglion, and three as other soft tissue injury or rheumatologic problem.
Table 1.
Comparative presentation
| Presenting symptom details | Calcaneus | Talus | ||
|---|---|---|---|---|
| Benign | Malignant | Benign | Malignant | |
| Number of patients | 21 | 13 | 20 | 3 |
| Mean length of symptoms (months with range) | 8 (1–36) | 10 (1–180) | 11 (1–48) | 5 (1–9) |
| Pain as presenting symptom | 19 (90%) | 11 (85%) | 20 (100%) | 3 (100%) |
| Severity of pain (median) | Mild-moderate | Severe | Mild-moderate | Severe |
| Swelling as presenting symptom | 9 (43%) | 8 (62%) | 4 (20%) | 3 (100%) |
| Pathological fracture | 2 (9.5%) | 2 (15%) | 0 (0%) | 0 (0%) |
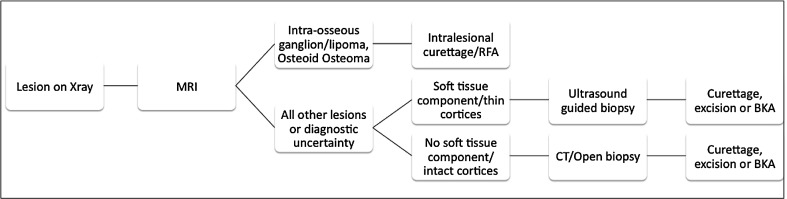
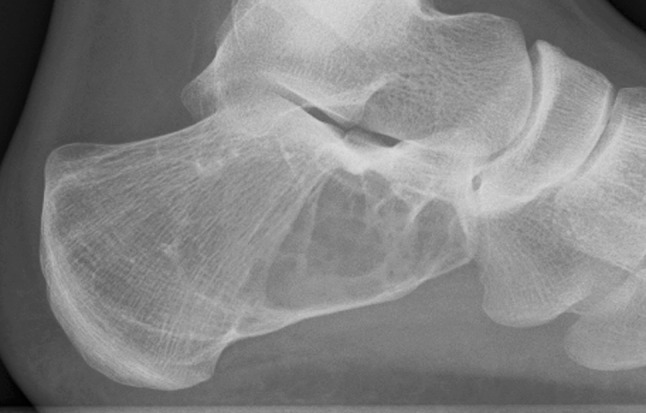
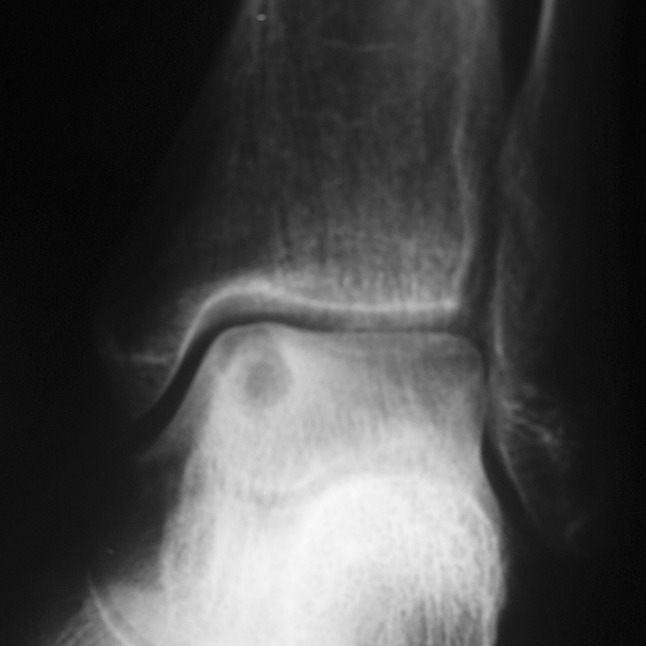
We found a greater ratio of malignant tumors of the calcaneus (benign:malignant ratio; calcaneus 1.6:1; talus 6.7:1). Of the 21 benign calcaneal tumors, there was a wide spectrum of pathology; however, the most common lesion was unicameral bone cyst (n = 6; Fig. 1; Table 2). In contrast, of the 20 benign talar lesions, by far the most common was osteoid osteoma (n = 7; Fig. 2) followed by chondroblastoma (n = 4) (Table 3). Of the 13 malignant calcaneal lesions, five were classified as low-grade histologically and eight high-grade (Table 4; Fig. 3). Two osteosarcomas presented in patients with Paget’s disease and one chondrosarcoma presented in a patient with known Maffucci syndrome. Of the three talar malignancies, two were low-grade and one high-grade (Table 3).
Fig. 1.
Lateral calcaneal radiograph showing a well-defined, radiolucent unicameral bone cyst (Table 1, Case 7)
Fig. 2.
AP ankle radiograph showing classic appearance of osteoid osteoma within the superomedial aspect of the talus (Table 3, Case 5)
Fig. 3.
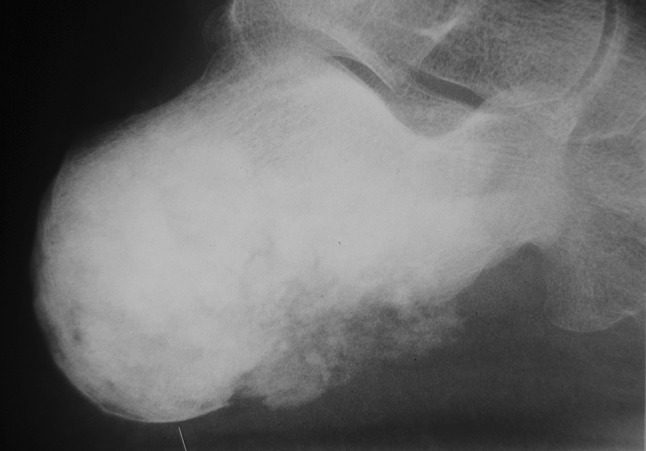
Lateral calcaneal radiograph showing extensive osteosarcoma with soft tissue involvement in osteosarcoma (Table 2, Case 11)
The benign tumors of both calcaneus (Table 2) and talus (Table 3) were treated by a variety of surgical options. Minimum followup of patients with benign tumors was 1 year (mean, 3 years; range, 1–12 years), 31 patients were discharged, one lost to followup after 4 months, and seven patients remain under surveillance. None of the benign tumors recurred as of last followup. Two patients with calcaneal chondrosarcoma underwent intralesional curettage by oncological surgeons as a result of an incorrect diagnosis made on imaging, one in 1974 for suspected os calcis infection based on radiographs only. The second patient with known Maffucci syndrome and previous os calcis enchondroma was suspected to have recurrence based on MRI. At minimum followup of 1 year (mean, 5 years; range, 1–10 years), there have been no recurrences, although two patients were lost to followup after 18 months and 8 years. Of the eight patients with high-grade malignancy, one died shortly after presentation with disseminated malignancy. Minimum followup was 6 weeks (mean, 7 years; range, 0–20 years). Seven of these eight underwent open biopsy followed by radical surgery (Table 4). Metastasis occurred in five of the eight patients, involving the lungs in all five cases. Two patients remained alive and disease-free at 20- and 10-year followup, respectively. All three patients with malignant talar tumors underwent below-knee amputation. One patient with chondrosarcoma died 7 years postoperatively with pulmonary metastasis. The other remains disease-free at 7 years. The patient with osteosarcoma was discharged after 14 years with no evidence of disease recurrence.
Discussion
Bony tumors of the hindfoot are rare with limited literature available [1, 5, 16, 19, 23]. Delay in diagnosis or missed diagnosis of hindfoot lesions is frequently reported [2, 6, 7, 9, 10, 15, 22]. This is largely attributed to the rarity and lack of familiarity of many clinicians to tumors of the hindfoot; however, early diagnosis is essential to outcome, especially in high-grade malignancy [2, 7, 17, 18, 24]. We therefore determined (1) how hindfoot tumors present and the specific reasons for delay in diagnosis; (2) whether the spectrum of disease varies between the talus and calcaneus; and (3) how these patients were treated.
Readers should be aware of the limitations of our study. First, it is a retrospective review that spans 56 years and therefore patients with historical investigations and treatments are included. We acknowledge that advances during this period in imaging, treatment (with neoadjuvant chemotherapy), and surgical techniques present difficulties in the analysis of this data. Investigations such as MRI and treatments such as radiofrequency ablation for osteoid osteoma have changed practice during this timeframe. Second, there may be a referral bias within the SBTR with some benign lesions being treated locally without notification to the registry. Third, bony tumors of the hindfoot are rare [1, 4, 5, 10, 16, 23]; we identified 57 patients over 56 years in a population base of approximately five million. This equates to approximately one case per year and represents 1% of all bony tumors recorded in the SBTR. As a result of the rarity of these lesions, the available literature is limited. However, our study has primarily focused on the clinical presentation of these lesions and comparison between the two osseous sites within the hindfoot.
Delay in diagnosis of bony hindfoot tumors has been frequently reported [2, 6, 7, 9, 10, 15, 22] and is attributable in our opinion to lack of clinician familiarity with these rare lesions and a low index of suspicion. We also found delays in treatment with a mean length of symptoms of 9 months for benign lesions and 7 months for malignant lesions. These patients present with considerable pain, typically around the heel in calcaneal lesions and the ankle in talar tumors. The pain is of a constant, dull, aching character, often associated with swelling and frequently misdiagnosed as soft tissue ankle or heel injuries [9, 15]. Rest pain, night pain, and, in the case of malignancy, systemic symptoms were common closer to the time of presentation. Four patients presented with pathological fracture of the calcaneus after several months of symptoms, half of which were high-grade sarcoma. Benign lesions present earlier (mean age, 25 years) than malignant lesions, particularly osteosarcoma (mean age, 40 years) in keeping with the literature [2, 7]. We also noted a higher prevalence among males, particularly in the talus (ratio 2.8:1) and malignant calcaneal tumors (ratio, 2.25:1), in keeping with the available literature [5, 10, 16].
The majority of primary tumors of the talus in this series were benign (ratio 6.67:1), consistent with most of the literature [10, 16]. Osteoid osteoma was predominant followed by chondroblastoma. In contrast, the spectrum of disease affecting the calcaneus in our study was diverse, although in keeping with the literature [20], unicameral bone cysts accounted for one-fourth of all benign pathology and one-third of these presented with pathological fracture, reinforcing their clinical relevance. Murrari et al. [16] reported a benign to malignant ratio of 5:1; however, our series suggests a much lower benign to malignant ratio (ratio, 1.6:1). Furthermore, this is in stark contrast to that of the talus, although we appreciate these findings are limited by the rarity of bony tumors both in our study and the literature as a whole. Three patients with sarcoma had preexisting pathology, Paget’s disease in two patients with osteosarcoma and Maffucci syndrome in one patient with chondrosarcoma, which caused a major delay in definitive diagnosis and management with poor outcome. Chondrosarcoma has been reported to have a predilection for the hindfoot [18], particularly the calcaneus, which we also noted.
Patients with low-grade chondrosarcoma seem to do well after surgery; only one patient had pulmonary metastasis noted in our study, although this is common with higher-grade chondrosarcoma [14]. Five of the eight patients with high-grade sarcoma had pulmonary or bony metastasis at diagnosis or within several years of diagnosis despite radical surgery in four patients. We reiterate the importance of early diagnosis of these aggressive tumors and the vital role of neoadjuvant chemotherapy.
We recommend that patients with persistent pain or swelling affecting the heel or that is unrelated to soft tissue injury should be investigated with appropriate radiographs, particularly patients with predisposing disease such as Paget’s disease. In the majority of patients, this will identify the presence of hindfoot lesions, although anatomical peculiarities are common in the calcaneus and talus [12]. Patients with osteoid osteoma may have normal radiographs and this diagnosis should be considered particularly in the talus. Further imaging with MRI or CT will allow definitive diagnosis of most lesions and can allow treatment without tissue diagnosis in characteristic benign lesions such as intraosseous ganglia, lipoma, or osteoid osteoma. After MRI, we recommend histological diagnosis by one of two methods (Fig. 4). Ultrasound-guided core biopsy is less invasive and appropriate in lesions with an easily penetrable outer cortex or soft tissue component. Where this is believed inappropriate, CT-guided or open biopsy is recommended. When performing open biopsy of the calcaneus, we recommend using a small lateral, longitudinal incision with orientation of the biopsy tract such that it facilitates subsequent removal, avoiding raising flaps of skin or muscle. The approach to the talus will depend on the anatomical location of the tumor; however, it can be limited by several factors, including the surrounding malleoli, neurovascular, and tendinous structures. After definitive diagnosis, appropriate and timely surgery by an experienced musculoskeletal oncology team is advised. Specific surgical intervention is beyond the scope of this article; however, the most important factor to long-term patient outcome in high-grade sarcoma is early neoadjuvant chemotherapy and radical resection.
Fig. 4.
Recommended diagnostic protocol for patients presenting with suspected hindfoot tumor. RFA = radiofrequency ablation; BKA = below-knee amputation
Clinicians should be aware that hindfoot tumors are often misdiagnosed as soft tissue injuries and suspicion should be raised in the presence of persistent or severe pain, swelling, or the absence of trauma. Lesions affecting the calcaneus are more likely to be malignant and surgical intervention is dependent on early diagnosis and adjuvant therapy.
Acknowledgments
We thank Dr Robin Reid, Mrs Jean Campbell, Dr Mary Catto, Dr David Richie, Dr Nigel Rabie, Dr Elaine MacDuff, Mr Mike Jain, and Mr Paul Herbert.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the registry was obtained.
This work was performed at the Western Infirmary, Glasgow, UK.
References
- 1.Bakotic B, Huvos AG. Tumors of the bones of the feet: the clinicopathologic features of 150 cases. J Foot Ankle Surg. 2001;40:277–286. doi: 10.1016/S1067-2516(01)80063-6. [DOI] [PubMed] [Google Scholar]
- 2.Biscaglia R, Gasbarrini A, Bohling T, Bacchini P, Bertoni F, Picci P. Osteosarcoma of the bones of the foot—an easily misdiagnosed malignant tumour. Mayo Clin Proc. 1998;73:842–847. doi: 10.4065/73.9.842. [DOI] [PubMed] [Google Scholar]
- 3.Bloem IC, Mulder JD. Chondroblastoma: a clinical and radiological study of 104 cases. Skeletal Radiol. 1985;14:1–9. doi: 10.1007/BF00361187. [DOI] [PubMed] [Google Scholar]
- 4.Campbell CJ, Leupold RG. Tumours and tumour-like conditions of the os calcis. Orthop Clin North Am. 1973;4:145–156. [PubMed] [Google Scholar]
- 5.Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumours of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76:47–62. [PubMed] [Google Scholar]
- 6.Casadei R, Ruggieri P, Moscato M, Ferraro A, Picci P. Aneurysmal bone cyst and giant cell tumour of the foot. Foot Ankle Int. 1996;17:487–495. doi: 10.1177/107110079601700810. [DOI] [PubMed] [Google Scholar]
- 7.Choong PFM, Qureshi AA, Sim FH, Unni KK. Osteosarcoma of the foot. Acta Orthop Scand. 1999;70:361–364. doi: 10.3109/17453679908997825. [DOI] [PubMed] [Google Scholar]
- 8.Davila JA, Amrami KK, Sundaram M, Adkins MC, Unni KK. Chondroblastoma of the hands and feet. Skeletal Radiol. 2004;33:582–587. doi: 10.1007/s00256-004-0762-1. [DOI] [PubMed] [Google Scholar]
- 9.DeBenedetti MJ, Waugh TR, Evanski PM, Jordon I, Krijger M. Chondrosarcoma of the talus: a case report. Clin Orthop Relat Res. 1978;136:234–237. [PubMed] [Google Scholar]
- 10.Dhillon MS, Singh B, Singh DP, Prabhu V, Nagi ON. Primary bone tumours of the talus. J Am Podiatr Med Assoc. 1994;84:379–384. doi: 10.7547/87507315-84-8-379. [DOI] [PubMed] [Google Scholar]
- 11.Fink BR, Temple T, Chiricosta FM, Mizel MS, Murphey MD. Chondroblastoma of the foot. Foot Ankle. 1997;18:236–242. doi: 10.1177/107110079701800410. [DOI] [PubMed] [Google Scholar]
- 12.Keats TE, Anderson MW. Atlas of Normal Roentgen Variants That May Simulate Disease. 7. St Louis, MO, USA: CV Mosby; 2001. [Google Scholar]
- 13.Kilgore WB, Parrish WM. Calcaneal tumours and tumour-like conditions. Foot Ankle Clin. 2005;10:541–565. doi: 10.1016/j.fcl.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Marco RAW, Gitelis S, Brebach GT, Healey JH. Cartilage tumours: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:292–304. doi: 10.5435/00124635-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Monroe MT, Manoli A., 2nd Osteoid osteoma of the lateral talar process presenting as a chronic sprained ankle. Foot Ankle Int. 1999;20:461–463. doi: 10.1177/107110079902000712. [DOI] [PubMed] [Google Scholar]
- 16.Murrari TM, Callaghan JJ, Berrey BH, Jr, Sweet DE. Primary benign and malignant osseous neoplasms of the foot. Foot Ankle. 1989;10:68–80. doi: 10.1177/107110078901000205. [DOI] [PubMed] [Google Scholar]
- 17.Nigrisoli M, Ferraro A, De Christofaro R, Picci P. Chondrosarcoma of the hand and foot. Chir Organi Mov. 1990;75:315–323. [PubMed] [Google Scholar]
- 18.Ogose A, Unni KK, Swee RG, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of hands and feet. Cancer. 1997;80:50–59. doi: 10.1002/(SICI)1097-0142(19970701)80:1<50::AID-CNCR7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowski ML, Spjut HJ. Lesions of the bones of the hand and feet. Am J Surg Pathol. 1997;216:676–690. doi: 10.1097/00000478-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Pogoda P, Priernel M, Linhart W, Stork A, Adam G, Windolf J, Rueger JM, Amling M. Clinical relevance of calcaneal bone cysts: a study of 50 cysts in 47 patients. Clin Orthop Relat Res. 2004;424:202–210. doi: 10.1097/01.blo.0000128297.66784.12. [DOI] [PubMed] [Google Scholar]
- 21.Ramappa AJ, Lee FY, Tang P, Carlson JR, Gebhardt MC, Mankin HJ. Chondroblastoma of bone. J Bone Joint Surg Am. 2000;82:1140–1145. doi: 10.1302/0301-620X.82B8.10791. [DOI] [PubMed] [Google Scholar]
- 22.Shereff MJ, Cullivan WT, Johnson KA. Osteoid osteoma of the foot. J Bone Joint Surg Am. 1983;65:638–641. [PubMed] [Google Scholar]
- 23.Unni KK, Dahlin DC. Dahlin’s Bone Tumours: General Aspects and Data on 11087 Cases. 5. Philadelphia, PA, USA: Lippincott-Raven; 1996. [Google Scholar]
- 24.Wu KK. Tumor review: osteogenic sarcoma of the foot. J Foot Surg. 1987;26:269–271. [PubMed] [Google Scholar]