Abstract
Background
Distal radius reconstruction after en bloc tumor resection remains a surgical challenge. Although several surgical techniques, either reconstructing the wrist or achieving a stable arthrodesis, have been described, it is unclear to what degree these restore function.
Description of Technique
We describe an updated technique making use of a tibia cortical strut autograft (TCSA) to perform a functional arthrodesis from the remaining radius to the first carpal row. This, in theory, could lead to less donor site morbidity while resulting in a stable but functional and pain-free arthrodesis of the wrist.
Methods
Between 1987 and 2010 we reconstructed the wrists of 17 patients using a TCSA arthrodesis (six primary and three revisions), seven with an osteoarticular allograft, three using an ulnar translocation, and one with a fibula autograft. Median age at diagnosis was 24 years (range, 9–58 years) and minimum followup was 2.7 years (median, 13.8 years; range, 2.7–24.5 years). Patients were evaluated using radiographs and clinical examination. We used Musculoskeletal Tumor Society (MSTS), Disabilities of the Arm, Shoulder, and Hand (DASH), and SF-36 questionnaires to assess function and quality of life.
Results
All TCSA reconstructions fused; one patient had a second surgery to expedite union with the carpal row. After osteoarticular allograft, five patients were revised (three to a TCSA) for nonunion, fracture, or joint collapse. ROM and grip strength were comparable in both AO and TCSA, all above 60% of the contralateral side. Median MSTS and DASH scores were 73% and 6, respectively, and did not differ between the groups. The SF-36 scores showed less pain after TCSA; otherwise, all patients presented with comparable function.
Conclusions
TCSA wrist arthrodesis resulted in a functional and painless wrist reconstruction with a relatively low complication and donor site morbidity rate and comparable functional results as other techniques.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The distal radius is a relatively uncommon location for primary skeletal tumors, because malignant bone tumors represent only 3% of all upper limb tumors [12, 16, 22]. However, it is the third most common site of benign giant cell tumors of bone. Approximately 12% of all giant cell tumors present in the distal part of the radius [5, 6].
A few decades ago, malignant tumors of the distal radius were usually treated with surgery alone, usually necessitating limb amputation in the majority of cases [1]. With the introduction of neoadjuvant chemotherapy, limb salvage surgery has also become a well-accepted treatment option for bone tumors about the wrist [1, 2, 4, 11, 16, 17]. In most cases the surgeon can now achieve sufficient margins after resection for malignant and locally aggressive benign bone tumors to achieve local control [16, 27] and maintain upper limb function [4, 18, 19, 30]. However, reconstruction of the wrist after en bloc resection of the distal radius has remained a surgical challenge, mainly because of high functional demands of the hand in young patients with relatively long life expectancies for which long-term stable and painless hand function has been a primary surgical goal. In addition, tumors of the distal radius present specific difficulties for reconstruction after resection as a result of limited cover of soft tissue of tendons, adjacent neurovascular structures, and carpal bones [5, 13].
Several surgical procedures for wrist reconstruction after wide resection have been described. In 1975 Campbell and Akbarnia [6] described an arthrodesis technique using bone graft harvested from the posteromedial surface of the proximal tibia. Another technique providing a possibly more functional reconstruction made use of a fresh-frozen osteoarticular allograft to reconstruct the wrist [4, 17, 28]. Recent reports suggest ulnar translocation can provide a functional reconstruction in young children [3, 7, 27]. The adjacent distal ulna was transferred into the bony defect left behind after resection of the distal radius. Finally, both vascularized and nonvascularized autologous-free fibula graft have been proposed to reconstruct or fuse the wrist after resection [11, 18, 20, 21]. These techniques all came with their unique possible advantages and complications, but a gold standard for distal wrist reconstruction has not been established.
Although reconstructing the wrist using either an osteoarticular allograft (adults) or vascularized ulna of fibula (children) may maintain wrist function, these reconstructions are prone to instability, dislocation, and early joint collapse [4, 17]. Reconstructing the distal radius with a free (vascularized) fibula with arthrodesis prevents complications at the cost of wrist flexion but can be accompanied by donor site morbidity [11].
The purpose of this study was to describe the adapted surgical technique for a tibia cortical strut autograft wrist arthrodesis and describe its indications and complications. Additionally, we evaluated the functional outcome and quality-of-life scores for this procedure and compared them with fresh-frozen osteoarticular allograft and the available literature.
Surgical Technique
The surgical technique for arthrodesis using an tibia cortical strut autograft (TCSA) was adapted by the senior author (AMH) following the article published by Campbell and Akbarnia in 1975 [6]. The indications for this TCSA reconstruction were: radical resection of the distal radius for a malignant bone or soft tissue tumor involving the distal radius; primary or recurrent giant cell tumor of the distal radius requiring an en bloc resection of the distal radius; and finally failure of a different reconstruction of the wrist (eg, osteoarticular allograft, allograft wrist arthrodesis). The contraindications were malignant tumor involvement of the radiocarpal joint and insufficient possibilities for soft tissue coverage.
The tibial strut graft was removed from the donor site (ipsilateral proximal tibia) from just under the tibial tuberosity with an average length of 21 cm (Fig. 1). This provided enough length for the graft and prevented for both tibial tuberosity pain and complications of the patella tendon insertion. The surgeon carefully dissected the periosteum to allow for primary closure over the defect, providing better bone healing and less postoperative pain resulting from swelling. The strut was then cut to size and the remaining tibia strut graft could later be used for volar support. The carpal bones were prepared using an oscillating saw and osteotome, creating a docking site freed of cartilage in the scaphoid and lunate bones (Fig. 1). The opening into the carpal bones was prepared to fit the size of the tibial strut. The surgeon placed two nonabsorbable sutures on both sides of the longitudinal osteotomy to later close the osteotomy and fix the strut graft. No additional fixation was necessary for the distal docking site because the strut was press-fit into the first carpal row, creating a primary stable construct (Fig. 1). After inserting and fixating the strut into the carpal bones, the surgeon fixed the proximal docking site to the remaining distal radius using two tricortical screws. He could then fix the remaining tibial strut on the volar side of the carpal bones, allowing for additional volar support and the bone-stock using nonabsorbable sutures (Fig. 2). The cancellous sides of the strut grafts are compressed to expedite union; no additional bone grafting was necessary in these cases. The distal radioulnar joint was not restored or stabilized using internal fixation. Postoperatively, the forearm was immobilized in a (removable) spica cast until we observed radiographic union. A Sarmiento brace is applied for 6 weeks protecting the donor site from possible fracture and pain. Full weightbearing is allowed from Day 1.
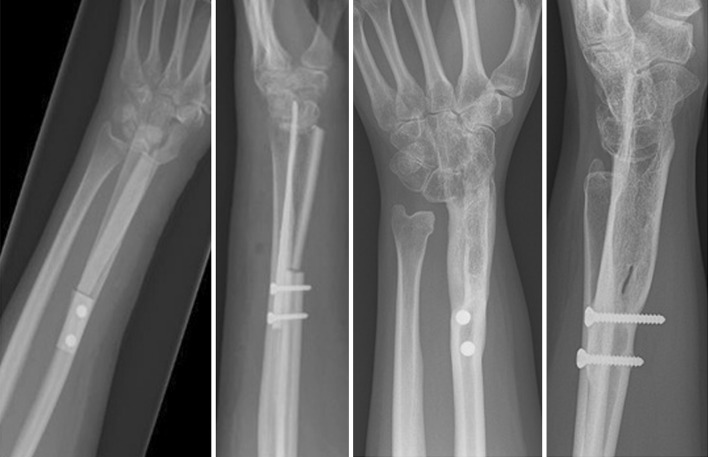
Fig. 1A–B.
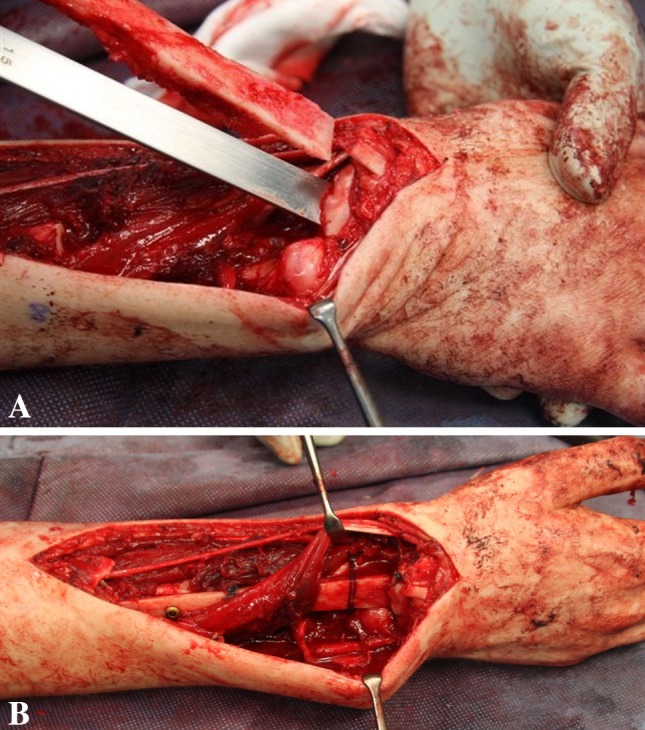
(A) Perioperative images show the introduction of the autograft into the carpal bones using an osteotome to create the docking site. (B) Perioperative images showing the final reconstruction with sutures closing the carpal osteotomy holding the graft in place at the docking site.
Fig. 2.
Postoperative radiographs taken directly postoperative and 6 years after a tibia cortical strut autograft were used to fuse the wrist. Final radiographs show full incorporation of the graft into the carpal bones and fusion between the two strut grafts.
Ulna transposition reconstruction was performed as described in previous studies [3, 7].
For osteoarticular reconstruction, we templated an osteoarticular allograft ordered preoperatively from the tissue bank (BIS Foundation, Leiden, The Netherlands). After aseptic recovery and processing, all allografts for these reconstructions were fresh-frozen at −80°C. To ensure optimal articular cartilage integrity, none was secondarily sterilized with chemicals or radiation. We performed this reconstruction using the technique earlier described by Kocher et al. [17].
Postoperatively, the forearm was immobilized in a cast until we observed the first signs of radiographic union. Wrist mobilization was started with initial supervised physiotherapy and home exercises. All patients were advised not to engage in contact sports or strenuous activities before full union was appreciated on followup radiographs.
Patients and Methods
Using our bone and soft tissue tumor database we identified 43 patients who were treated between 1987 and 2009 for a tumor about the wrist. Of these, 17 underwent wide resection and reconstruction of the distal radius/wrist (Fig. 1). All patients were operated on by the senior author (AHMT). There were 10 female and seven male patients with a median age at diagnosis of 24 years (range, 9–58 years). The right radius was affected in 11 patients and the left in six. The dominant extremity was involved in nine cases. The diagnostic protocol consisted of standard radiographs of the wrist, dynamic MRI, and sharp needle biopsy. The histological diagnoses included: giant cell tumor (eight patients), osteosarcoma (five), chondrosarcoma (two), malignant fibrous histiocytoma (one), and malignant fibromatosis (one) (Table 1). We attempted to contact all 17 patients by letter and telephone, informing them of this study. Three patients were lost to followup because they had moved abroad, and one patient had died of disease. The 13 remaining patients were free of disease at last followup. Because clinical and radiographic review was part of our standard clinical followup, no patients were recalled specifically for this study. The minimum followup was 2.7 years (median, 13.8 years; range, 2.7–24.5 years). Our local ethics committee deemed that signed informed consent for participation was not necessary.
Table 1.
Patient demographics
| Patient number | Sex* | Age (years) | Side | Diagnosis | Length of resection (cm) | Available for final followup | Followup (years) |
|---|---|---|---|---|---|---|---|
| 1 | Male | 13 | Right | AF | 11 | Yes | 25 |
| 2 | Female | 58 | Right* | OS | 17 | Yes | 16 |
| 3 | Male | 41 | Left | GCT | 8 | Yes | 24 |
| 4 | Male | 54 | Left | ChS | 8 | No | 5† |
| 5 | Male | 9 | Left* | OS | 11 | Yes | 20 |
| 6 | Female | 28 | Left | GCT | 5 | Yes | 21 |
| 7 | Female | 25 | Right* | ChS | 15 | Yes | 24 |
| 8 | Female | 23 | Right* | MFH | 5 | Yes | 19 |
| 9 | Female | 22 | Right | OS | 12 | No | 8 |
| 10 | Male | 20 | Right | GCT | 7 | No | 13 |
| 11 | Female | 35 | Right* | GCT | 8 | Yes | 20 |
| 12 | Female | 32 | Left | GCT | 6 | Yes | 6 |
| 13 | Female | 24 | Right | GCT | 7 | Yes | 9 |
| 14 | Female | 17 | Right* | OS | 12 | Yes | 3 |
| 15 | Male | 55 | Right* | GCT | 8 | Yes | 14 |
| 16 | Female | 19 | Left* | OS | 8 | Yes | 4 |
| 17 | Male | 24 | Right* | GCT | 9 | No | 3 |
* Dominant extremity; †death resulting from disease; AF = aggressive fibromatosis; OS = osteosarcoma; GCT = giant cell tumor; ChS = chondrosarcoma; MFH = malignant fibrous histiocytoma.
Of the original 17 patients, seven were initially treated with an osteoarticular allograft, five primarily, and two after curettage and filling with bone cement (Fig. 3). Six patients received a TCSA, four were performed primarily, and two after revision for tumor recurrence. These two had been previously treated with curettage, phenol, and bone cement. One patient received a nonvascularized fibula autograft and three underwent ulna interposition, one as primary treatment and two as secondary reconstruction after curettage. TCSA was only used from 1995 on; other procedures were only used before 1995. After 1995 all patients were reconstructed with TCSA. The mean length of the resection was 9.3 cm (TCSA group, 8.5 cm; osteoarticular allograft group, 9.5 cm).
Fig. 3.
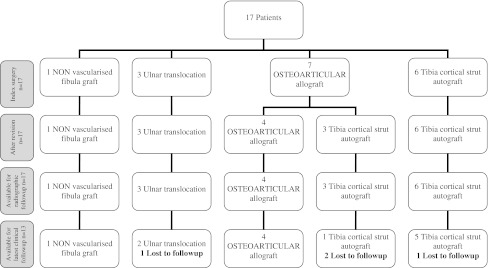
Flowchart for included patients. Three of seven patients that were initially reconstructed using an osteoarticular allograft were revised to TCSA for nonunion (two) and painful joint collapse.
Appropriate neoadjuvant therapy was provided for patients with osteosarcoma within the EURAMOS protocol [33]. One patient (Patient 1) received radiation therapy for local recurrence before the reconstruction.
We routinely scheduled visits at 6 weeks, 3, 6, 9, 12, and 18 months after surgery, and yearly thereafter until last followup. During these standard followup visits, we initially assessed wound healing and local control and when functional rehabilitation was allowed also ROM using a goniometer. Until bony union [9] was appreciated both clinically (no pain to palpation or axial compression at the osteotomy site[s]) and radiographically (presence of bridging callus at least three cortices), a plain radiograph was taken at every visit and yearly thereafter. We assessed these orthogonal plain radiographs for the presence of local recurrence, bony union, and possible subluxation of the radioulnar and radiocarpal joints. Bony union was evaluated using plain radiographs in two planes. Union was described as bridging callus over the osteotomy or cortical continuity at more than three cortices.[9] During the latest scheduled followup, when all patients were informed on this study, we obtained grip strength measurement of both hands, expressing strength as a percentage of the unaffected side, and completed the necessary questionnaires for wrist function and quality of life. All 13 patients had radiographic examination. All patients were evaluated by an independent research fellow blinded to primary surgical treatment during last followup.
Outcome assessments included the SF-36 [32], Disabilities of the Arm, Shoulder, and Hand (DASH) [14] and the Musculoskeletal Tumor Society scoring system (MSTS) [10]. The SF-36 consisted of 36 items and measured each of eight general health concepts: physical functioning, role limitations due to physical health problems, bodily pain, general health perception, social functioning, vitality, limitations due to emotional problems, and mental well-being. This resulted in a final score between 0 and 100 with the higher score defining a more favorable state of health [32]. We used the MSTS to measure functional outcome. The latest version of this system evaluated factors that cover the whole patient such as pain, emotional acceptance, and functional activities [10]. The DASH [14, 31], developed by the American Academy of Orthopaedic Surgeons and Institute for Work and Health, measured the ability to perform different daily care activities and monitored symptoms associated with the condition of the upper extremity. A score of 0 meant no disability and 100 indicated maximum disability.
As a result of the small patient groups, we computed only descriptive statistics in SPSS 17.0 (Chicago, IL, USA).
Results
Of the patients treated with either osteoarticular allograft or TCSA, two had local recurrence after reconstruction (Table 2). In one of these (Patient 4), the chondrosarcoma recurred in the surrounding soft tissue within 7 months after reconstruction. After a reresection, the chondrosarcoma recurred again, and the patient finally underwent an above-the-elbow amputation 14 months after the index surgery. In the second case (Patient 11), the giant cell tumor recurred 11 months after the osteoarticular allograft was revised to an arthrodesis because of a fracture. One patient with an ulnar translocation had persistent nonunion; she was revised using cancellous autograft and plate fixation (Table 2).
Table 2.
Procedures and complications
| Patient number | Primary procedure | Reconstruction procedure | Recurrence | Complication | Treatment | Time to union for index surgery (months) |
|---|---|---|---|---|---|---|
| 1 | No | Autologous nonvascularized fibula grafting | No | Nonunion | Iliac bone grafting | 7 |
| 2 | No | Ulnar translocation | No | Radiocarpal arthritis | none | 8 |
| 3 | Yes | Ulnar translocation | No | Nonunion | AO plate with persistent asymptomatic pseudoarthrosis | 19 |
| 4 | No | Ulnar translocation | Yes | Metastasis | Above-elbow amputation | Amputation before union was appreciated§ |
| 5 | No | Osteoarticular allograft | No | Nonunion, mild arthritis | DC plate iliac bone graft Mild complaints |
16 |
| 6 | Yes | Osteoarticular allograft | No | Mild arthritis | Mild complaints | 29 |
| 7 | No | Osteoarticular allograft | No | Plate failure, mild arthritis | Iliac bone grafting, no complaints | 22 |
| 8 | Yes | Osteoarticular allograft | No | Severe arthritis | None | 15 |
| 9 | No | Osteoarticular allograft | No | Instability, nonunion | Iliac bone grafting, arthrodesis and TCSA arthrodesis | 13*,† |
| 10 | No | Osteoarticular allograft | No | Arthritis, joint collapse | TCSA arthrodesis | 14*,† |
| 11 | No | Osteoarticular allograft | Yes | Fracture, nonunion | TCSA arthrodesis | 11*,† |
| 12 | Yes | Tibia autograft, interposition arthrodesis | No | None | 12 | |
| 13 | No | Tibia autograft, interposition arthrodesis | No | None | 12 | |
| 14 | Yes | Tibia autograft, interposition arthrodesis | No | None | 8 | |
| 15 | Yes | Tibia autograft, interposition arthrodesis | No | None | 9 | |
| 16 | Yes | Tibia autograft, interposition arthrodesis | No | Fracture | New tibial strut from contralateral tibia | 12*,†,‡ |
| 17 | No | Tibia autograft, interposition arthrodesis | No | None | 9 |
* Nonunion revised to arthrodesis; †time to union after revision to tibial autograft interposition arthrodesis; ‡nonunion after fracture, revised and fused after 12 months. Multiple soft tissue metastases developed 3.9 years after tumor resection about the elbow in the subcutis. A distal transhumeral amputation was performed. The retrieval specimen showed stable fusion between the carpal bones and the tibia autograft; §Patient 4 had an amputation before union was noted. Patients 9, 10, and 17 were lost to followup long after their wrist was clinically and radiographically united and free of complaints or pain; TCSA = tibia cortical strut autograft.
Using clinical examination and plain film radiographs, we observed bony union for the index surgery at one or both sides of the graft in 12 of the initial 17 patients. Median time to final union was 13 months (range, 7–29 months): 16 (range, 13–29) for the osteoarticular allograft group and 11 (range, 9–14) for the arthrodesis group (Table 2).
We observed no intraoperative or early postoperative complications. After reconstruction, we saw no wound healing problems or infections (Table 2). In the group of patients reconstructed with osteoarticular allograft, we diagnosed one plate loosening, one fracture, and three nonunions on postoperative radiographs. The patient with plate loosening was successfully revised with iliac bone grafting and refixation. In one case, we treated the nonunion with additional fixation using a DC plate. Three patients (Patients 9–11) eventually received a TCSA interposition arthrodesis because of persistent nonunion or painful joint collapse. The one complication in the TCSA group was a traumatic fracture of the tibial strut, resulting in a nonunion. This strut graft was revised 2 years after the index surgery using a new strut from the contralateral tibia. Almost 4 years after tumor resection (3 months after final followup for this study), this patient developed multiple soft tissue metastases around the elbow in the subcutaneous tissue. A distal transhumeral amputation was performed after we had completed final followup measurements. The retrieval specimen showed stable fusion between the carpal bones and the tibia autograft (Fig. 4). Besides some mild discomfort around the scar and the expected discomfort caused by the Sarmiento brace, no other donor site morbidity was noted.
Fig. 4.
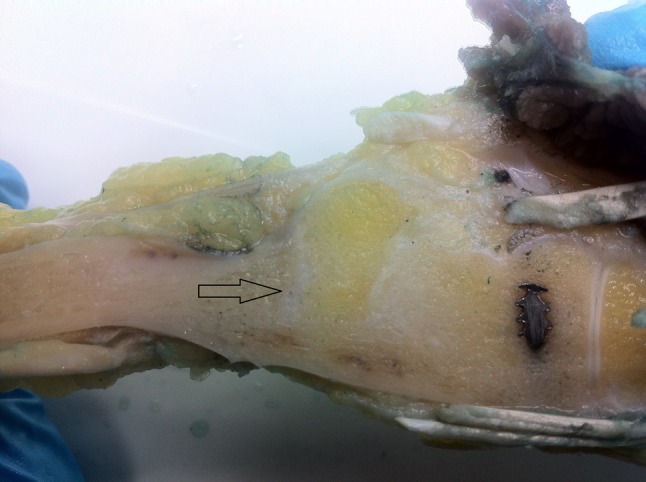
Photograph shows the retrieval specimen of Patient 16 after a distal transhumeral amputation for soft tissue metastasis about the elbow. Full incorporation between the two grafts and fusion between the graft and the carpal bones can be appreciated.
We assessed the function in 13 patients (Table 3), three of whom were initially treated with an osteoarticular allograft but had a TCSA after allograft failure (Fig. 3). Pro- and supination were almost absent after ulnar translocation and were only possible in Patient 3 as a result of a pain-free nonunion. After TCSA arthrodesis, radiocarpal function was negligible, but pro- and supination were possible and comparable to patients with osteoarticular allografts (Table 3). Grip strength was above 60% of the collateral side in all patients. Median grip strength was 75% of the contralateral side in the TCSA group and 80% for the osteoarticular allograft group.
Table 3.
Range of motion and grip strength
| Patient number | Pro-/supination | Percentage of pro-/supination of contralateral side | Extension/flexion | Percentage of extension/flexion of contralateral side | Radial/ulnar deviation | Percentage of radial/ulnar deviation of contralateral side | Grip strength in kg (% of contralateral side) | MSTS score |
|---|---|---|---|---|---|---|---|---|
| 1 | 0/0/0 | 0/0/25 | 0–50 | 20/0/25 | 100/63 | 30 (60) | 18 | |
| 2 | 0/0/0 | 0/0/0 | 0/0/0 | 40 (67) | 20 | |||
| 3 | 90/0/75 | 100/94 | 25/0/50 | 63–83 | 20/0/5 | 100/14 | 32 (64) | 30 |
| 4† | ||||||||
| 5 | 60/0/30 | 67/33 | 25/0/60 | 83–100 | 15/0/30 | 75/86 | 32 (64) | 28 |
| 6 | 90/0/75 | 100/100 | 30/0/40 | 71/89 | 25/0/35 | 100/100 | 23 (85) | 29 |
| 7 | 90/0/20 | 100/22 | 20/0/50 | 50/83 | 7/0/35 | 35/88 | 28 (82) | 12 |
| 8 | 90/0/90 | 100/100 | 35/0/55 | 78/92 | 20/0/35 | 100/88 | 30 (79) | 16 |
| 9† | ||||||||
| 10† | ||||||||
| 11 | 80/0/40 | 89/45 | 0/0/0 | 0/0 | 10/0/5 | 50/13 | 21 (72) | 21 |
| 12 | 70/0/75 | 78/83 | 0/0/0 | 0/0 | 0/0/0 | 0/0 | 30 (67) | 27 |
| 13 | 90/0/90 | 100/100 | 0/0/0 | 0/0 | 10/0/25 | 50/63 | 32 (80) | 27 |
| 14 | 90/0/0 | 100/0 | 0/0/15 | 0/21 | 0/0/15 | 0/63 | 35 (73) | 26 |
| 15 | 90/0/45 | 100/50 | 0/0/0 | 0/0 | 0/0/0 | 0/0 | 32 (78) | 22 |
| 16* | 50/0/45 | 56/50 | 0/0/0 | 0/0 | 0/0/0 | 0/0 | 28 (61) | 12 |
| 17† | ||||||||
* Before amputation; †lost to followup; MSTS = Musculoskeletal Tumor Society.
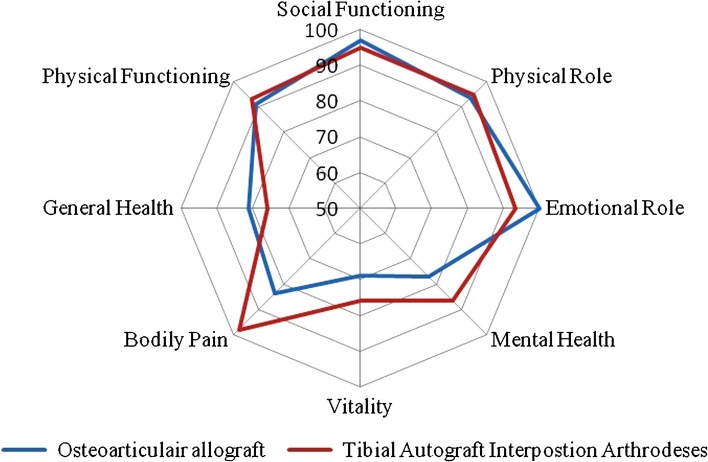
The median overall MSTS score was 73% compared with the contralateral side (range, 40%–100%). The median DASH score, calculated over the remaining 13 patients, was 5.8 (range, 0–55). Seven patients demonstrated no pain at all (SF-36 bodily pain), but two patients who received an osteoarticular allograft had moderate pain in the last 4 weeks before last followup. All patients who had been employed outside the home preoperatively continued their jobs postoperatively. Median MSTS score was 21.5 for the TCSA group and 22 for the osteoarticular allograft group. The median DASH score in the TCSA group was 9.2 and 10.9 in the osteoarticular allograft. The SF-36 web plot (Fig. 5) shows the slight advantages of TCSA arthrodesis when compared with patients undergoing osteoarticular allograft, particularly for bodily pain scores. Mental health, social functioning, and general health were very dependent on personal factors such as important life events and comorbidity; therefore, we did not discuss them in this analysis. Physical functioning and functional limitations due to physical health problems were similar for both techniques.
Fig. 5.
Chart shows SF-36 outcomes for both patients undergoing tibia cortical strut autograft arthrodesis and osteoarticular allograft.
Discussion
The surgical treatment of tumors about the distal radius presents a substantial challenge in orthopaedic oncology. There has been no consensus in the limited literature about the best surgical reconstruction of the distal radius after tumor resection; studies have described multiple methods of reconstruction in small patient populations [18–22]. However, a standard procedure has not yet been formulated as a result of small numbers of patients, differences in surgical techniques, and patient characteristics. The aim of this study was to describe an updated reconstruction and compare its functional outcome with the commonly used osteoarticular allograft reconstruction of the wrist. For this technique, a tibial strut autograft was fused with the first carpal row to perform an arthrodesis as first described by Campbell and Akbarnia in 1975 [6]. The original article clearly describes the use of the proximal tibia to reconstruct the distal radius. It includes the proximal medial tibia in the donor site, imposing a possible risk for patella tendon disruption or complaints of the extensor apparatus. Additionally, the tibia strut was fixed with Kirschner wires in the original technique.
We recognize the limitations of this study. First are the retrospective character and small number of patients, which precluded any relevant statistical analyses. However, described indications for distal radius reconstruction are relatively rare. Second, the evaluation of bony union entailed some degree of subjectivity. Even with the most technical support, it was difficult to state definitively that a host-graft junction showed union. Clinical examination combined with radiographs are still most commonly used to assess union [9], but CT has been proposed more a reliable entity [9]. Therefore, the time of union that we obtained should be considered an approximation. Third, the relative absence of comparable data in the literature on DASH, SF-36, and MSTS scores for both tumor cases or other patients in need of a distal radius reconstruction or arthrodesis made a reliable literature comparison difficult. Several studies [4, 25, 26, 28, 29] have recently published function (ROM, MSTS/DASH) of surgical reconstruction of the distal radius at mean followups of 4–14 years (Table 4). Our data falls within the ranges reported by these authors. Although the ROM for wrist flexion and extension after joint reconstruction using an osteoarticular allograft is superior to arthrodesis, favorable functional scores (MSTS/DASH) for arthrodesis techniques, especially after longer followup, were noted [1, 4, 15, 23–26, 28, 29]. This difference may be explained by the increased risk for joint collapse of the osteoarticular allograft in the long term [26, 28, 29].
Table 4.
Functional results for distal radius reconstruction in recent literature
| Authors | Reconstruction | Number | Mean followup | ISOLS-MSTS | MSTS | Mean DASH | Flexion/extension | Pro-/supination | Grip (percent contralateral side) |
|---|---|---|---|---|---|---|---|---|---|
| Scoccianti et al., 2010 [26] | Osteoarticular allograft | 17 | 5 years | 86% (range, 63–97) | NA | NA | 56/58 | 80/84 | – |
| Saini et al., 2011 [25] | Ipsilateral fibula arthrodesis | 12 | 6 years | 91% (range, 77–93) | NA | NA | 42/31 | 37/52 | 71 (range, 42–86) |
| Jaminet et al., 2012 [15] | Fibuloscapholunate arthrodesis | 3 | 6–60 months | NA | NA | NA | 33/13 | 67/56 | 73 (range, 70–80) |
| van Isacker et al., 2011 [29] | Osteoarticular allograft | 5 | 14 years (range, 5–19) | 62 (range, 50–74) | 18.6 (range, 13–22) | NA | NA | NA | NA |
| Peng-Fei and Yu-Hua, 2011 [23] | Vascularised fibular graft joint reconstruction | 18 | NA | NA | 26 (range, 21–29) | NA | 33/67 | 34/13 | 75% |
| Puloski et al., 2007 [24] | Arthrodesis after resection | 21 | NA | NA | 27 (range, 23–31) | 20 (5–35) | 0/0 | 76/68 | 75% |
| Szabo et al., 2006 [28] | Osteoarticular allograft + Sauve-Kapandji | 9 | 100 months (range, 39–219) | 75% (range, 40–90) | NA | 15 (2–41) | 50/52 | 80/67 | 77% |
| Bianchi et al., 2005 [4] | Osteoarticular allograft | 12 | 52 months (range, 26–145) | NA | NA | NA | 514/37 | NA | NA |
| Current study | Tibia cortical strut autograft arthrodesis | 9 | 8.9 years (range, 3–20) | 75% (range, 40–90) | 23 (range, 12–27) | 14.3 (0–55) | 0/0 | 78/50 | 72 |
ISOLS = International Society of Limb Salvage; MSTS = Musculoskeletal Tumor Society; DASH = Disability of the Arm, Shoulder, and Hand; NA = not applicable.
Finally, the possible introduction of inclusion bias, resulting from the difference in timeframes between the compared techniques, should be considered. Although the osteoarticular allograft group showed a considerably longer followup, this did not directly result in higher complication rates because all complications, excluding joint collapse, occurred in the first 2 years of followup.
Although a painless, stable, and functional reconstruction of the wrist could be achieved using an osteoarticular allograft, this reconstruction has been preferred for smaller forearm resections with possible preservation of the wrist extensors [17]. In larger segment resections with substantial soft tissue extension, a primary arthrodesis using either auto- or allograft may have provided a better chance for union and a stable wrist resulting from the lack of soft tissues stabilizing the wrist [16]. In addition, an arthrodesis could be a successful salvage procedure after failed joint reconstruction using either osteoarticular allograft or tumor prosthesis. An arthrodesis offered inherent stability, pain relief, adequate hand function, and good grip strength despite the loss of flexion and extension. An additional advantage of a wrist arthrodesis was the possibility to bear substantial loads [5, 7, 8, 20]. Other authors have critically commented on the use of an arthrodesis because it results in limited wrist function as a result of loss in ROM. However, this limited function, in our experience, can still result in a functional hand and lower arm. Additionally, an osteoarticular allograft only partly restored wrist function but was accompanied by a high rate of nonunion, ulnar subluxation, and joint collapse [4, 13, 17].
Other techniques using either the ulna or a free vascularized fibula to reconstruct the distal radius have also resulted in satisfactory functional results, but it has been anticipated that a one-bone forearm is possibly weaker and, therefore, one would be hesitant to let patients engage in strenuous activities [3, 7, 27]. Also, this type of reconstruction generally did not allow for any pro- or supination. One study reported substantial donor site morbidity in vascularized or nonvascularized autologous fibula grafts as a risk of pain in the leg, valgus deformity of the ankle, or looseness of the collateral ligament of the knee [21]. Additionally, when using autologous vascularized fibula grafts, there is a need for specialized know-how in microsurgical procedures.
The limited reports on tibial strut autografts have probably been the result of concerns about potential donor site morbidity, fractures of the tibia, and functional deficit after arthrodesis in young patients. Our patients demonstrated no tibial fractures, and complaints concerning the donor site were limited or of short duration. Additionally, we found only one postoperative complication (one fracture resulting in nonunion of the bone graft) in the arthrodesis group. Nonunion occurred in three cases treated by osteoarticular allograft reconstruction. Results presented in the literature, although small in numbers, agreed with our results for union, complication rates, and postoperative function [4, 17, 28].
If we considered functional outcome as an end point, the results differed between the methods used. Supination was slightly better in the osteoarticular allograft group, but pronation was better in the TCSA group. Flexion and extension were absent in almost all patients reconstructed with a TCSA. No patients had restrictions in ROM of the elbow or shoulder. These results were comparable to other studies reported using an osteoarticular allograft [4, 17, 28]. The outcome measures of the MSTS, SF-36, and DASH indicated a high quality of life in patients treated with TCSA, which was remarkable given the limited ROM. The median DASH and MSTS scores were comparable in patients treated with osteoarticular allograft and TCSA. A small benefit in terms of pain was seen in patients treated with TCSA.
In conclusion, TCSA of the wrist after tumor resection of the distal radius resulted in a stable and painless wrist and presented comparable results for quality-of-life measurement using the SF-36 score when compared with osteoarticular allograft reconstruction of the wrist. With our reported low complication rate for infection, nonunion, and postoperative pain, we now consider TCSA a reasonable alternative to osteoarticular allograft in primary and secondary reconstruction of the distal radius.
Footnotes
Each author certifies that he, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bacci G, Ferrari S, Lari S, Mercuri M, Donati D, Longhi A, Forni C, Bertoni F, Versari M, Pignotti E. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84:88–92. doi: 10.1302/0301-620X.84B1.12211. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Mercuri M, Bertoni F, Picci P, Manfrini M, Gasbarrini A, Forni C, Cesari M, Campanacci M. Predictive factors for local recurrence in osteosarcoma: 540 patients with extremity tumors followed for minimum 2.5 years after neoadjuvant chemotherapy. Acta Orthop Scand. 1998;69:230–236. doi: 10.3109/17453679809000921. [DOI] [PubMed] [Google Scholar]
- 3.Bhan S, Biyani A. Ulnar translocation after excision of giant cell tumour of distal radius. J Hand Surg Br. 1990;15:496–500. doi: 10.1016/0266-7681(90)90102-A. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi G, Donati D, Staals EL, Mercuri M. Osteoarticular allograft reconstruction of the distal radius after bone tumour resection. J Hand Surg Br. 2005;30:369–373. doi: 10.1016/j.jhsb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 6.Campbell CJ, Akbarnia BA. Giant-cell tumor of the radius treated by massive resection and tibial bone graft. J Bone Joint Surg Am. 1975;57:982–986. [PubMed] [Google Scholar]
- 7.Chalidis BE, Dimitriou CG. Modified ulnar translocation technique for the reconstruction of giant cell tumor of the distal radius. Orthopedics. 2008;31:608. doi: 10.3928/01477447-20080601-05. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CY, Shih HN, Hsu KY, Hsu RW. Treatment of giant cell tumor of the distal radius. Clin Orthop Relat Res. 2001;383:221–228. doi: 10.1097/00003086-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Corrales LA, Morshed S, Bhandari M, Miclau T., III Variability in the assessment of fracture-healing in orthopaedic trauma studies. J Bone Joint Surg Am. 2008;90:1862–1868. doi: 10.2106/JBJS.G.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enneking WF, Dunham W, Gebhardt MC, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 11.Friedrich JB, Moran SL, Bishop AT, Wood CM, Shin AY. Free vascularized fibular graft salvage of complications of long-bone allograft after tumor reconstruction. J Bone Joint Surg Am. 2008;90:93–100. doi: 10.2106/JBJS.G.00551. [DOI] [PubMed] [Google Scholar]
- 12.Garcia J, Bianchi S. Diagnostic imaging of tumors of the hand and wrist. Eur Radiol. 2001;11:1470–1482. doi: 10.1007/s003300000751. [DOI] [PubMed] [Google Scholar]
- 13.Harness NG, Mankin HJ. Giant-cell tumor of the distal forearm. J Hand Surg Am. 2004;29:188–193. doi: 10.1016/j.jhsa.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Jaminet P, Rahmanian-Schwarz A, Pfau M, Nusche A, Schaller HE, Lotter O. Fibulo-scapho-lunate arthrodesis after resection of the distal radius for giant-cell tumor of the bone. Microsurgery. 2012 Mar 21 [Epub ahead of print]. DOI: 10.1002/micr.21971. [DOI] [PubMed]
- 16.Kesani AK, Tuy B, Beebe K, Patterson F, Benevenia J. Single-bone forearm reconstruction for malignant and aggressive tumors. Clin Orthop Relat Res. 2007;464:210–216. doi: 10.1097/BLO.0b013e318156fb30. [DOI] [PubMed] [Google Scholar]
- 17.Kocher MS, Gebhardt MC, Mankin HJ. Reconstruction of the distal aspect of the radius with use of an osteoarticular allograft after excision of a skeletal tumor. J Bone Joint Surg Am. 1998;80:407–419. doi: 10.2106/00004623-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Maruthainar N, Zambakidis C, Harper G, Calder D, Cannon SR, Briggs TW. Functional outcome following excision of tumours of the distal radius and reconstruction by autologous non-vascularized osteoarticular fibula grafting. J Hand Surg Br. 2002;27:171–174. doi: 10.1054/jhsb.2001.0707. [DOI] [PubMed] [Google Scholar]
- 19.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68:235–242. [PubMed] [Google Scholar]
- 20.Minami A, Kato H, Iwasaki N. Vascularized fibular graft after excision of giant-cell tumor of the distal radius: wrist arthroplasty versus partial wrist arthrodesis. Plast Reconstr Surg. 2002;110:112–117. doi: 10.1097/00006534-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Murray JA, Schlafly B. Giant-cell tumors in the distal end of the radius. Treatment by resection and fibular autograft interpositional arthrodesis. J Bone Joint Surg Am. 1986;68:687–694. [PubMed] [Google Scholar]
- 22.Nishida J, Sim FH, Wenger DE, Unni KK. Malignant fibrous histiocytoma of bone. A clinicopathologic study of 81 patients. Cancer. 1997;79:482–493. doi: 10.1002/(SICI)1097-0142(19970201)79:3<482::AID-CNCR9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 23.Peng-Fei S, Yu-Hua J. Reconstruction of distal radius by fibula following excision of grade III giant cell tumour: follow-up of 18 cases. Int Orthop. 2011;35:577–580. doi: 10.1007/s00264-010-0967-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puloski SK, Griffin A, Ferguson PC, Bell RS, Wunder JS. Functional outcomes after treatment of aggressive tumors in the distal radius. Clin Orthop Relat Res. 2007;459:154–160. doi: 10.1097/BLO.0b013e318059b91f. [DOI] [PubMed] [Google Scholar]
- 25.Saini R, Bali K, Bachhal V, Mootha AK, Dhillon MS, Gill SS. En bloc excision and autogenous fibular reconstruction for aggressive giant cell tumor of distal radius: a report of 12 cases and review of literature. J Orthop Surg Res. 2011;6:14. doi: 10.1186/1749-799X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scoccianti G, Campanacci DA, Beltrami G, Caldora P, Capanna R. The use of osteo-articular allografts for reconstruction after resection of the distal radius for tumour. J Bone Joint Surg Br. 2010;92:1690–1694. doi: 10.1302/0301-620X.92B12.25121. [DOI] [PubMed] [Google Scholar]
- 27.Seradge H. Distal ulnar translocation in the treatment of giant-cell tumors of the distal end of the radius. J Bone Joint Surg Am. 1982;64:67–73. [PubMed] [Google Scholar]
- 28.Szabo RM, Anderson KA, Chen JL. Functional outcome of en bloc excision and osteoarticular allograft replacement with the Sauve-Kapandji procedure for Campanacci grade 3 giant-cell tumor of the distal radius. J Hand Surg Am. 2006;31:1340–1348. doi: 10.1016/j.jhsa.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 29.van Isacker T, Barbier O, Traore A, Cornu O, Mazzeo F, Delloye C. Forearm reconstruction with bone allograft following tumor excision: a series of 10 patients with a mean follow-up of 10 years. Orthop Traumatol Surg Res. 2011;97:793–799. doi: 10.1016/j.otsr.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Vander Griend RA, Funderburk CH. The treatment of giant-cell tumors of the distal part of the radius. J Bone Joint Surg Am. 1993;75:899–908. [DOI] [PubMed]
- 31.Veehof MM, Sleegers EJ, van Veldhoven NH, Schuurman AH, van Meeteren NL. Psychometric qualities of the Dutch language version of the Disabilities of the Arm, Shoulder, and Hand questionnaire (DASH-DLV) J Hand Ther. 2002;15:347–354. doi: 10.1016/S0894-1130(02)80006-0. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Whelan J, Seddon B, Perisoglou M. Management of osteosarcoma. Curr Treat Options Oncol. 2006;7:444–455. doi: 10.1007/s11864-006-0020-y. [DOI] [PubMed] [Google Scholar]