Abstract
Background
The dual-mobility concept was proposed as an alternative to prevent postoperative dislocation events. However, intraprosthetic dislocation (IPD) is a troublesome and specific complication induced by the loss of the polyethylene retentive rim and escape of the femoral head from the polyethylene liner. The factors associated with IPD are unknown as only isolated cases have been reported and do not provide a clear understanding of the mechanisms of failure.
Questions/purposes
We therefore (1) identified features related to different types of IPD and (2) determined factors related to the timing of IPD.
Methods
We identified 81 cases (80 patients) with IPD from among 1960 primary THAs performed between January 1985 and December 1998. To classify the types of IPD we considered perioperative (presence of arthrofibrosis, cup loosening, and type of liner wear) and radiographic (radiographic cup loosening or migration, and ossification) features.
Results
We identified three types of IPD with the following causal mechanisms: Type 1 was pure IPD without arthrofibrosis and without cup loosening (n = 26), Type 2 was IPD secondary to blocking of the liner (n = 41), and Type 3 was IPD associated with a cup loosening (n = 14). The mean times of onset were, 11, 8, and 9 years after THA, respectively. We found no difference according to the stem design regarding timing of the IPD.
Conclusions
This new IPD classification allows clinicians to anticipate the possible conditions they will encounter with revision surgery and plan surgery (cup removal, liner exchange, synovectomy). The implant characteristics and this new classification accounted for the differences in the timing of occurrence.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The dual-mobility concept was invented and developed by Bousquet in 1974 to reduce the postoperative dislocation rate [6]. The reported rates of postoperative instability ranging from 0% to 2% in primary [2, 6, 11, 17, 19, 20] and 2% to 15% in revision THA [13, 18] confirmed the success of dual mobility in achieving low rates of dislocation.
The original concept has evolved during the past 30 years; bone anchorage of the acetabular cup was reinforced in 2000 and the alumina surface was replaced in 2006 with a dual layer coating of hydroxyapatite and titanium plasma spray. After initial enhancement of primary fixation and secondary osseointegration of the acetabular cup achieved in 1993, the reported survival rates of 90% to 96% at 12 to 22 years [2, 17, 19, 20] were similar to those reported with noncemented implants [4, 9, 10]. In 1998 the polyethylene (PE) liners were changed by increasing crosslinking to reduce PE wear rate [7] although before that time the wear rates (approximately 70 mm3/year [1, 8]) were comparable to those reported in the literature with metal and conventional PE bearing couples [1]. Finally, in 2001 the design of the acetabular cup was improved by decreasing the anterior overhang and increasing the press fit to achieve better primary fixation and avoid mechanical conflict with the iliopsoas tendon.
Despite improvements, intraprosthetic dislocation (IPD) remains a troublesome dual mobility-specific complication induced by the loss of the PE retentive rim and escape of the femoral head from the PE liner [12, 16]. Although IPD dislocation is rare (series from the literature have reported a 2% to 4% long-term incidence [2, 17, 19, 20]), it requires surgical treatment. A multicenter series from Massin et al. [14] reported a lower incidence (0.3% at 10 years followup) of IPD than those reported in previous studies [2, 17, 19, 20]. However, the relationship between the decrease of the IPD rate and the different liner modifications remains unclear, partly owing to the lack of data for clinical, radiographic, and material characteristics of each IPD.
Our purposes therefore were to (1) devise a classification of IPD based on clinical and radiographic findings; and (2) determine the factors influencing the timing of appearance of IPD.
Patients and Methods
We prospectively followed all 1960 revisions performed on 1850 patients who had dual-mobility prostheses implanted between January 1985 and December 1998. During that time, all patients were treated with this type of implant for THA. Eighty-one (80 patients) of the 1960 hips subsequently had an IPD (4%); one patient had bilateral IPDs. The mean interval between implantation of the dual-mobility prostheses and onset of IPD was 9 ± 4 years (range, 1–18 years). The last reported case occurred in 2011. Thirty-two of the 80 patients with IPDs were females and 48 were males. Their mean age at the time of the implantation of the prostheses was 51 ± 15 years (range, 21–85 years). Their mean BMI was 26 ± 4 kg/m2 (range, 18–36 kg/m2). Initial etiologies at the time of the implantation included 48 patients with primary osteoarthritis of the hip, seven with posttraumatic arthritis, 18 with aseptic osteonecrosis of the femoral head, six with developmental dysplasia, and one had THA after hip arthrodesis. No patients were lost to followup. The minimum followup of the 1850 patients was 11 years (mean, 14 ± 4 years; range, 11–25 years). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
We performed THA only through a posterior approach. The characteristics of the prostheses used during the initial surgery, including size and features of the dual-mobility acetabular cup, prosthetic neck, and stem were noted. From 1985 to 1998 three types of prostheses were used (Fig. 1): (1) the first featured a stainless steel PF® (Serf, Decines, France) femoral stem combined with a stainless steel NOVAE® (Serf) dual-mobility cup, from 1985 to 1990; (2) the second featured a titanium PRO® (Serf) femoral stem combined with a 316L stainless steel NOVAE® dual-mobility cup, from 1988 to 1998; and (3) the third featured a titanium PRO® femoral stem combined with a titanium NOVAE® dual-mobility cup, used in 120 THAs from 1990 to 1992. Both stems differed in femoral neck diameter and material, which was 16 mm and stainless steel, respectively, for the PF® femoral stems, and 13 mm and titanium, respectively, for the PRO (Serf) femoral stems (Fig. 2). Among the 81 IPDs, 31 occurred in patients with PF® stems and 50 in patients with PRO® stems. All dual-mobility cups were cementless, press-fit NOVAE-1® Serf components with AL2O3 porous alumina surface treatment. All cups were identical except for being either stainless steel or titanium. These tripod cups featured two impacted anchorage studs and one superior fixation screw (Fig. 3). The mobile liner provided dual mobility and was free to articulate between the metal-backed shell and the femoral head. This retentive component (Fig. 3) manufactured from PE was vigorously impacted over the femoral head. Cobalt-chromium femoral heads were either 22.2 mm or 26 mm in diameter. Two types of acetabular cups were used and only differed in the metal-backed material manufactured from stainless steel or titanium.
Fig. 1.
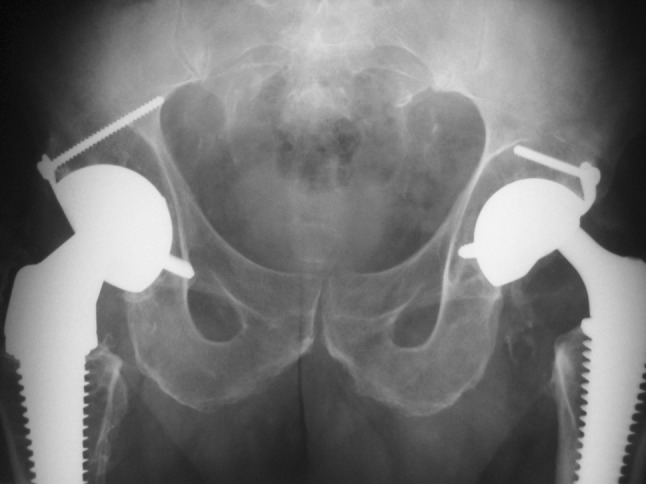
The AP postoperative radiograph shows a PF® (Serf) stem combined with a Novae® (Serf) dual-mobility cup on the right side. On the left side there is a Pro® stem combined with a Novae® dual-mobility cup. Type I IPD is evident on the left side.
Fig. 2.

Both stems differed in the femoral neck diameter and material, which was 16 mm and stainless steel, respectively, for the PF® (Serf) femoral stems on the left and 13 mm and titanium, respectively, for the PRO® (Serf) femoral stems on the right.
Fig. 3.

The press-fit NOVAE-1® Serf components are shown. These tripod cups feature two impacted anchorage studs and one superior fixation screw. (Reproduced with permission from Serf Dedienne Santé Implants Orthopédiques & Dentaires, Décines, France.)
We performed an intraoperative assessment at revision surgery for IPD and two types of data were systematically evaluated: (1) the possible causes for blockage of the larger articulation and thus accelerated wear of the PE retentive rim. Various causes such as the presence of a periprosthetic fibrosis, a cam effect, or calcifications leading to impingement have been suggested; and (2) possible cup loosening occurring before IPD associated with wear and metallosis debris, which might account for a third-body reaction. Based on intraoperative findings, IPD was classified into three types: (1) Type I was pure IPD secondary to wear of the PE retentive rim and associated with normal functioning of the dual-mobility prosthesis (Fig. 4); (2) Type II was IPD secondary to blocking of the larger articulation of the dual-mobility prosthesis (Fig. 5); and (3) Type III was IPD secondary to loosening (Table 1).
Fig. 4.
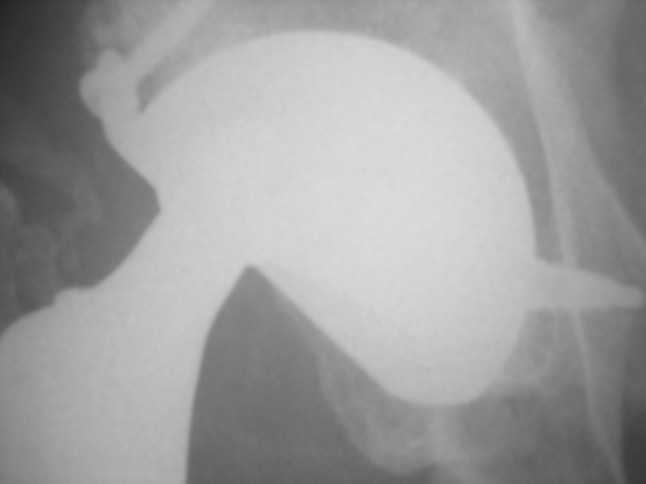
This postoperative radiograph shows a Type I IPD in a patient with 15 years followup. No acetabular loosening is seen.
Fig. 5.
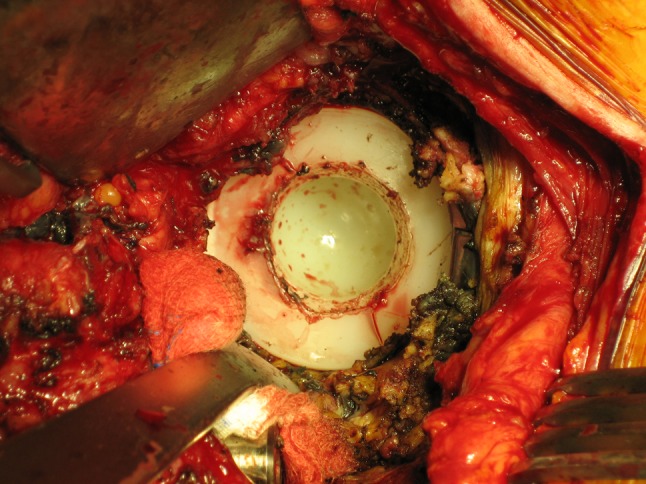
The perioperative photograph shows a Type II IPD. There is blockage of the large articulation of the dual-mobility system with extensive fibrosis.
Table 1.
Radiologic and perioperative findings according the type of IPD
| Type of IPD (number of hips) | Radiologic considerations | Perioperative findings | Delay of occurrence (years ± SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cup loosening (number) | Ossification (number) | GTP | Cup inclination | Fibrosis | GTI | Ossification impingement | Metallosis | Homogeneous wear of PE | ||
| Type I N = 26 |
0 | B1 : 1 B2 : 3 B3 : 1 B4 : 1 |
0 | 47 ± 4.2 | 1 | 0 | 0 | 0 | 26 | 10.6 ± 3.9 |
| Type II N = 41 |
0 | B1 : 2 B2 : 6 B3 : 10 B4 : 4 |
5 | 45 ± 5.7 | 21 | 5 | 15 | 0 | 0 | 7.5 ± 3.5 |
| Type III N = 14 |
10 | B1 : 2 B2 : 2 B3 : 1 B4 : 0 |
1 | 46 ± 6.2 | 0 | 0 | 1 | 14 | 0 | 9.2 ± 4 .1 |
| p value (comparison between Type II and Type III) | 0.06 | 0.001 | ||||||||
| p value (comparison between Type I and Type III) | 0.07 | 0.002 | ||||||||
IPD = intraprosthetic dislocation; GTP = greater trochanter pseudarthrosis; GTI = greater trochanter impingement; PE = polyethylene; B = Brooker stage.
All patients were allowed to walk with crutches the day after surgery. No other physiotherapy except walking aids was provided during the hospital stay; patients either were discharged to their home or referred to a nursing home until they achieved independence. They were instructed to avoid high flexion activities combined with adduction of the involved hip for 6 weeks postoperatively, after which they were allowed to walk without crutches and resume their usual activities. Patients could return to employment 3 months after surgery.
Clinical and radiographic assessments were performed at 2 months, 6 months, 1 year, and every 2 years postoperatively. To supplement information from the medical records of the routine followups, one of us (BB) examined the following variables radiographically before revision surgery of the dislocated prostheses: cup inclination determined on AP radiographs as described by McArthur et al. and with intraobserver and interobserver reliabilities greater than 95% [15]; ectopic calcifications defined according to Brooker et al. [3]; and an interobserver Kappa of 0.43 as reported by Della Valle et al. [5]. Trochanteric nonunion was assessed radiographically before revision surgery; when migration was greater than 5 mm, the patient was classified as having a nonunion.
All explants were collected and analyzed by one of the authors (BB). Parts of the data already were reported [8].
Quantitative variables were expressed using means, SDs, median, and extreme values. Qualitative variables were expressed by means of percentages. We determined differences of timing between the three types of IPD with Kruskal-Wallis tests and between timing of the two types of stems with a Mann-Whitney U test. Statistical analysis was performed using the StatView 5.0 software (Abacus Concepts, Inc, Berkeley, CA, USA).
Results
Using preoperative radiographic analysis and perioperative findings (presence of arthrofibrosis, cup loosening, and type of liner wear) we established the following classification; IPD Type 1 was characterized by the absence of arthrofibrosis and cup loosening, Type 2 was defined as blockage of the liner owing to an extrinsic phenomena (arthrofibrosis, ectopic ossification), and Type 3 was associated with cup loosening. We observed no intraoperative evidence of reduced mobility of the larger articulation or metallosis in the joint in 26 patients. In these 26 patients, dual-mobility prostheses showed normal function and wear of the PE liner retentive rim appeared circular and homogeneous. Forty-one hips had complete loss or a major decrease in mobility of the larger articulation of the dual-mobility prosthesis as a result of extrinsic blocking. Obstruction was induced by ectopic calcifications in 15 hips, major fibrosis surrounding the acetabular cup and PE component in 21 hips, and impingement associated with trochanteric nonunion in five hips. In 14 hips, severe metallosis associated with a large number of intraarticular wear particles secondary to aseptic loosening of the acetabular cup could be observed. These intraoperative findings were used to classify IPD according to its etiologic mechanism of occurrence: 26 Type I, 41 Type II, and 14 Type III.
The mean time to IPD with PF® stems was 9 years (range, 4–17 years) and 8.5 years (range, 1–15 years) with PRO® stems. Regarding the mean time to IPD according to the stem design, two subgroups were made: the PF® stem subgroup (n = 31 hips) and the PRO® stem subgroup (n = 50 hips). We found no difference (p = 0.08) between the two subgroups. If we considered the relationship between the type of IPD and the time of occurrence of the event, we found differences according to the type of IPD: IPD Type 2 occurred earlier than Type 3 (p = 0.06) and IPD Type 3 occurred earlier than Type 1 (p = 0.07).
Discussion
Although using dual-mobility prostheses achieves maximum hip ROM it is associated with a risk of IPD. Many changes have been made to the PE liner, thus resulting in a decrease in the rate of IPD. IPD appears to have different etiologies and clinical presentations. Our purposes in this series were to classify the different types of IPD and to determine the factors influencing the timing of occurrence of IPD.
Although our series is relatively large, the study has some limitations. First, concerning the 1960 THAs, we did not have an exhaustive description of all clinical and radiographic parameters (design features of the acetabular and femoral components, morphologic features of the patients, postoperative implant positioning), which therefore did not allow us to compare the IPD group with the global population. Second, the perioperative findings were identified by different surgeons and would be prone to biases, therefore we focused on a few specific perioperative criteria to decrease these biases.
We identified three main types of IPD. Questions still remain regarding the presence of periprosthetic arthrofibrosis in patients with Type II IPD, including the chronologic order of periprosthetic arthrofibrosis occurrence relative to PE wear, and the individual factors sensitive to wear debris or implant materials that are responsible for fibrosis. The mobility of dual-mobility systems might have highlighted a phenomenon found in any type of arthroplasty but which remains asymptomatic because ankylosis is beneficial in simple mobility components regarding the dislocation risk. Types I and III IPD were more understandable; they corresponded to wear of the PE retentive rim, which appeared normal in Type I and accelerated in Type III IPD. Our classification may explain the lower incidence of IPD (0.3%) reported in one study [13], which is 10 times lower than the incidence reported in another series [17].
What were the most substantial concept changes that could account for this low rate of IPD? First, the PE liner design underwent several modifications between 1995 and 2000. We noted the increase in manufacturing tolerances of external and internal diameters, which were intended to reduce blockage of the PE liner under loading conditions. The design of the retentive rim also was modified. The PE liner was improved to provide homogeneous distribution of impaction and extraction forces between the various PE liner diameters available in the range of implants. All these improvements account for the decrease in Type 2 IPD. Second, the UHMWPE molecular density was modified from 4.5 to 7 mg/mol. According to one study [7], an increase in PE density is responsible for the reduced PE wear as long as such an increase is not associated with an increase in the free radicals, which are produced during the increase in PE reticulation. UHMWPE has been sterilized using a vacuum sterilization process since 2001, which decreases the rate of free radical reaction [7]. This accounts for the decrease in Type 1 IPD. Third, the bioactive treatment surface enhances secondary osseointegration thus reducing the long-term loosening rates [4]. There is a strong relationship between the reduction in implant loosening rate and the reduced incidence of Type III IPDs.
We found no difference in time of onset of IPD between PF® stems (large neck diameter, 316 L stainless steel) and PROFIL stems (smaller neck diameter manufactured from Ti6A14V). The lack of any difference between the IPD rate of the PF® and PROFIL stems might result from the negative effects produced by the large neck diameter of the PF® stem (frequent impingement) and those induced by the unpolished titanium surface treatment of the PROFIL stem (poor tribologic choice) that cancelled each other out. The change in the femoral implant (and more precisely the femoral neck) seems to have had an effect. Massin et al. reported [14] that all implanted stems featured a highly polished small-diameter neck, which might explain the low rate of IPD (0.3%). To date, no study has confirmed our hypotheses; however, the changes in liner design, quality of the PE, and femoral stem reduced the time of occurrence of IPD and incidence of IPD from a range of 2% to 3% to 0.3% to 1% [14, 17, 19, 20].
We found a low incidence of IPD. A thorough study of IPD explants may help identify more precisely the causes of this complication. Further studies are needed to define the process of IPD, because current dual-mobility prostheses are intended to achieve higher survival rates than previous generation prostheses as a result of the increase in life expectancy and improvements in implant anchorage. We advocate the use of dual-mobility prostheses in patients older than 60 years or at particular risk for dislocation. The enhancement of the tribologic properties of this implant, based on a thorough analysis of the mechanisms of failures, could expand the indications of this implant.
Acknowledgments
We thank the department of biostatistics (Saint Etienne University, Saint Etienne, France, E. Presle) for the statistical analysis.
Footnotes
One of the authors (RP) certifies that he has or may receive payments or benefits, during the study period, an amount of $10,000–$100,000, from Serf, Decines, France, a company related to this work. One of the authors (FF) certifies that he has or may receive payments or benefits, during the study period, an amount of $10,000–$100,000, from Serf, Decines, France, a company related to this work.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Adam P, Farizon F, Fessy MH. [Dual articulation retentive acetabular liners and wear: surface analysis of 40 retrieved polyethylene implants][in French] Rev Chir Orthop Reparatrice Appar Mot. 2005;91:627–636. doi: 10.1016/S0035-1040(05)84466-6. [DOI] [PubMed] [Google Scholar]
- 2.Boyer B, Philippot R, Geringer J, Farizon F. Primary total hip arthroplasty with dual mobility socket to prevent dislocation: a 22-year follow-up of 240 hips. Int Orthop. 2012;36:511–518. doi: 10.1007/s00264-011-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 4.Chatelet JC, Setiey L. [Long term bone behavior in total primary hip arthroplasty with a fully hydroxyapatite-coated femoral stem: a continuous series of 120 cases with twelve years follow-up][in French] Rev Chir Orthop Reparatrice Appar Mot. 2004;90:628–635. doi: 10.1016/S0035-1040(04)70723-0. [DOI] [PubMed] [Google Scholar]
- 5.Della Valle AG, Ruzo PS, Pavone V, Tolo E, Mintz DN, Salvati EA. Heterotopic ossification after total hip arthroplasty: a critical analysis of the Brooker classification and proposal of a simplified rating system. J Arthroplasty. 2002;17:870–875. [DOI] [PubMed]
- 6.Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility: a twelve-year follow-up study. Int Orthop. 1998;22:219–224. doi: 10.1007/s002640050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galvin A, Kang L, Tipper J, Stone M, Ingham E, Jin Z, Fisher J. Wear of crosslinked polyethylene under different tribological conditions. J Mater Sci Mater Med. 2006;17:235–243. doi: 10.1007/s10856-006-7309-z. [DOI] [PubMed] [Google Scholar]
- 8.Geringer J, Boyer B, Farizon F. Understanding the dual mobility concept for total hip arthroplasty: investigations on a multiscale analysis-highlighting the role of arthrofibrosis. Wear. 2011;271:2379–2385. doi: 10.1016/j.wear.2011.02.027. [DOI] [Google Scholar]
- 9.Hallan G, Lie SA, Furnes O, Engesaeter LB, Vollset SE, Havelin LI. Medium- and long-term performance of 11,516 uncemented primary femoral stems from the Norwegian arthroplasty register. J Bone Joint Surg Br. 2007;89:1574–1580. doi: 10.1302/0301-620X.89B12.18969. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH. Long-term results of the cementless porous coated anatomic total hip prosthesis. J Bone Joint Surg Br. 2005;87:623–627. doi: 10.1302/0301-620X.87B5.15554. [DOI] [PubMed] [Google Scholar]
- 11.Lautridou C, Lebel B, Burdin G, Vielpeau C. [Survival of the cementless Bousquet dual mobility cup: minimum 15-year follow-up of 437 total hip arthroplasties][in French] Rev Chir Orthop Reparatrice Appar Mot. 2008;94:731–739. doi: 10.1016/j.rco.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Lecuire F, Benareau I, Rubini J, Basso M. [Intra-prosthetic dislocation of the Bousquet dual mobility socket][in French] Rev Chir Orthop Reparatrice Appar Mot. 2004;90:249–255. doi: 10.1016/S0035-1040(04)70101-4. [DOI] [PubMed] [Google Scholar]
- 13.Leiber-Wackenheim F, Brunschweiler B, Ehlinger M, Gabrion A, Mertl P. Treatment of recurrent THR dislocation using of a cementless dual-mobility cup: a 59 cases series with a mean 8 years’ follow-up. Orthop Traumatol Surg Res. 2011;97:8–13. doi: 10.1016/j.otsr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop Relat Res. 2012;470:1932–1940. doi: 10.1007/s11999-011-2213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McArthur B, Cross M, Geatrakas C, Mayman D, Ghelman B. Measuring acetabular component version after THA: CT or plain radiograph? Clin Orthop Relat Res. 2012;470:2810–2818. doi: 10.1007/s11999-012-2292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammed R, Cnudde P. Severe metallosis owing to intraprosthetic dislocation in a failed dual-mobility cup primary total hip arthroplasty. J Arthroplasty. 2012;27:493.e1–3. [DOI] [PubMed]
- 17.Philippot R, Adam P, Farizon F, Fessy MH, Bousquet G. [Survival of cementless dual mobility sockets: ten-year follow-up][in French] Rev Chir Orthop Reparatrice Appar Mot. 2006;92:326–331. doi: 10.1016/S0035-1040(06)75762-2. [DOI] [PubMed] [Google Scholar]
- 18.Philippot R, Adam P, Reckhaus M, Delangle F, Verdot FX, Curvale G, Farizon F. Prevention of dislocation in total hip revision surgery using a dual mobility design. Orthop Traumatol Surg Res. 2009;95:407–413. doi: 10.1016/j.otsr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Philippot R, Camilleri JP, Boyer B, Adam P, Farizon F. The use of a dual-articulation acetabular cup system to prevent dislocation after primary total hip arthroplasty: analysis of 384 cases at a mean follow-up of 15 years. Int Orthop. 2009;33:927–932. doi: 10.1007/s00264-008-0589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philippot R, Farizon F, Camilleri JP, Boyer B, Derhi G, Bonnan J, Fessy MH, Lecuire F. [Survival of dual mobility socket with a mean 17 years follow-up][in French] Rev Chir Orthop Reparatrice Appar Mot. 2008;94:43–48. doi: 10.1016/j.rco.2007.10.011. [DOI] [PubMed] [Google Scholar]


