Abstract
Background
Many surgeons perform a varus femoral or Salter pelvic osteotomy in patients with Legg-Calvé-Perthes (LCP) disease. However, more severely deformed femoral heads show greater congruency in adduction rather than in abduction. Therefore, a valgus-(flexion) femoral osteotomy (VFO) seems preferable rather than a varus femoral or Salter pelvic osteotomy.
Questions/purposes
We evaluated whether the VFO improves (1) femoral head roundness, (2) radiographic parameters reflecting hip subluxation, and (3) function.
Methods
We treated 25 patients (25 hips; 18 lateral pillar C and seven B) in the late fragmentation stage by VFO. Seven patients had additional pelvic procedures. VFO was performed at a mean age of 9.8 years. Three hips were Stulberg Class II, 20 were Class III, and two were Class IV. The following components of femoral head roundness were calculated from preoperative MRI and final radiographs: lateral and medial head roundness (LHR and MHR); anterior and posterior head roundness (AHR and PHR); central head height; and the ratios MHR/LHR and PHR/AHR. Continuity of Shenton’s line, medial gap ratio were evaluated. Function was determined with the Iowa hip score. Minimum followup was 3.1 years (mean, 6.3 years; range, 3.1–11.2 years).
Results
All femoral head roundness measurements improved, with greatest improvement in the lateral and anterior head. Pillar C hips showed greater relative improvement than pillar B hips. The continuity of Shenton’s line improved and the mean medial gap ratio decreased. Mean Iowa hip score improved from 71 before surgery to 90 at the last followup.
Conclusions
VFO appears to help the deformed femoral head in the fragmentation stage to remodel to fit the acetabulum.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
It generally is believed containment surgery in Legg-Calvé-Perthes (LCP) disease can be performed safely as soon as the severity of the disease has been determined [13, 16, 17]. For various reasons it is not uncommon for the physician to see patients with advanced collapse of the femoral epiphysis even at the first visit. An MRI study [18] found that 25% of pillar B hips [12, 14] and 62% of pillar C hips showed greatest congruency in adduction and incongruency in abduction. Even when the hip shows no improvement of congruency in abduction, many surgeons will use one of several surgical alternatives to contain the hip and achieve subsequent remodeling: a varus femoral osteotomy [1, 9–11, 15, 23, 29, 35], a Salter pelvic osteotomy [21, 25, 36], or a triple [22, 37] pelvic osteotomy. The question arises whether containment surgery can create a congruent hip in all patients. If the remodeling potential of the femoral head is insufficient, for example in the older (≥ 8 years) or severely affected patient (ie, Catterall Group III or IV [4], lateral pillar group B/C border or C [12]), then a satisfactory outcome (Stulberg Class I or II [12]) should not be expected. These patients commonly show worsening fragmentation of the ossific nucleus and an ovoid, flattened, or bilobed femoral head at the healing stage [19, 20] because containment surgery does not halt the ongoing pathologic process. A different approach is needed for such patients.
Valgus femoral osteotomy (VFO) reportedly is useful in the treatment of the nonspherical femoral head in adolescent and adult hips [5, 6, 26, 28, 33], but it has not been widely used in patients with LCP disease. Some studies suggest VFO is indicated when there is hinge abduction [7, 27, 30–32, 38] in severe LCP disease as a salvage operation. However, it is not clear at what amount of hinge abduction the VFO should be considered. A multicenter study [13] concluded containment of the severely affected (lateral pillar C) femoral head into the acetabulum, either by brace or surgery, is apparently of limited value at any age. However, VFO was not used in that study. We have found improved hip congruency with adduction, using imaging studies of the deformed femoral head in patients with severe LCP disease [18]. This suggests a VFO might achieve better congruency than the alternatives.
We therefore addressed the following questions: Does VFO (1) improve femoral head roundness for patients with LCP disease in the fragmentation or early reossification stage who show the greatest hip congruency in adduction; (2) improve Shenton’s line and medial gap ratio which may have been disrupted and/or increased before surgery, and worsened immediately after VFO; and (3) improve function?
Patients and Methods
We retrospectively reviewed 103 patients (106 hips) with LCP disease who were treated by us between 1998 and 2007. All of these patients had at least one MRI study at the maximal fragmentation stage; a total of 151 MRI sets were performed. During that time we treated a total of 40 patients with VFO, and 25 patients with healed femoral heads were selected for the study; the other 15 patients did not show complete healing of the femoral head. The indications for VFO were: (1) ROM MRI and intraoperative arthrography showing an ovoid to flattened femoral head; (2) in abduction, an increased medial joint space and hinge abduction with the labral direction horizontal or upward and/or distorted; and (3) decreased medial joint space and greatest congruency in adduction. Contraindicated were hips with best congruency in abduction or which showed similar congruency in both positions, and which could be treated with abduction ROM exercise or brace, or by a traditional femoral varus, Salter, or triple pelvis osteotomy. Another contraindication was an irreducible stiff hip. Our inclusion criteria for patients in this study were: (1) hips classified as lateral pillar B or C treated with VFO at the late fragmentation or early reossification stage, with or without a disrupted Shenton’s line and an increased medial joint gap observed on the radiograph; (2) preoperative MRI before VFO and arthrography during the operation to evaluate the femoral head and its relationship with the acetabulum at five different positions of the hip (neutral, abduction, abduction-internal rotation, abduction-internal rotation-flexion, and adduction); and (3) at or near skeletal maturity. From the total of 40 patients with VFOs, 25 met our inclusion criteria. Three of these patients had bilateral involvement with one hip becoming involved first but treated nonoperatively at the time of VFO on the other side. Twenty-three of the 25 patients were treated with VFO in the late fragmentation stage, and two in the early reossification stage. Eighteen patients underwent VFO alone, six underwent VFO plus Chiari pelvic osteotomy, and one had VFO plus shelf acetabuloplasty. Eighteen patients had lateral pillar C involvement and seven had lateral pillar B (16 Catterall Group IV and nine Catterall Group III). Twenty-two patients were male and three were female. The mean age of the patients at their first visit to our clinic was 8.2 years (range, 4.7–13.8 years). VFO was performed at a mean age of 9.8 years (range, 6.8–13.8 years). None of the 25 patients was lost to followup. Minimum followup after VFO for all patients was 3.1 years (mean, 6.3 years; range, 3.1–11.2 years), and the mean age at the last followup (by which time all femoral heads had healed) was 16.1 years (range, 11.3–20.8 years). Twelve of the 25 patients were past skeletal maturity (older than 16 years) at last followup. No patients were recalled specifically for this study; all data were obtained from medical records and imaging.
ROM MRI [18] was performed before surgery and deformity of the cartilaginous femoral head and the presence of hinge abduction were noted. Each ROM MRI consisted of a set of scans of the hip in neutral, abduction, abduction-internal rotation, abduction-internal rotation-flexion, and adduction. Arthrography was performed in the operating room before VFO and the images were compared with ROM MR images of the same positions. Correction was planned in the coronal and sagittal planes (Fig. 1).
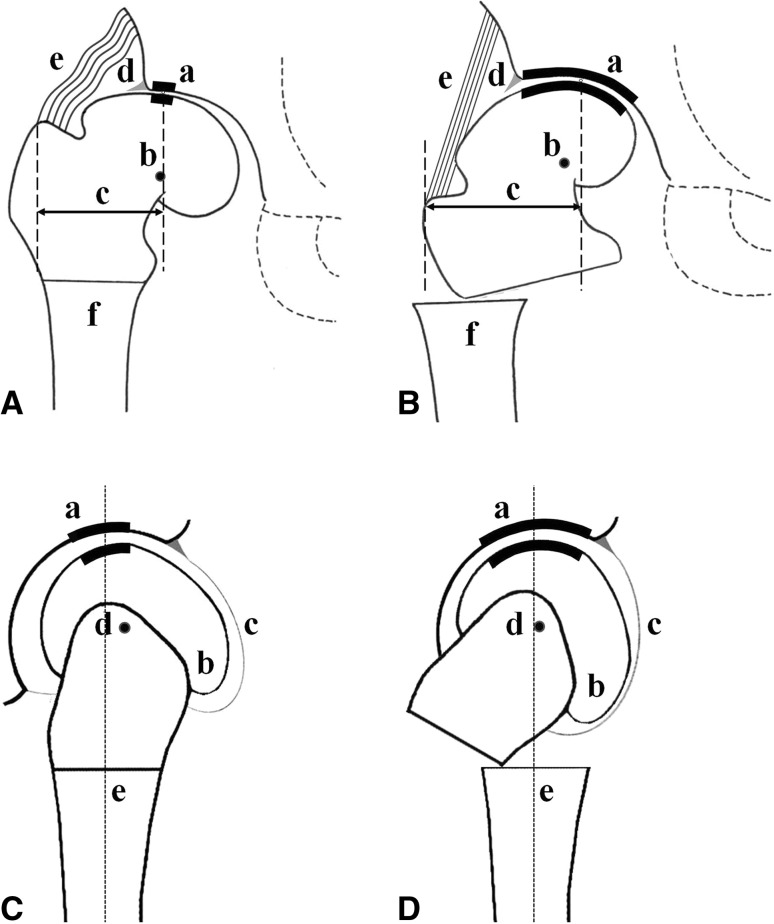
Fig. 1A–D.
The schematic drawings illustrate the effect of a VFO. (A) Before VFO, the hip shows subluxation with joint incongruity and a decreased weightbearing surface, and overload in the region of the acetabular corner in the AP plane. (B) The VFO aims to increase joint congruity (a) and the weightbearing surface; medialize the center of rotation (b); lengthen the lateral lever arm of the abductors (c); reduce overload in the region of the acetabular corner (d); and increase tension of the abductors (e). Lateral displacement of the distal femur in the coronal plane is required to maintain a normal relationship with the proximal femur (f). (C) In the lateral plane, before VFO, the hip shows a decreased weightbearing surface and an anteriorly protruding portion of the femoral head. (D) The flexion osteotomy increases the congruous weightbearing surface and decreases shear (a); moves the anteriorly protruding portion of the femoral head away from the acetabular rim (b); tightens the anterior portion of the capsule (c); and shifts the center of rotation posteriorly (d). Anterior displacement of the distal femur in the sagittal plane is required to maintain a normal relationship with the proximal femur (e).
Soft tissue release (psoas or adductor tenotomy) was performed when abduction was limited by soft tissue contracture, but not by a cartilaginous or bony hinge between the femoral head and the acetabulum in the arthrogram. The VFO was an open-wedge type using a blade plate angle of 130°. To minimize the resultant deformity during plate fixation, we translated the distal femur laterally (and anteriorly when a flexion component was included) using a no-wedge technique (without resection of the triangular fragment), which allowed us to maintain the original mechanical axis. The mean valgus correction was 18.0° (range, 15°–20°) and the mean flexion component was 14.0° (range, 10°–20°). Pelvic procedures (Chiari osteotomy or shelf acetabuloplasty) were indicated when arthrography showed decreased femoral head coverage and subluxation after the VFO. All surgeries included greater trochanteric apophysiodesis by simple drilling and curettage to prevent trochanteric overgrowth.
As pain subsided the patients were permitted to sit and to do nonweightbearing walking using crutches from 1 week after surgery. Partial weightbearing walking using a crutch was permitted from 6 weeks when a callus was identified at the osteotomy site. Progressive ROM exercise also was recommended. After bony union, full weightbearing walking was permitted when the patients showed comfortable leg-raising in the supine position and the hip ROM was approximately equal to the preoperative range. When the patients were allowed full weightbearing walking the senior author (HTK) recorded pain and activities of daily living (such as the distance they could walk, discomfort when sitting, climbing stairs, and putting on shoes and socks). They were evaluated at every visit based on the Iowa hip score [24], including hip motion, deformity, limping.
Complications after VFO, according to the classification by Dindo et al. [8], were as follows: three patients had Grade I complications (two had ileus and one had fever) that did not need pharmacologic or surgical treatment, and 17 had Grade II complications (16 had lowered hemoglobin level and one had hepatic dysfunction) which required blood transfusions and medication. No other complications occurred.
Three of us (H.T.K, J.H.J, S.H.B) evaluated each hip twice according to a modified lateral pillar classification [12] and twice according to a modified classification of Stulberg outcomes [12]. In each case the first and second viewings were 2 weeks apart. For the lateral pillar classification, the three observers’ mean weighted Kappa value for intraobserver reliability was 0.857. Pairwise comparisons of the three observers (interobserver reliability) gave a mean weighted Kappa value of 0.816. For Stulberg classes, the intraobserver weighted Kappa values averaged 0.820, while interobserver weighted Kappa values averaged 0.825.
Medial gap ratio [34] and breakage in Shenton’s line were recorded before and after surgery from AP radiographs. We measured femoral head roundness on preoperative coronal and sagittal MR images and on the final AP and frog-leg lateral radiographs (Fig. 2). We used Adobe® Photoshop® (Photoshop CS2; Adobe system version, San Jose, CA, USA) and Microsoft PowerPoint® (Microsoft, Redmond, WA, USA). We placed the clearest MR and radiograph images (in the JPG file) side by side on the same Power Point image and made their sizes equal using the magnification tool. Best-fitting circles for the lateral and medial third of the femoral head was drawn on both images. The radius of each circle (r and R) was defined as the lateral head roundness (LHR) and the medial head roundness (MHR). In the middle third of the femoral head, the central head height (CHH) was the distance (m and M) from the midpoint of the baseline to the uppermost point of the femoral head. The method for calculating roundness components (posterior, anterior, and central head height) on sagittal MR images and frog-leg lateral radiographs was the same as that used on AP images. The relative changes of LHR and MHR were calculated as (r-R)/R × 100 (%), where r = the radius of the best-fitting circle on the MR image and R = the corresponding radius on the radiograph. The relative change of CHH was (m-M)/M × 100 (%). The amount of collapse in the lateral portion of the deformed femoral head was quantified by comparing the radius of its circle with that of the medial circle as the ratio MHR/LHR. A higher MHR/LHR ratio meant a rounder femoral head.
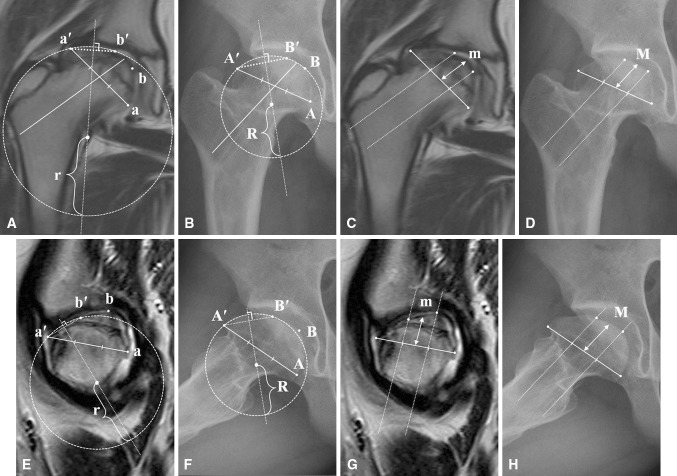
Fig. 2A–H.
Our method of measuring femoral head roundness is shown. (A) The most superolateral and inferomedial points of the femoral head (a’ and a) in the coronal MR image were located where a line parallel to the femoral neck axis would be tangential to the femoral head. A base line was created by connecting these two points and divided into thirds. We then drew lines parallel to the femoral neck axis which ran through the 1/3 and 2/3 points on the baseline, and drew the best-fitting circles for the lateral third of the femoral head passing through points a′ and b′ (the point where the upper line parallel to the femoral neck axis meets the articular cartilage of the femoral head). The same approach was used for the medial third of the femoral head (not shown here). The length of the radius (r) for the best-fitting circle of the lateral third is termed the lateral head roundness (LHR), and the corresponding radius for the medial circle is the medial head roundness (MHR). (B) The same measurement methods were used to measure the radius (R) of the lateral third on the AP radiograph (using A and A’, and B and B’). (C) In the middle third, since any circle approximating it would be very large and subject to error, we defined central head height (CHH) as the height from the baseline, measured from its midpoint (m). (D) The same measurement method was used to measure CHH on the radiograph (M). (E) On the sagittal MR image, the anterior head roundness (AHR) and posterior head roundness (PHR) were measured using the same methods that were used in the coronal MR image. (F) On the lateral radiograph, AHR and PHR also were measured with the same methods that were used for the AP radiograph. (G) The CHH on the sagittal MR image was measured with the same method that was used for the coronal MR image. (H) The CHH on the lateral radiograph was measured with the same method that was used for the AP radiograph.
Results
Femoral head roundness parameters improved after VFO. The regions of the femoral head that were most improved by VFO included the lateral 1/3 in the AP plane (p = 0.001) and the anterior 1/3 in the lateral plane (p = 0.001) (Table 1); in both cases they become less flattened, ie, their radius of curvature is decreased. The medial 1/3 in the AP plane and posterior 1/3 in the lateral plane were not changed, consequently the ratios MHR/LHR (in the AP plane) (p = 0.003) and PHR/AHR (in the lateral plane) (p = 0.019) were increased after VFO.
Table 1.
Improvement in femoral head roundness parameters after VFO
| Plane | Roundness component | Before VFO (mm) (range) | Latest followup (mm) (range) | p* |
|---|---|---|---|---|
| AP (n = 25) | LHR | 48.0 (30–72) | 36.5 (27–47) | 0.001 |
| MHR | 28.3 (21–35) | 27.3 (22–33) | 0.105 | |
| CHH | 24.4 (17–29) | 26.0 (18––35) | 0.037 | |
| MHR/LHR | 0.65 (0.32–1.10) | 0.81 (0.49–0.97) | 0.003 | |
| Lateral (n = 14) | AHR | 36.6 (25–56) | 32.9 (22–50) | 0.001 |
| PHR | 20.6 (15–30) | 19.6 (14–26) | 0.137 | |
| CHH | 19.1 (15–26) | 19.9 (16–25) | 0.059 | |
| PHR/AHR | 0.61 (0.43–0.85) | 0.75 (0.43–0.85) | 0.019 |
LHR = lateral head roundness; MRH = medial head roundness; CHH = central head height; AHR = anterior head roundness; PHR = posterior head roundness; * Wilcoxon signed-rank test.
The continuity of Shenton’s line, which was disrupted before surgery and/or worsened after VFO, improved in 14 patients (56%) as observed on the final radiographs. The mean medial gap ratio decreased from 1.66 preoperatively (1.60 after surgery) to 1.45 on the final radiographs. When considered by lateral pillar group (Table 2), in the AP plane we see that patients with pillar B involvement had higher initial and final values of the MHR/LHR ratio, but patients with pillar C involvement showed a greater change. This also was true in the lateral plane. In terms of percent change, the overall results in each plane follow the same trend as for absolute change. In the AP and lateral planes, the greatest relative change was found in LHR and AHR after VFO (Table 3).
Table 2.
Improvement of MHR/LHR and PHR/AHR ratios by lateral pillar group
| Roundness component and pillar group | Mean ratio | |||
|---|---|---|---|---|
| Before VFO (range) | Latest followup (range) | Improvement | p* | |
| MHR/LHR AP plane (n = 25) |
||||
| Pillar B (n = 7) | 0.76 (0.63–0.97) | 0.88 (0.71–0.97) | 0.12 | 0.016 |
| Pillar C (n = 18) | 0.59 (0.32–1.1) | 0.78 (0.48–0.97) | 0.19 | 0.009 |
| PHR/AHR Lateral plane (n = 14) |
||||
| Pillar B (n = 2) | 0.68 (0.51–0.85) | 0.79 (0.55–0.85) | 0.11 | 0.655 |
| Pillar C (n = 12) | 0.57 (0.37–0.68) | 0.73 (0.42–0.76) | 0.16 | 0.016 |
MHR = medial head roundness; LHR = lateral head roundness; PHR = posterior head roundness; AHR = anterior head roundness; * Wilcoxon signed-rank test.
Table 3.
Relative change of femoral head roundness parameters after VFO
| Plane | Relative change | ||
|---|---|---|---|
| AP (n = 25) | LHR (%) | MHR (%) | CHH (%) |
| Mean (Range) | 21.4 (0.1–56.5) | 2.7 (−14.8 to 14.3) | 7.8 (−18.2 to 52.9) |
| Lateral (n = 14) | AHR (%) | PHR (%) | CHH (%) |
| Mean (Range) | 10.6 (3.7–19.4) | 3.4 (−6.3 to 16.7) | 6.3 (−0.5 to 17.6) |
LHR = lateral head roundness; MHR = medial head roundness; CHH = central head height; AHR = anterior head roundness; PHR = posterior head roundness.
At the latest followup, 10 patients said they experienced limitation of motion of the hip during everyday life. Hip pain was not evident in any of the patients but three reported discomfort after long-distance walking or hiking. Limping occurred in 10 patients based on the patients’ statements and their parents’ observations. The mean Iowa hip score improved from 71 before VFO to 90 at the latest followup.
Discussion
With LCP disease, it is not uncommon to see poor results (Stulberg Class III or IV) in severely affected hips (Catterall III or IV, lateral pillar B/C border or C) and/or in older patients (≥ 8 years) after surgery aimed at containment of the affected femoral head in the acetabulum. For these patients a different approach from the conventional treatment is required. In this study we used VFO as our main surgical approach for patients showing best congruency in adduction. We evaluated whether the VFO (1) improves femoral head roundness and determined regions that were most improved; (2) improves Shenton’s line and medial gap ratio; and (3) improves function.
Our study does have some limitations. First, some patients had a pelvic osteotomy along with the VFO. We believed this was important to remedy the acetabular (head coverage) deficiency in seven patients, so that they could be compared with the other 18 patients on the basis of VFO and femoral head roundness alone. Second, we were able to examine only sagittal MR images for 14 of our 25 patients. This is because the (neutral) MR and (frog) radiographic images were too different to be compared for our other patients. Third, we are assuming that when femoral head roundness is improved, then so is congruency. In actual practice, congruency in the various hip positions is easy to judge qualitatively, but for quantitative, statistically testable results, roundness is easier to measure and analyze. Thus, we focused on the various components of femoral head roundness in our analysis. Fourth, not all patients were observed to skeletal maturity. However, we were able to see and measure the femoral head roundness in all patients.
Our observations suggest femoral head roundness is improved by VFO, especially the lateral and anterior parts of the femoral head. We believe improved congruency and the increase in the weightbearing surface are the main reasons for the improvement in femoral head roundness after VFO in the late fragmentation stage, even in severely affected (Catterall III or IV, lateral pillar B/C border or C), relatively older (≥ 8 years) patients with LCP disease. By rotating the ovoid head into a valgus position, the flattened lateral part of the head moves downward and relieves the pressure in the region of the acetabular corner, and the labrum assumes a more normal downward direction. A flexion component of the osteotomy may be desirable because it establishes a more vertical compressive force between the posteromedial true femoral head and the acetabulum, thus reducing shear [20]. Apparently the valgus and flexion components of the VFO bring more of the uninvolved, rounder parts of the femoral head into contact with the acetabulum, where they help support it; thus the diseased part of the femoral head is more able to remodel to fit the acetabulum [27, 38].
Regarding the improvement in Shenton’s line and of the medial joint gap ratio, we believe reduced hinge abduction between the labrum (the acetabular margin) and the femoral head and the increased tension of the abductor muscles after VFO reduce the risk of its slipping out of the acetabulum and facilitate reduction. In addition, the increased abductor tension facilitates remodeling of the femoral head by displacing its center of rotation medially, and by favorably altering the lever arm of the hip. We contemplated Chiari osteotomy when femoral head subluxation appeared substantial on preoperative radiographs and on MR images in adduction, and when the head coverage in adduction (the position of best congruency) was approximately 70% on the arthrogram; and we performed the Chiari osteotomy when the same findings were identified after VFO. Therefore it should be helpful when combined with the VFO because some patients may have an unstable hip with subluxation after VFO. The Chiari osteotomy often is effective in improving articular congruency and even femoral head roundness [3].
Several studies [2, 10, 32] have used VFO with varying ranges of outcomes reported for Stulberg Classes II (range, 11.4%–29%), III (range, 33%–62.9%), IV (range, 24%–27%), and V (range, 14%–26%). The Iowa hip score in these studies at the last followup ranged from 86 to 95.2. Our results are similar, with a mean Iowa hip score of 91 at the last followup, but with more Stulberg Class III (80%), fewer Class IV (8%), and no Class V outcomes. We believe favorable remodeling of the hip is promoted when VFO is performed before there is permanent deformity of the femoral head.
Severely affected patients with LCP disease who undergo VFO, rather than a conventional varus femoral osteotomy or Salter’s or triple pelvic osteotomy, show clear improvement in femoral head roundness, particularly in the lateral and anterior areas of the femoral head. Our patients also showed improvement in continuity of Shenton’s line and decreased medial joint space, and definite improvement in Iowa hip scores.
Footnotes
One or more of the authors (HTK) has received funding from Pusan National University Hospital.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution has approved the human use protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for clinical participation in the study was obtained.
This work was performed at the Pediatric Orthopedics, Pusan National University Hospital, Pusan, Korea.
References
- 1.Axer A, Gershuni DH, Hendel D, Mirovski Y. Indications for femoral osteotomy in Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 1980;150:78–87. [PubMed] [Google Scholar]
- 2.Bankes MJ, Catterall A, Hashemi-Nejad A. Valgus extension osteotomy for “hinge abduction” in Perthes’ disease: results at maturity and factors influencing the radiological outcome. J Bone Joint Surg Br. 2000;82:548–554. doi: 10.1302/0301-620X.82B4.10339. [DOI] [PubMed] [Google Scholar]
- 3.Bennett JT, Mazurek RT, Cash JD. Chiari’s osteotomy in the treatment of Perthes’ disease. J Bone Joint Surg Br. 1991;73:225–228. doi: 10.1302/0301-620X.73B2.2005144. [DOI] [PubMed] [Google Scholar]
- 4.Catterall A. The natural history of Perthes’ disease. J Bone Joint Surg Br. 1971;53:37–53. [PubMed] [Google Scholar]
- 5.Catterall A. Adolescent hip pain after Perthes’ disease. Clin Orthop Relat Res. 1986;209:65–69. [PubMed] [Google Scholar]
- 6.Clohisy JC, Schoenecker PL. Proximal femoral osteotomy. In: Callaghan JJ, Rosenberg AG, Rubash HE, editors. The Adult Hip. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2007. pp. 781–794. [Google Scholar]
- 7.Choi IH, Yoo WJ, Cho TJ, Moon HJ. The role of valgus osteotomy in LCPD. J Pediatr Orthop. 2011;31(2 suppl):S217–S222. doi: 10.1097/BPO.0b013e318223b404. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedlander JK, Weiner DS. Radiographic results of proximal femoral varus osteotomy in Legg-Calvé-Perthes disease. J Pediatr Orthop. 2000;20:566–571. doi: 10.1097/01241398-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Heikkinen E, Puranen J. Evaluation of femoral osteotomy in the treatment of Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 1980;150:60–68. [PubMed] [Google Scholar]
- 11.Herceg MB, Cutright MT, Weiner DS. Remodeling of the proximal femur after upper femoral varus osteotomy for the treatment of Legg-Calvé-Perthes disease. J Pediatr Orthop. 2004;24:654–657. doi: 10.1097/01241398-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part I: classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am. 2004;86:2103–2120. [PubMed] [Google Scholar]
- 13.Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86:2121–2134. [PubMed] [Google Scholar]
- 14.Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pediatr Orthop. 1992;12:143–150. doi: 10.1097/01241398-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Hoikka V, Poussa M, Yrjönen T, Osterman K. Intertrochanteric varus osteotomy for Perthes’ disease: radiographic changes after 2–16-year follow-up of 126 hips. Acta Orthop Scand. 1991;62:549–553. doi: 10.3109/17453679108994494. [DOI] [PubMed] [Google Scholar]
- 16.Joseph B, Nair NS, Narasimha Rao KL, Mulpuri K, Varghese G. Optimal timing for containment surgery for Perthes disease. J Pediatr Orthop. 2003;23:601–606. doi: 10.1097/01241398-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Joseph B, Varghese G, Mulpuri K, Narasimha Rao KL, Nair NS. Natural evolution of Perthes disease: a study of 610 children under 12 years of age at disease onset. J Pediatr Orthop. 2003;23:590–600. doi: 10.1097/01241398-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kim HT, Oh MH, Lee JS. MR imaging as a supplement to traditional decision-making in the treatment of LCP disease. J Pediatr Orthop. 2011;31:246–253. doi: 10.1097/BPO.0b013e31820fc63c. [DOI] [PubMed] [Google Scholar]
- 19.Kim HT, Wenger DR. “Functional retroversion” of the femoral head in Legg-Calvé-Perthes disease and epiphyseal dysplasia: analysis of head-neck deformity and its effect on limb position using three-dimensional computed tomography. J Pediatr Orthop. 1997;17:240–246. doi: 10.1097/00004694-199703000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Kim HT, Wenger DR. Surgical correction of “functional retroversion” and “functional coxa vara” in late Legg-Calvé-Perthes disease and epiphyseal dysplasia: correction of deformity defined by new imaging modalities. J Pediatr Orthop. 1997;17:247–254. doi: 10.1097/00004694-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Kitakoji T, Hattori T, Kitoh H, Katoh M, Ishiguro N. Which is a better method for Perthes’ disease: femoral varus or Salter osteotomy? Clin Orthop Relat Res. 2005;430:163–170. doi: 10.1097/01.blo.0000137549.60694.63. [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Bache CE, O’Hara JN. Interlocking triple pelvic osteotomy in severe Legg-Calvé-Perthes disease. J Pediatr Orthop. 2002;22:464–470. [PubMed] [Google Scholar]
- 23.Lack W, Feldner-Busztin H, Ritschl P, Ramach W. The results of surgical treatment for Perthes’ disease. J Pediatr Orthop. 1989;9:197–204. doi: 10.1097/01241398-198903000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Larson CB. Rating scale for hip disabilities. Clin Orthop Relat Res. 1963;31:85–93. doi: 10.1097/00003086-196300310-00011. [DOI] [PubMed] [Google Scholar]
- 25.Moberg A, Hansson G, Kaniklides C. Results after femoral and innominate osteotomy in Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 1997;334:257–264. doi: 10.1097/00003086-199701000-00033. [DOI] [PubMed] [Google Scholar]
- 26.Müller ME. Intertrochanteric osteotomy: indication, preoperative planning, technique. In: Schatzker J, ed. The Intertrochanteric Osteotomy. Berlin, Germany: Springer-Verlag; 1984:25–66.
- 27.Myers GJ, Mathur K, O’Hara J. Valgus osteotomy: a solution for late presentation of hinge abduction in Legg-Calvé-Perthes disease. J Pediatr Orthop. 2008;28:169–172. doi: 10.1097/BPO.0b013e3181653b13. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S, Ninomiya S, Morimoto S, Moro T, Takatori Y. Combined intertrochanteric valgus and rotational acetabular osteotomy. Clin Orthop Relat Res. 2001;384:176–188. doi: 10.1097/00003086-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Noonan KJ, Price CT, Kupiszewski SJ, Pyevich M. Results of femoral varus osteotomy in children older than 9 years of age with Perthes disease. J Pediatr Orthop. 2001;21:198–204. [PubMed] [Google Scholar]
- 30.Patil S, Sherlock D. Valgus osteotomy for hinge abduction in avascular necrosis. J Pediatr Orthop B. 2006;15:262–266. doi: 10.1097/01202412-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Quain S, Catterall A. Hinge abduction of the hip: diagnosis and treatment. J Bone Joint Surg Br. 1986;68:61–64. doi: 10.1302/0301-620X.68B1.3941142. [DOI] [PubMed] [Google Scholar]
- 32.Raney EM, Grogan DP, Hurley ME, Ogden MJ. The role of proximal femoral valgus osteotomy in Legg-Calvé-Perthes disease. Orthopedics. 2002;25:513–517. doi: 10.3928/0147-7447-20020501-18. [DOI] [PubMed] [Google Scholar]
- 33.Schneider R. Intertrochanteric osteotomy in osteoarthritis of the hip joint. In: Schatzker J, editor. The Intertrochanteric Osteotomy. Berlin, Germany: Springer-Verlag; 1984. pp. 135–168. [Google Scholar]
- 34.Song HR, Lee SH, Na JB, Tymowsi GI, Cho SH, Koo KH. Relationship between lateral subluxation and widening of medial joint space in Legg-Calvé-Perthes disease. J Pediatr Orthop. 1998;18:637–642. doi: 10.1097/00004694-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Than P, Halmai V, Shaikh S, Kranicz J, Bellyei A. Long-term results of derotational femoral varus osteotomy in Legg-Calvé-Perthes disease: 26-year follow-up. Orthopedics. 2003;26:487–491. doi: 10.3928/0147-7447-20030501-13. [DOI] [PubMed] [Google Scholar]
- 36.Vukasinovic Z, Slavkovic S, Milickovic S, Siqeca A. Combined Salter innominate osteotomy with femoral shortening versus other methods of treatment for Legg-Calvé-Perthes disease. J Pediatr Orthop B. 2000;9:28–33. doi: 10.1097/01202412-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Wenger DR, Pring ME, Hosalkar HS, Caltoum CB, Lalonde FD, Bastrom TP. Advanced containment methods for Legg-Calvé-Perthes disease: results of triple pelvic osteotomy. J Pediatr Orthop. 2010;30:749–757. doi: 10.1097/BPO.0b013e3181f5a0de. [DOI] [PubMed] [Google Scholar]
- 38.Yoo WJ, Choi IH, Chung CY, Cho TJ, Kim HY. Valgus femoral osteotomy for hinge abduction in Perthes’ disease: decision-making and outcomes. J Bone Joint Surg Br. 2004;86:726–730. doi: 10.1302/0301-620X.86B5.13897. [DOI] [PubMed] [Google Scholar]