Abstract
Background
Joint-preserving surgery is performed in select patients with bone sarcomas of extremities and allows patients to retain the native joint with better joint function. However, recurrences may relate to achieving adequate margins and there is frequently little room for error in tumors close to the joint surface. Further, the tumor margin on preoperative CT and/or MR images is difficult to transpose to the actual extent of tumor in the bone in the operating room.
Questions/purposes
We therefore determined whether joint-preserving tumor surgery could be performed accurately under image-guided computer navigation and determined local recurrences, function, and complications.
Methods
We retrospectively studied eight patients with bone sarcoma of extremities treated surgically by navigation with fused CT-MR images. We assessed the accuracy of resection in six patients by comparing the cross sections at the resection plane with complementary prosthesis templates. Mean age was 17 years (range, 6–46 years). Minimum followup was 25 months (mean, 41 months; range, 25–60 months).
Results
The achieved resection was accurate, with a difference of 2 mm or less in any dimension compared to that planned in patients with custom prostheses. We noted no local recurrence at latest followup. The mean Musculoskeletal Tumor Society score was 29 (range, 28–30). There were no complications related to navigation planning and procedures. There was no failure of fixation at the remaining epiphysis.
Conclusions
In selected patients, the computer-assisted approach facilitates precise planning and execution of joint-preserving tumor resection and reconstruction. Further followup assessment in a larger study population is required in these patients.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-012-2536-8) contains supplementary material, which is available to authorized users.
Introduction
In the resection of primary bone sarcomas, sparing the articular end of the affected bone enables patients to retain their native joints and ligaments. This may result in better proprioception and a more normal joint function after reconstruction. With the advent of effective neoadjuvant chemotherapy and accurate MRI [8], a close but tumor-free margin resection is possible while sparing the juxtaarticular bone and joint as in some metaphyseal osteosarcomas around the knee [2, 9–11, 15].
However, it is difficult to relate the tumor margins on preoperative CT and/or MR images to the actual extent of the tumor in the bone and to precisely carry out the resection in the operating room without the use of an image guidance or surgical navigation system. Computer-assisted tumor surgery may facilitate the precise resection of the bone tumor and may enable an accurate reconstruction to be performed [14, 16–18]. Joint-preserving resections require a high level of precision and may be facilitated with the use of a surgical navigation system [4, 17]. Short- and intermediate-term studies show no increase in local recurrence rates when compared to conventional joint-sacrificing surgery [4, 16].
Custom-designed knee-sparing prostheses have been used to reconstruct and secure the small distal femoral bone remnant after joint-preserving resections in malignant bone sarcomas [2, 9]. The prosthesis allows preservation of the juxtaarticular bone and ligaments and achieves Musculoskeletal Tumor Society (MSTS) scores of 25.1 to 28.8 (of 30) [2, 9]. However, the resection is technically demanding as the surgeon needs to make sure the margin is sufficiently wide and the orientation of the resection plane precisely matches that of the custom prosthesis. However, it is unclear how accurately a surgeon can transfer a bone resection planned by CT and/or MRI to the operating room. Fusing MR-CT images and then using these images with the bony landmarks registered during navigation offer the potential to increase the accuracy of the resections relative to the tumor margins.
We therefore evaluated (1) the accuracy of planned bone resection using image-guided computer navigation with fused CT-MR images and fit of custom prostheses to the remaining articular bone, (2) the number of local recurrences, (3) functional scores, and (4) complications after joint-preserving tumor surgery performed under image-guided computer navigation.
Patients and Methods
Between January 2006 and June 2009, we surgically treated eight patients with long-bone sarcomas with joint-preserving tumor resection and reconstruction with the assistance of image-guided computer navigation. The mean age of the patients was 17 years (range, 6–46 years) (Table 1). There were four patients with conventional high-grade osteosarcoma affecting the distal femur (all received neoadjuvant chemotherapy), two patients with parosteal osteosarcoma (one in the proximal tibia, one in the distal femur), and two patients with low-grade chondrosarcoma (one in the proximal femur, one in the proximal humerus). En bloc resection was performed in two patients with chondrosarcoma because the sarcoma was Grade 2 and endosteal scalloping and cortical erosion were present on CT images. Patients were selected for the joint-preserving surgery provided they satisfied the following criteria: (1) there was no transphyseal extension of the tumor on MRI (T1-weighted coronal section that best showed the maximum extent of the tumor); (2) a residual epiphyseal segment of at least 1 cm would be available after tumor resection so as to allow adequate bone fixation; and (3) in patients with high-grade osteosarcoma, there was no evidence of tumor progression clinically or on MRI during neoadjuvant chemotherapy. No patients were lost to followup. The minimum followup was 24.7 months (mean, 40.7 months; range, 24.7–59.8 months). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Table 1.
Details of eight patients with joint-preserving tumor resection and reconstruction under the guidance of computer navigation
| Patient | Age (years) | Sex | Diagnosis | Location | Surgery | Bone reconstruction | AB (mm) | ROM (°) | MSTS score (points) | Followup (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | Female | Parosteal osteosarcoma | Proximal tibia (posterior aspect) | Joint-preserving resection (hemicortical) | Vascularized fibular graft | 10 | 0–130 | 28 | 59.8 | |
| 2 | 14 | Male | Conventional osteosarcoma | Femur (subtrochanteric region to distal metaphysis) | Joint-preserving resection after neoadjuvant chemotherapy | Custom tumor prosthesis | 14 | 0–120 | 30 | 52.3 | |
| 3 | 6 | Male | Conventional osteosarcoma | Distal femur (metaphysis) | Joint-preserving resection after neoadjuvant chemotherapy | Custom extendable tumor prosthesis | 10 | 0–90 | 5.0 | Died of pulmonary metastases at 5 months postsurgery | |
| 4 | 8 | Male | Conventional osteosarcoma | Distal femur (metaphysis) | Joint-preserving resection after neoadjuvant chemotherapy | Custom extendable tumor prosthesis | 8 | 0–130 | 30 | 45.7 | |
| 5 | 21 | Female | Low-grade chondrosarcoma | Proximal femur (at greater trochanter) | Joint-preserving resection (multiplanar) | Custom tumor prosthesis | 50 | 0–135 | 28 | 41.0 | |
| 6 | 24 | Male | Parosteal osteosarcoma | Distal femur (metaphysis) | Joint-preserving resection (multiplanar) | Custom tumor prosthesis | 11 | 0–95 | 28 | 35.3 | Proximal femur stem loosened at 2.5 years; only proximal component revised |
| 7 | 6 | Male | Conventional osteosarcoma | Distal femur (metaphysis) | Joint-preserving resection after neoadjuvant chemotherapy | Custom extendable tumor prosthesis | 5 | 0–130 | 30 | 26.0 | |
| 8 | 16 | Female | Low-grade chondrosarcoma | Proximal humerus (metaphysis) | Joint-preserving resection (hemicortical) | Iliac crest bone graft | 25 | 0–180 | 30 | 24.7 |
AB = shortest length of the remaining epiphysis measured to the joint line; MSTS score = Musculoskeletal Tumor Society score, with a total score of 30 points.
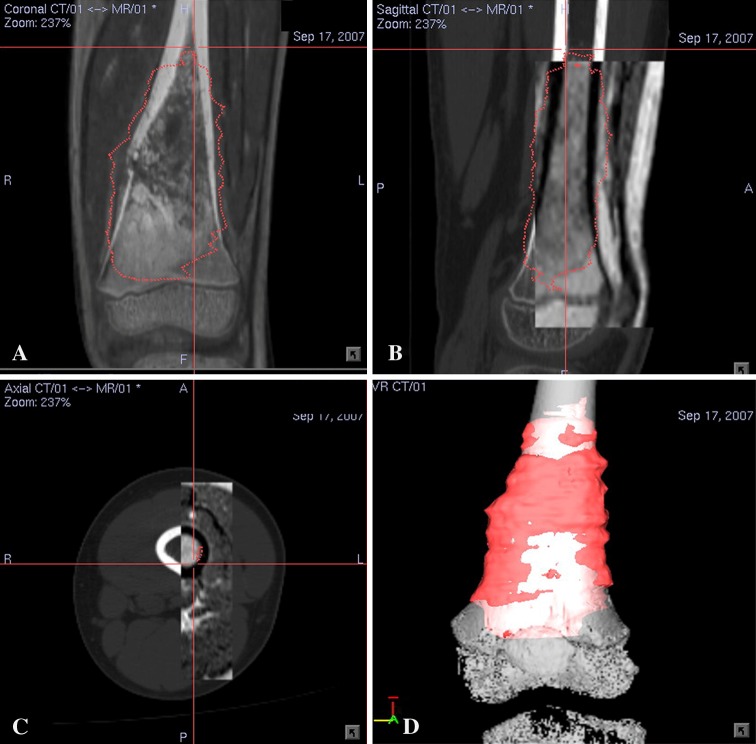
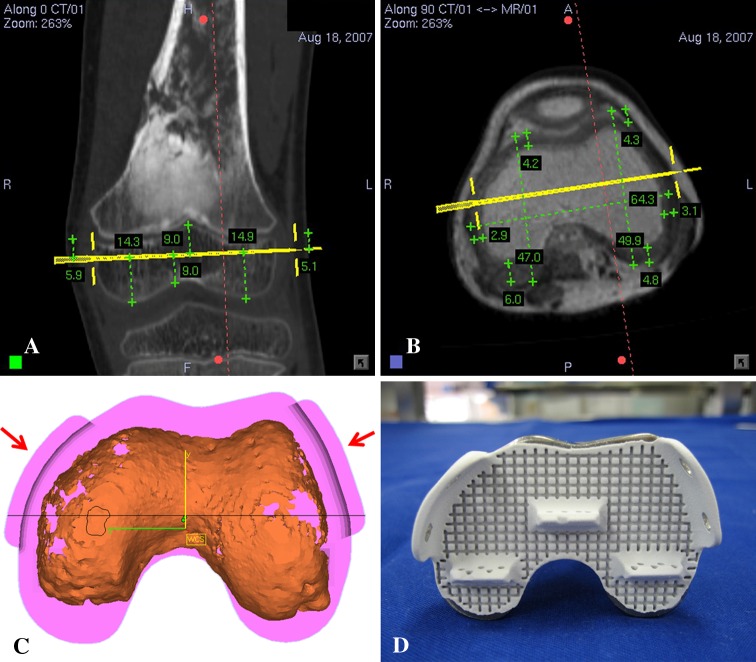
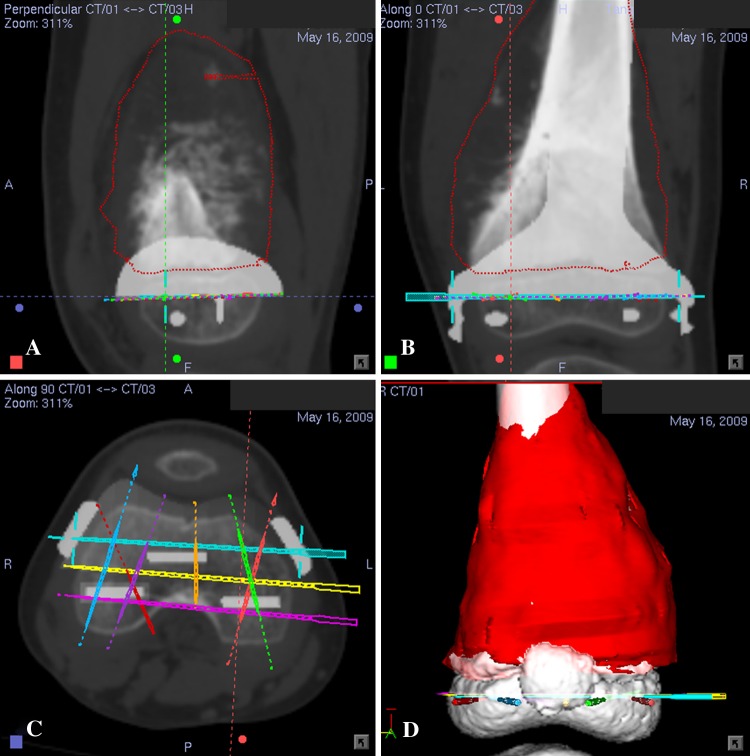
Preoperative CT and MRI examinations of each patient were performed. Axial CT images of the lesion and surrounding area were acquired using a 16-detector scanner (General Electric LightSpeed™; GE Medical Systems, Milwaukee, WI, USA). Slices with 0.625-mm or 1.25-mm thickness were obtained using a soft tissue algorithm. MR images of the corresponding region were acquired using a 1.5-T unit (Siemens Sonata; Siemens Medical Solutions, Erlangen, Germany). Postcontrast T1-weighted axial images (TR, 512 milliseconds; TE, 13 milliseconds; 2-mm-thick slices) were used for fusion with CT images because of better bone-soft tissue contrast. Radiographic data with DICOM format were obtained and imported into a CT-based navigation system (CT Spine, Version 1.6; Stryker Navigation, Freiburg, Germany). The CT and MR images were fused using the navigation software. This navigation system was used for the first three patients while another navigation system (iNtellect Cranial, Version 1.1; Stryker Navigation) was used for the rest. The process of fusing multimodal image data sets has been described [16]. We used the cranial navigation software for image fusion in the latter part of the study as it allowed automatic fusion of various image data sets regardless of imaging modalities and scan orientation. The fused image data sets then allowed preoperative surgical planning in the CT spine navigation software (Fig. 1A–C). A three-dimensional (3-D) bone model was created by adjusting the contrast level of the CT images. We defined the extent of the tumor and segmented tumor volume from MR images. We determined the tumor edge by looking at the transition of marrow signal from abnormal to normal in T1-weighted MR images. We regarded areas of intermediate signal intensity adjacent to tumor edge that may represent microscopic metastases or marrow hyperplasia as being part of the tumor. A 3-D bone tumor model was generated (Fig. 1D). All reconstructed two-dimensional (2-D) and 3-D images were used for preoperative surgical planning. The plane of bone resection was planned at least 5 mm (for low-grade chondrosarcoma) and 10 mm (for osteosarcoma) from the intramedullary extent of the tumor and was marked with virtual screws in the navigation software. The planning provided information about the exact length of bone resection and bone dimensions at the site of joint-preserving resection (Fig. 2A–B). Together with patients’ CT data, the manufacturer (Stanmore Implants Worldwide Ltd, Middlesex, UK) then designed and manufactured custom-made joint-preserving prostheses for six patients (Figs. 2C–D, 3A–B). In Patients 6 and 7, we virtually simulated preoperative tumor resection (Fig. 4A) and prosthetic reconstruction (Fig. 4B) using computer-aided design (CAD) software (MIMICS®; Materialise, Leuven, Belgium). The surgical plan of tumor resection and CAD prosthesis reconstruction in CAD format were back-converted to CT data sets in DICOM format [19]. Both original CT data sets and virtual surgical planning CT data sets were fused in the navigation software. Virtual screws were then easily placed along the plane and orientation of planned tumor resection in the fused image data sets (Fig. 5).
Fig. 1A–D.
Preoperative (A) coronal, (B) sagittal, and (C) axial sections of CT-MR fusion images and (D) a 3-D bone model for Patient 4 with a left distal femur osteosarcoma are shown. (A) A fusion image is in the fusion mode, which has slightly higher CT weighting than MR images. (B–C) Fusion images are in the splitting mode in which the rectangular window displays overlaid MR images on the base CT images. An image fusion is accurate and acceptable for navigation planning if the bony contours on the CT-MR images at the region of interest match within a 1-mm margin of error as visually assessed by the authors. Tumor extent (red color) is outlined on each MR image. (D) The 3-D bone tumor model reconstructed from CT images and MR segmented tumor volume is shown.
Fig. 2A–D.
(A) A coronal section of a CT image for Patient 4 with a left distal femur osteosarcoma is shown. The resection level is marked by a virtual pedicle screw (yellow color). The distance between planned resection and joint line is measured. (B) An axial section of a CT-MR fused image with predominant MR weighting at the planned resection level is shown. The thickness of cartilage is measured. The planning provides information to implant engineers about the exact level and bone dimensions at the site of planned resection. (C) A diagram shows the cross section of the final CAD prosthesis that matched to the cartilaginous surface at the remaining epiphysis of distal femur. CT images show better information on bone (orange color) than cartilage. CT-MR image fusion helps determine the exact dimension of the distal prosthetic junction in young patients who have thick cartilage at the distal epiphysis. Extracortical plates (red arrows) and screws are used for distal bone fixation. (D) A photograph shows the cross section of the actual CAD prosthesis coated with HA for enhancing osteointegration at the bone-implant interface.
Fig. 3A–B.
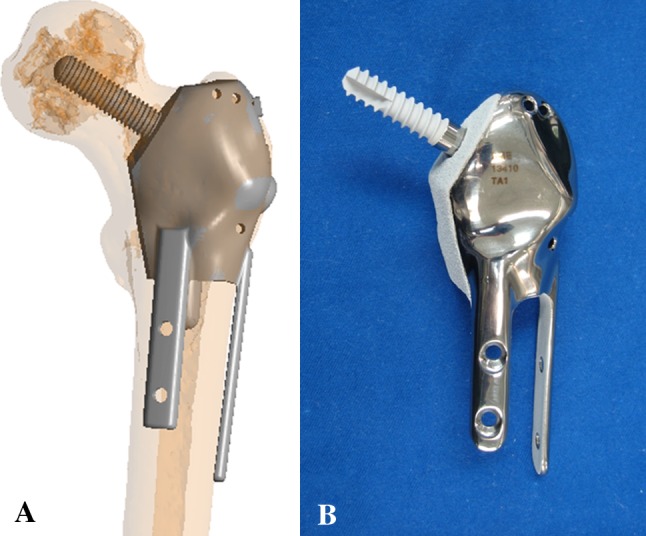
(A) A diagram shows the preoperative position of the CAD prosthesis in Patient 5 with left proximal femur Grade 2 chondrosarcoma and multiplanar osteotomies. Reprinted with permission from Wong KC, Kumta SM, Tse LF, Ng EW, Lee KS. Image fusion for computer-assisted tumor surgery (CATS). In: Ukimura O, ed. Image Fusion. InTech; 2011. Available at: http://cdn.intechopen.com/pdfs/12998/InTech-Image_fusion_for_computer_assisted_tumor_surgery_cats_.pdf. Accessed June 27, 2012. (B) A photograph shows the anterior view of the actual CAD prosthesis. Two extracortical plates and screws are used for fixation of the femoral shaft while one screw is used for fixation of the femoral head and neck. All implant junctions in contact with host bone are coated with HA (white color).
Fig. 4A–B.
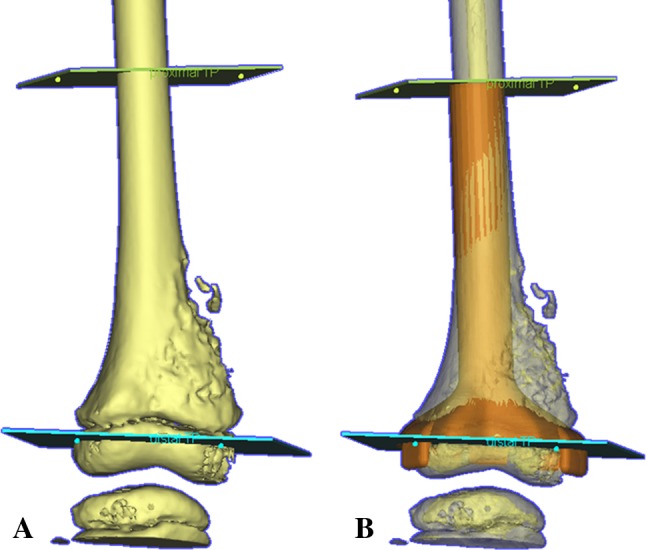
A preoperative virtual simulation of joint-preserving tumor resection and prosthetic reconstruction using CAD software is shown for Patient 7 with right distal femur osteosarcoma at the metaphysis. (A) A diagram shows an anterior view of the 3-D bone model generated from the preoperative CT image data set. The 3-D model and reformatted views of CT images are manipulated and analyzed. The level and orientation of proximal and distal resection are marked, based on the MR measurements of the tumor extent with reference to the joint line. The simulation project file of the CAD software is then transferred to implant engineers who design a CAD prosthesis with the exact dimensions as decided by the surgeons. (B) The final design of CAD prosthesis (orange in color) is imported back to the original simulation file and a final check of the design before approval to manufacture is made.
Fig. 5A–D.
Preoperative (A) sagittal, (B) coronal, and (C) axial images and (D) a 3-D model of navigation planning on the navigation monitor are shown for Patient 7 with right distal femur osteosarcoma at the metaphysis. The CAD prosthesis can be integrated into the navigation planning and visualized on CT images. This integration facilitates the precise definition of intended resection planes by virtual pedicle screws.
Intraoperative navigation has been described [16–18]. After appropriate surgical exposure, a dynamic reference patient tracker was attached to the bone in which the tumor was located. We minimized soft tissue dissection at the capsule or ligaments and preserved blood supply to the epiphysis. We planned the tracker’s location at least 2 cm from the tumor margin, estimating on MR images so as to avoid tumor contamination at the surgical field during attachment of the tracker. An image-to-patient registration to match precisely the operative anatomy and preoperative virtual CT images was performed by paired points and surface points matching. We next calibrated the navigation probe or operative instruments (drill) mounted with navigation trackers to the navigation system. This allowed real-time tracking of the spatial location of the tip of these instruments in relation to the patient’s anatomy on the virtual preoperative images. The anatomic locations of virtual pedicle screws were identified, and intended resection level and plane were marked using navigated tools. An oscillating saw of 0.9-mm thickness or thin osteotome was used to make the osteotomy, and the tumor was removed en bloc. We reconstructed skeletal defects using custom joint-preserving prostheses for six patients and biologic graft for the other two (the intraoperative navigation procedure in Patient 4 is illustrated in Video 1; supplemental materials are available with the online version of CORR®). Extendable custom tumor prostheses were used to reconstruct the skeletal defect in young patients (n = 3) who were predicted to have a substantial limb length discrepancy after skeletal maturity.
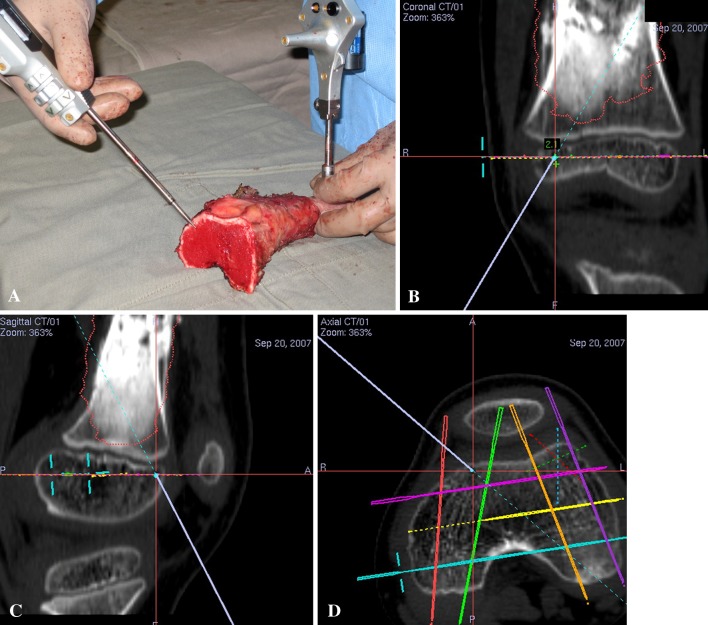
In Patients 2 to 7, the accuracy of the planned bone resection was determined intraoperatively by comparing the cross sections at the resection plane with complementary prosthesis templates (Fig. 6A) and assessing the fit of the custom prostheses to the remaining bone at the surgery (Fig. 6B). An additional method was used to assess the accuracy in Patients 3, 4, 6, and 7. As their dynamic reference trackers were still attached to the tumor specimens after resection and image-to-patient registration remained valid, the achieved resection margin at the two ends of tumor specimens were validated intraoperatively under computer navigation. By positioning the tip of navigation probe at the achieved bone resection (Fig. 7A), we measured the distance between the virtual tip of navigation probe and the planned resection planes on the virtual preoperative images (Fig. 7B–D) (Video 2; supplemental materials are available with the online version of CORR®). However, this validation method was not used in Patients 1 and 8 with hemicortical resection and in Patient 5 with multiplanar resection. Their trackers were attached to the remaining distal bone after resection. The thin bone joining the proximal and distal bone fragments might deform, resulting in discrepancies between preoperative anatomic data and real-time surgical anatomy. The planned bone resection was considered accurate if the remaining epiphysis matched the prosthesis template, fitted to the custom prosthesis, or the achieved bone resection deviated from the planned one on navigation validation with a difference of 2 mm or less in any dimension. We did not validate the accuracy of planned bone resection by the above methods for Patients 1 and 8 with hemicortical resection as their resection planes were irregular and curved. The resected specimens were sectioned longitudinally in 5-mm thickness. The two largest slabs of sectioned specimens were further divided and paraffin embedded into 2- × 2.5-cm tissue blocks. Representative blocks were extensively sampled from the remaining slabs. The blocks containing the margins of resection (medial, lateral, anterior, posterior, proximal, and distal) were secured. The specific margins of particular concern by surgeons were also taken. All the tissue blocks were histologically evaluated for clear resection margins.
Fig. 6A–B.
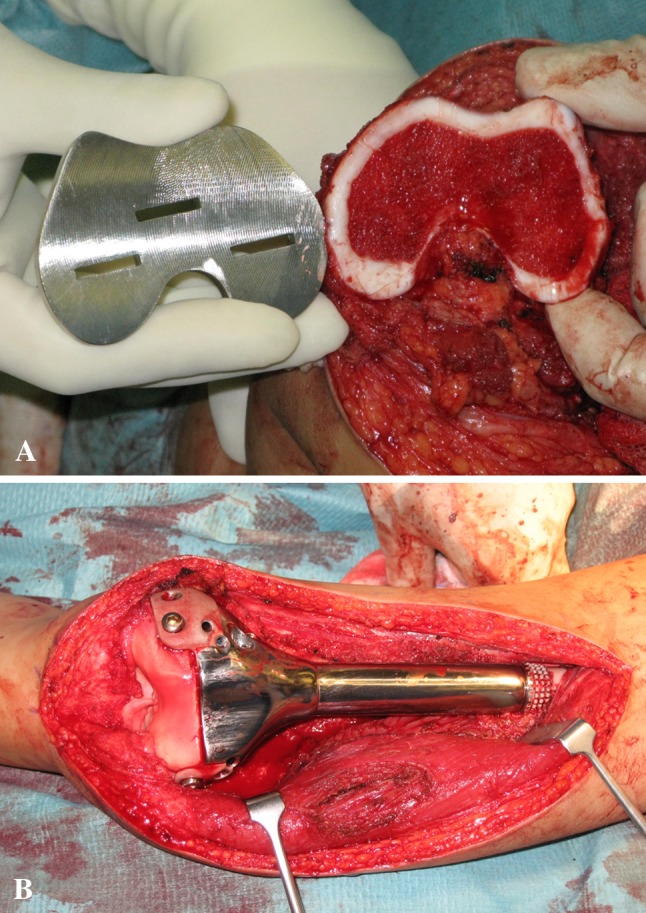
Photographs show (A) the complementary prosthesis template to measure the accuracy of the bone resection near the joints in Patient 7 and (B) the precise fitting of the prosthesis to the small residual bone fragments in Patient 4. We preserve the soft tissue and ligament attachments, in particular at the posterior intercondylar notch where the middle genicular artery enters and supplies the remaining distal femoral epiphysis [13].
Fig. 7A–D.
(A) A photograph shows the tumor specimen for Patient 4 after computer-assisted joint-preserving resection. The image-to-patient registration remains valid as the dynamic reference tracker is still attached to the tumor specimen after resection. The tip of the navigation probe is on the distal bone end of the tumor specimen. The corresponding (B) coronal, (C) sagittal, and (D) axial views on the navigation monitor are shown. The virtual tip of the navigation probe (red cross) is exactly at the resection level virtually planned on the preoperative CT images. The distance between the achieved resection (at the virtual tip of the navigation probe) and planned resection (at the virtual pedicle screws) is measured. For a better illustration, see Video 2 (supplemental materials are available with the online version of CORR®).
Postoperatively, the patients had early physiotherapy with both active and passive joint mobilization. They were allowed to walk with a protective brace and partial weightbearing for 4 weeks and were then allowed to bear weight fully. Postoperative chemotherapy was resumed in patients with conventional high-grade osteosarcoma soon after their wounds healed. The skeletally immature patients with minimally invasive extendable prostheses had lengthening when a leg length discrepancy of greater than 1 cm had occurred.
All patients were followed at 1 month, 2 months, every 3 months for 2 years, every 6 months until 5 years, and annually thereafter. We performed clinical examinations to look for local recurrence and recorded the limb function by measuring the joint ROM and MSTS scores [7]. We also obtained orthogonal views of plain radiographs of the operated area at each visit. Bone scans were taken at 6 months after joint-preserving resection to detect the complication of avascular necrosis in the remaining epiphysis. The signal uptake of the remaining epiphysis comparable to the normal side was regarded as viable bone. In two patients younger than 10 years (Patients 4 and 7), CT examination of both knees was performed at the latest followup to document the growth of the remaining epiphysis. We excluded the complication of growth arrest in the remaining epiphysis if its bone dimension in coronal and sagittal views was not smaller than that of the normal side.
We also recorded complications, including image fusion as failure to match the bony contour on CT-MR images at the region of interest within 1-mm margin of error as visually assessed by surgeons; intraoperative navigation procedures that included failure of navigation hardware/software, displacement of dynamic reference patient and inaccurate image-to-patient registration for registration errors of greater than 1 mm; intraoperative fracture of the remaining epiphysis; postoperative wound infection; and failure of bony reconstruction requiring revision surgery.
Results
In patients with joint-preserving prostheses (Patients 2–7), the bone resections matched the prosthesis templates and fitted to the prostheses with a difference of 2 mm or less in any dimension. In patients whose patient trackers were still attached to the resected tumor specimens (Patients 3, 4, 6, and 7), the achieved bone resection margins could be visualized intraoperatively under computer navigation and were within a 2-mm difference from the planned resection. Histologic examination of all resected specimens showed a clear tumor margin.
At latest followup, we found no cases of local tumor recurrence (Fig. 8).
Fig. 8A–B.
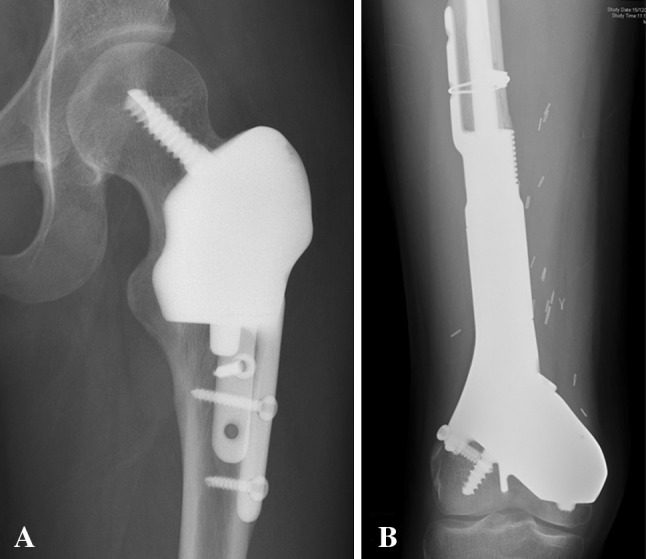
Postoperative radiographs taken at (A) 30 and (B) 38 months after surgery show the multiplanar resection and prosthetic reconstruction for Patients 5 and 6, respectively. No local tumor recurrence is noted.
The mean MSTS score was 29.1 (range, 28–30). The mean knee flexion was 115° (range, 90°–130°). Full hip flexion and shoulder movement could be achieved in Patients 5 and 8, respectively.
No major complications were noted. Preoperative image fusion was successful in all cases as bony contour on the CT-MR images at the region of interest could be matched within 1-mm margin of error. We could perform all navigation procedures as planned. No failure of hardware/software of the navigation system or displacement of patient tracker was recorded. All image-to-patient registrations were possible with a mean error of 0.47 mm (range, 0.34–0.8 mm). We noted no intraoperative fracture of the remaining epiphysis, which had a mean shortest length of 16.6 mm (range, 5–50 mm). No postoperative wound infection was found. No failure of bony reconstruction occurred, except in Patient 6 with multiplanar bone resection for his distal femur parosteal osteosarcoma; he developed aseptic loosening at the stem of the proximal femur component of his joint-preserving prosthesis because of failure of bone ingrowth into the hydroxyapatite (HA) collar. The prosthesis originally consisted of two separate components. As the distal component with the multiplanar junction was still integrated well with the remaining distal femur condyle, the proximal loosened component of the prosthesis was revised at 2.5 years after his first surgery and was replaced by a new component with addition of bone graft at the HA collar. No osteonecrosis of the remaining epiphysis was detected in bone scans taken after surgery. Two patients (Patients 4 and 7) with joint-preserving extendable prostheses had minimally invasive lengthening of 34 and 15 mm, respectively. In their CT scans taken at latest followup, the remaining distal femur epiphysis demonstrated no growth arrest and had growth comparable to that of the normal side (Fig. 9).
Fig. 9A–B.
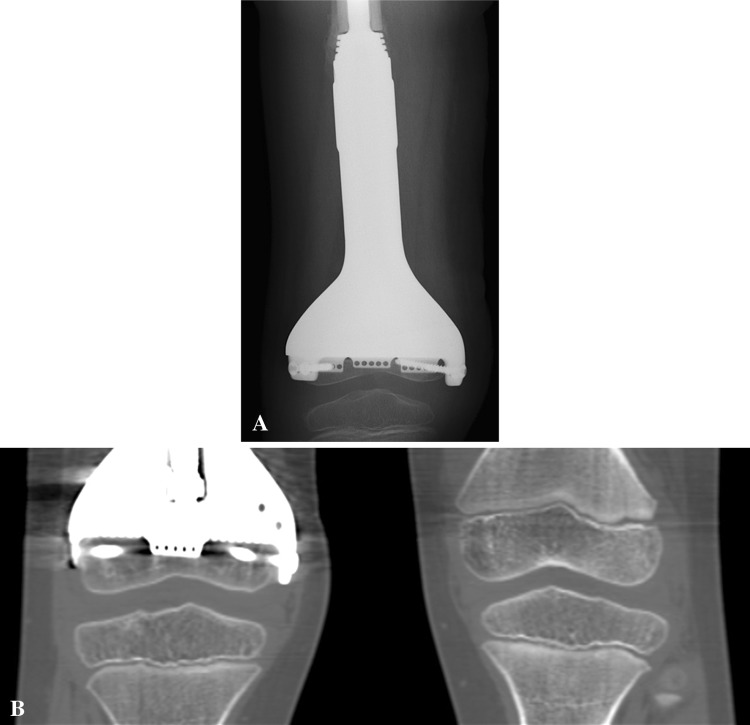
(A) A radiograph taken at 4 weeks after surgery shows the joint-preserving distal femur prosthesis in Patient 7. (B) A CT scan of both knees taken 2 years after surgery shows continuous growth of the small remaining distal femur epiphysis. The growth of the proximal tibia epiphysis is also preserved, which would have been affected in a conventional standard arthroplasty after distal femoral tumor resection.
Discussion
Computer-assisted surgery has been applied in the field of orthopaedic oncology in recent years. It may facilitate resection and reconstruction in patients with complex bone tumors [4, 16–18]. Inadequate resection margins are associated with higher risk of local tumor recurrence and poorer patient survival [12]. With the advent of effective chemotherapy and accurate MRI [8], the real extent of tumor in the bone is known and it may allow a joint-preserving tumor resection. However, there is no report in the literature that has specifically studied how accurate surgeons can transfer the surgical planning and execute this technically demanding bone resection in the operating room. In select patients with bone sarcomas who underwent joint-preserving resection and reconstruction under computer navigation, we determined the accuracy of planned bone resection, local recurrences, functional outcomes, and complications after the procedure.
Our study has several limitations. First, the patient population is small with heterogeneous diagnosis and short study duration for drawing any firm conclusions, and the single-group study design allows no comparative assessment of local recurrence rates and limb function to those of conventional joint-sacrificing surgery. Therefore, our findings may not imply any improvement in the treatment delivered by more conventional planning and surgery. Second, the computer-assisted joint-preserving surgery was applied only to patients who could meet the inclusion criteria. The surgery may not be suitable for the majority of patients with bone sarcomas who are not good responders to neoadjuvant chemotherapy or have a remaining epiphysis of less than 1-cm thickness after bone resection. Third, theoretically, the best way of measuring the accuracy of the resection is to relate the planned resection to the histology. However, we found it difficult to slice the tumor specimens so that the cross section of the slice coincided exactly with the corresponding views in the navigation planning for comparison. We believe, if surgeons can minimize the potential errors of the computer technology (Table 2), assessing the accuracy of the resection by measuring the difference in dimensions between achieved and planned bone resection will be comparable to relating planned resection to the histology of tumor specimens.
Table 2.
Potential errors during computer-assisted tumor surgery
| Stage | Type of error | Error |
|---|---|---|
| All | System | Inherent hardware and software error of position measuring of the surgical navigation system |
| Preoperative | Imaging | Error of the imaging modality in geometrically correct depiction of the anatomic structures |
| Preoperative | Navigation planning | Accuracy and quality of planning are limited by the quality of the original preoperative imaging data sets Image fusion: bony contour on the CT-MR images at the region of interest must be matched within 1-mm margin of error as visually assessed by surgeons Misplacement of virtual screws to simulate the planes of resection |
| Intraoperative | Image-to-patient registration | Dynamic reference patient tracker should be stably fixed on the patient’s bone in which tumor is located The calculated registration errors from the navigation system cannot be trusted completely as they only represent the mismatch between the planned and chosen registration points The registration accuracy should always be verified by touching anatomic points or tracing on the bone surface; the registration should be accepted only if the calculated position on the computer screen is comparable with the real position on the patient |
| Intraoperative | Application | Displacement of the dynamic reference patient tracker should be avoided Thin saw and osteotome should be used for bone cut Operators’ errors should be avoided, including visual misinterpretation during navigation procedures, hand tremors, and errors in final bone resection with freehand oscillating saw and osteotome |
We are unaware of any reports that specifically determine how accurately surgeons can perform joint-preserving surgery regarding tumor margins and prosthetic fit. Muscolo et al. [11] have described using anatomic landmarks and correlating with measurements on preoperative MR images to perform intraepiphyseal bone resection. Deijkers et al. [6] used fluoroscopic guidance intraoperatively to define the bone resection. However, the methods rely on 2-D measurements or fluoroscopic images and may result in errors between the perceived anatomy and that seen during actual surgery. Our experience shows the computer-assisted approach could facilitate joint-preserving tumor surgery as the difference of 2 mm or less between the achieved and planned bone resection was considered accurate for the surgery. The fused images and 3-D bone-tumor model allowed better mental pictures of tumor extent and its surrounding anatomy. With the navigation guidance, we could reproduce the planned resection without any help of landmark measurements or intraoperative fluoroscopy. Some authors have reported the use of multiplanar osteotomies or hemicortical resection to treat select patients with bone sarcoma [1, 3, 5]. Our results in Patients 1, 5, 6, and 8 also suggest multiplanar osteotomies or hemicortical resection are possible around bone tumors. For these osteotomies, it is even more difficult to correlate the information obtained from the preoperative MRI studies with the real tumor limits at the time of surgery. Therefore, navigation guidance may facilitate performing technically demanding bone resections.
For our series, the rate of local tumor recurrence (0%) was comparable to rates (0%–8%) reported in other studies [6, 10, 11] in which bone sarcomas were treated with joint-preserving resection and allograft reconstruction. This indicates joint-preserving surgery using computer navigation may be a viable and safe option for select patients with bone sarcomas.
The functional results of our series, with a mean MSTS score of 29, were better than those of other studies with joint-preserving tumor resection and allograft reconstruction that had a mean MSTS score of 26.8 [11] and 23.7 [6]. The superior functional results in our series may be attributed to the use of custom-designed prostheses that were anatomically and accurately fit to the bone defect after tumor resection using computer navigation. The stable bony construct and optimal soft tissue tension after surgery enabled patients to have earlier and more vigorous physiotherapy for better functional recovery of operated limbs. Gupta et al. [9] reported a study of eight patients with bone sarcoma at the distal femur who underwent joint-preserving tumor resection and prosthetic reconstruction without computer navigation. The mean MSTS score was 25.1 with the mean length of remaining epiphysis of 35.6 mm. Our results suggest, using the computer-assisted approach, we may be able to achieve excellent MSTS functional scores even in patients with a smaller remaining epiphysis (mean length, 16.6 mm).
We did not have major complications related to intraoperative navigation procedures. So et al. [14] reported two failures of image-to-patient surface registration during computer-assisted tumor resection in two patients with long-bone sarcomas. It was postulated the thin cortices caused by the tumor were not optimal for surface registration. In this study, we did not experience failure of registration. Our select patients had a remaining epiphysis with a thickness of at least 1 cm after tumor resection. This provided sufficient normal bone surface for registration points and made the registration successful. Other complications of joint-preserving surgery, such as osteonecrosis and growth arrest of the remaining epiphysis, were not noted. We are unaware of any reports describing the viability and growth of the remaining epiphysis after joint-preserving surgery. With the help of computer navigation, the epiphysis and its capsular and ligamentous attachment no longer had to be fully exposed for reference in marking the resection plane, which helped to preserve the blood supply to the remaining epiphysis [13]. This might explain the viable epiphysis in the bone scan examination and our observation that, for Patients 4 and 7, the retained blood supply supported the continuous growth of the remaining distal femoral epiphysis.
In carefully selected patients, the computer-assisted approach facilitated the precise planning and execution of joint-preserving tumor resection and reconstruction. Resections that spare part of the native joints may allow more conservative reconstruction and this may lead to a better joint function. Further followup assessment is required to determine the long-term rates of local recurrence and limb function in these patients.
Electronic supplementary material
Acknowledgments
We thank Mr. Eric Wai-Kin Ng and Mr. Keith Kam-Shing Lee (ACAOS-ITAV team, Department of Orthopaedics and Traumatology, Prince of Wales Hospital, The Chinese University of Hong Kong) for setup of the navigation system and documentation of operative procedures. We also thank Mr. Sudha Shunmugam, the design team, and Dr. Paul Unwin (Stanmore Implants Worldwide Ltd) for design and manufacture of the CAD custom prostheses. We acknowledge the great assistance of Mr. Rock Hu and Ms. Yukin Zhao (Bio-Medical Engineering, Materialise China, Shanghai, China) in using the MIMICS® software.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Agarwal M, Puri A, Anchan C, Shah M, Jambhekar N. Hemicortical excision for low-grade selected surface sarcomas of bone. Clin Orthop Relat Res. 2007;459:161–166. doi: 10.1097/BLO.0b013e318059b8eb. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal M, Puri A, Gulia A, Reddy K. Joint-sparing or physeal-sparing diaphyseal resections: the challenge of holding small fragments. Clin Orthop Relat Res. 2010;468:2924–2932. doi: 10.1007/s11999-010-1458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avedian RS, Haydon RC, Peabody TD. Multiplanar osteotomy with limited wide margins: a tissue preserving surgical technique for high-grade bone sarcomas. Clin Orthop Relat Res. 2010;468:2754–2764. doi: 10.1007/s11999-010-1362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho HS, Oh JH, Han I, Kim HS. Joint-preserving limb salvage surgery under navigation guidance. J Surg Oncol. 2009;100:227–232. doi: 10.1002/jso.21267. [DOI] [PubMed] [Google Scholar]
- 5.Deijkers RL, Bloem RM, Hogendoorn PC, Verlaan JJ, Kroon HM, Taminiau AH. Hemicortical allograft reconstruction after resection of low-grade malignant bone tumours. J Bone Joint Surg Br. 2002;84:1009–1014. doi: 10.1302/0301-620X.84B7.13032. [DOI] [PubMed] [Google Scholar]
- 6.Deijkers RL, Bloem RM, Kroon HM, Van Lent JB, Brand R, Taminiau AH. Epidiaphyseal versus other intercalary allografts for tumors of the lower limb. Clin Orthop Relat Res. 2005;439:151–160. doi: 10.1097/00003086-200510000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 8.Gillespy T, Manfrini M, Ruggieri P, Spanier SS, Pettersson H, Springfield DS. Staging of intraosseous extent of osteosarcoma: correlation of preoperative CT and MR imaging with pathologic macroslides. Radiology. 1988;167:765–767. doi: 10.1148/radiology.167.3.3163153. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Pollock R, Cannon SR, Briggs TW, Skinner J, Blunn G. A knee-sparing distal femoral endoprosthesis using hydroxyapatite-coated extracortical plates: preliminary results. J Bone Joint Surg Br. 2006;88:1367–1372. doi: 10.1302/0301-620X.88B10.17756. [DOI] [PubMed] [Google Scholar]
- 10.Kumta SM, Chow TC, Griffith J, Li CK, Kew J, Leung PC. Classifying the location of osteosarcoma with reference to the epiphyseal plate helps determine the optimal skeletal resection in limb salvage procedures. Arch Orthop Trauma Surg. 1999;119:327–331. doi: 10.1007/s004020050420. [DOI] [PubMed] [Google Scholar]
- 11.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Partial epiphyseal preservation and intercalary allograft reconstruction in high-grade metaphyseal osteosarcoma of the knee. J Bone Joint Surg Am. 2005;87(suppl 1 pt 2):226–236. doi: 10.2106/JBJS.E.00253. [DOI] [PubMed] [Google Scholar]
- 12.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 13.Scapinelli R. Vascular anatomy of the human cruciate ligaments and surrounding structures. Clin Anat. 1997;10:151–162. doi: 10.1002/(SICI)1098-2353(1997)10:3<151::AID-CA1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.So TY, Lam YL, Mak KL. Computer-assisted navigation in bone tumor surgery: seamless workflow model and evolution of technique. Clin Orthop Relat Res. 2010;468:2985–2991. doi: 10.1007/s11999-010-1465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchiya H, Abdel-Wanis ME, Sakurakichi K, Yamashiro T, Tomita K. Osteosarcoma around the knee: intraepiphyseal excision and biological reconstruction with distraction osteogenesis. J Bone Joint Surg Br. 2002;84:1162–1166. doi: 10.1302/0301-620X.84B8.13330. [DOI] [PubMed] [Google Scholar]
- 16.Wong KC, Kumta SM, Antonio GE, Tse LF. Image fusion for computer-assisted bone tumor surgery. Clin Orthop Relat Res. 2008;466:2533–2541. doi: 10.1007/s11999-008-0374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision tumour resection and reconstruction using image-guided computer navigation. J Bone Joint Surg Br. 2007;89:943–947. doi: 10.1302/0301-620X.89B7.19067. [DOI] [PubMed] [Google Scholar]
- 18.Wong KC, Kumta SM, Chiu KH, Cheung KW, Leung KS, Unwin P, Wong MC. Computer assisted pelvic tumor resection and reconstruction with a custom-made prosthesis using an innovative adaptation and its validation. Comput Aided Surg. 2007;12:225–232. doi: 10.3109/10929080701536046. [DOI] [PubMed] [Google Scholar]
- 19.Wong KC, Kumta SM, Leung KS, Ng KW, Ng EW, Lee KS. Integration of CAD/CAM planning into computer assisted orthopaedic surgery. Comput Aided Surg. 2010;15:65–74. doi: 10.3109/10929088.2010.514131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.