Abstract
Chronic inflammation is one of the primary causes of colorectal cancer (CRC), and major inflammatory pathways implicated in CRC are COX2 and iNOS; both regulated by NF-κB suggesting that inhibitors of these pathways could be ideal against CRC. Silibinin has shown promising efficacy against various malignancies including CRC, and therefore here we assessed whether silibinin targets NF-κB activation and associated signaling as a mechanism of its anti-inflammatory and anti-cancer effects in CRC. Our results indicated that silibinin treatment (50–200 μM) of human CRC SW40, LoVo and HT29 cells strongly inhibits TNFα-induced NF-κB activation together with decreased nuclear levels of both p65 and p50 sub-units. Silibinin also significantly increased IκBα level with a concomitant decrease in phospho-IκBα, without any effect on TNFR1, TRADD and RIP2, indicating its inhibitory effect on IKKα kinase activity. Next we assessed the effect of oral silibinin feeding on NF-κB pathway in SW480 (COX-2 negative) and LoVo (COX-2 positive) tumor xenografts in nude mice. Together with its inhibitory efficacy on tumor growth and progression, silibinin inhibited NF-κB activation in both xenografts. The protein levels of various NF-κB-regulated molecules such as Bcl-2, COX2, iNOS, VEGF and MMPs were also decreased by silibinin in both cell culture studies and xenograft analyses, suggesting its potential to alter NF-κB transcriptional activity. Together, these findings are highly significant in establishing for the first time that silibinin suppresses CRC growth and progression possibly through its anti-inflammatory activity by interfering with NF-κB activation and thus has potential against human CRC.
Keywords: NF-κB signaling, colorectal cancer, chemoprevention, silibinin
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths in the United States [1] and statistical estimates by the American Cancer society for the year 2010 indicated that there would have been 142,570 new cases of CRC and 51,370 associated deaths in the United States alone [1]. Chronic inflammation is one of the primary causes of CRC owing to increased CRC riskwith longer duration of inflammatory bowel diseases (IBD: ulcerative colitis and crohn’s disease) and other inflammatoryresponses [2,3]. Anti-inflammatory drugs are, thus, the drugs of choice to prevent CRC [4,5]; however, there are several side effects associated with their long-term use suggesting that more efforts are needed to identify non-toxic agents, possibly from dietary/non-dietary sources, that can be used to prevent/intervene CRC [4–6]. In terms of scientific quintessentiality, CRC also represents a paradigm for the connection between inflammation and cancer, based on the i) epidemiological studies which indicate a higher incidence of CRC in patients with IBD, ii) protective function of nonsteroidal anti-inflammatory drugs (NSAIDs) against CRC, and iii) preclinical mechanistic studies which indicate a causal association of CRC with genes encoding for pro-inflammatory mediators [3,7,8].
Physiological/pathological conditions recognized as predisposing to CRC (IBD) or genetic events leading to neoplastic transformation maneuver the construction of an inflammatory microenvironment which is infiltrated with inflammatory cells and mediators [7–10]. The inflammatory mediators that have been implicated in the development of CRC include key transcription factors (e.g., NF-κB, STAT3); proinflammatory cytokines (e.g., TNF α, IL-6); cyclooxygenase-2 (COX-2) and selective CC-chemokines (CCl2) [2,3,9,11–16]. The negative mediators that keep this inflammation in check being IL-10, TGFβ, toll like receptor and the IL-1 receptor inhibitor TIR8/SIGIRR, and chemokine decoy and scavenger receptor D6 [3,9]. NF-κB is an inflammation-associated transcription factor, known to activate a wide variety of anti-apoptotic/pro-survival/inflammatory genes in response to viral and bacterial infections, inflammation, and stressful situations requiring a rapid reprogramming of gene expression [14,15,17–19]. NF-κB is activated constitutively in many tumors/tumor cells, including human CRC cells [3,8,19]. This is one of the major reasons why the chemotherapeutic agents are ineffective in inducing apoptosis in cancer cells including CRC [8,15]. Constitutively active NF-κB provides growth and survival signals in human malignancies, suggesting that the agents that inhibit NF-κB activation could be effective in CRC prevention and therapy [8,11]. NF-κB transcription complexes are comprised of homo- and heterodimers formed by five different subunits viz., p50, p52, p65 (RelA), RelB, and c-Rel subunits; the most common form being the p50 and p65 heterodimer[14,17,20]. This complex is sequestered in the cytoplasm (inactive state), where it is bound to a group of inhibitory proteins known as inhibitors of NF-κB (IκBs) which in turn are regulated through phosphorylation by the IκB kinase (IKK) complex, targeting it for ubiquitin dependent degradation [3,11]. In response to a variety of stimuli, such as the cytokine TNFα, the IκB proteins are phosphorylated by the IKK, ubiquitinated, and then undergo subsequent proteasomal degradation [3,11]. Degradation of IκB releases NF-κB, which then translocates to the nucleus. The resulting nuclear translocation of the active NF-κB leads to transcription, translation, and expression of a large number of NF-κB-dependent genes, which interestingly, also include the genes encoding for TNFα [11,12]
Since in patients with IBD, the risk of CRC generation is higher than in general population [3], we chose to assess the effect of silibinin, a non-toxic polyphenolic flavonolignan isolated from the seeds of milk thistle (Silybum marianum) with known cancer preventive efficacy against a variety of epithelial cancers including CRC, on inflammatory mechanisms that are associated with CRC [21–36]. Through detailed in vitro studies and employing tissues from in vivo studies, we assessed the effect of silibinin on both constitutive as well as TNFα-induced NF-κB activation in human CRC cells together with upstream/downstream effectors in this pathway. Our results suggest that silibinin inhibits CRC growth and progression by targeting the inflammatory NF-κB pathway.
MATERIALS AND METHODS
Cell Line and Reagents
SW480, HT-29 and LoVo human CRC cell lines were obtained from the AmericanType Culture Collection (Manassas, VA). Media and other cell culture materialswere from Invitrogen (Carlsbad, CA). Silibinin was purchased from Sigma (St. Louis, MO), and dissolvedin DMSO. Unless specified otherwise, the final concentration of DMSO in the culture medium during different treatments did not exceed 0.1% (v/v). Specific oligonucleotidesand the gel shift assay system were from Promega Corp (Madison, WI).
Cell Culture and Treatments
CRC cells were cultured in their respective media (Leibovitz L-15 media for SW480 cells, DMEM media for HT-29, and F-12/Hams mixture media for LoVo cells) containing 10% FBS and 1% penicillin-streptomycin under standard culture conditions. At 60% confluency, cells were serum starved for 24 h, and then treated with silibinin (Sb) for indicated time as specified in the experiments. Cells were stimulated with TNFα (10 ng/mL for different time periods) and harvested. Whole-cell/cytoplasmic /nuclear extracts were prepared as described previously [37].
Xenograft tissues
Xenograft tissues of LoVo and SW480 cells [22,23], stored at −80°C, of athymic (nu/nu) nude male mice orally gavaged either with control [0.2 ml of 0.5% (w/v) carboxymethyl cellulose (CMC)/day] or silibinin (dose: 200 mg/kg body weight) in 0.2 ml of 0.5% CMC, respectively, 5 days/week for six weeks, were used in the present study. All retrospective studies have been previously approved by our Institutional Animal Care and Use Committee. Individually and randomly selected frozen tumor samples, three from each group, were homogenized and lysates (nuclear/whole cell) were prepared as described previously [38].
Electrophoretic Mobility Shift Assay (EMSA)
Consensus sequences of double stranded NF-κB oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′ and 3′-TCA ACT CCC CTG AAA GGG TCC G-5′ from Santa Cruz) were end labeled with γ-P32-ATP (3000 Ci/mmol at 10m Ci/mL) as per manufacturer’s protocol (Promega, Madison, WI). Labeled probe was separated from freeγ-P32-ATP using G-25 Sephadex column. Nuclear extract (10 μg) along with 5X gel shift binding buffer (Promega) was incubated with 1–2 μl (20,000 cpm) of P32-labeled NF-κB probe for 20 min at 37°C. In super shift and competition assays, nuclear extract was incubated with anti-p65 or p50 antibody or unlabeled-oligo before adding labeled NF-κB oligo. DNA retardation gel (6%) was used to resolve DNA-protein or DNA-protein-antibody complexes followed by gel drying and autoradiography.
Immunoblotting
Lysates from in vitro experiments and tumor tissue lysates from control and silibinin-fed groups of mice were analyzed by immunoblotting as previously described [24]. Briefly, 50–60 μg protein per lysate was denatured with 2x-sample buffer and equal amount of protein was resolved on 8%, 12% or 16% tris-glycine gels by SDS-PAGE. Separated proteins were transferred onto nitrocellulose membrane by western blotting The membranes were blocked in blocking buffer for 1 h at room temperature and then incubated with specific primary antibody followed by peroxidase conjugated appropriate secondary antibody. Finally, proteins were visualized by enhanced chemiluminescence (ECL) detection assay (Amersham, Piscataway, NJ). Primary antibodies were anti-Cyclin D1, anti-VEGF, anti-p50, and anti COX-2 (Santa Cruz technology, Santa Cruz, CA); anti-iNOS (Abcam, Cambridge, MA); anti-phoshpo IκB, anti-IκB, anti-Bcl2, anti p65, anti-TNFR1, anti-TRADD, anti-RIP2, and anti-MMP9 (Cell Signaling, Danvers, MA). Secondary antibodies were anti-rabbit IgG (Cell Signaling, Danvers, MA) or anti-mouse IgG (Amersham, Piscataway, NJ). Equal protein loading was confirmed by stripping and re-probing membranes with either anti-α-tubulin (Neomarkers, Fremont, CA) or anti-β-actin primary antibody (Sigma, St. Louis, MO).
Immunohistochemical Analyses
Paraffin-embedded sectionswere deparaffinized and stained using specific primary antibody for total p65 (Santa cruz) as previously described [24]. Thereafter, sections were incubated with biotinylated secondary antibody (1:250 dilution) for 1 h at room temperature, followed by 45 min incubation with conjugated horseradish peroxidase (HRP) streptavidin, and with 3, 3-diaminobenzidine for 10 min at room temperature. Sections were counterstained with Harris hematoxylin. To rule out nonspecific staining, sections were incubated with N-Universal Negative Control rabbit antibody (Dako Cytomation, Denmark). Immunoreactivity (represented by intensity of brown staining) was scored as 0 (no staining), +1 (very weak and scattered cytoplasmic staining), +2 (weak but uniform cytoplasmic staining), +3 (moderate with peripheral tumor areas with strong patchy cytoplasmic staining) and +4 (strong with both nuclear and cytoplasmic staining).
Statistical and Microscopic Analyses
All statistical analyses were carried out with Sigma Stat software version 2.03 (Jandel Scientific, San Rafael, CA) and two sided P values <0.05 were considered significant. The difference between respective controls versus silibinin-treated groups was analyzed by unpaired two-tailed Student’s t-test. Densitometric analysis of EMSA blots or of immunoblots (adjusted with β-actin/α tubulin as loading control) was done by Scion Image program (NIH, Bethesda, MD) and the values obtained are indicated below each blot in the respective figures. All microscopic immunohistochemical analyses were done by Zeiss Axioscope 2 microscope (Carl Zeiss, Inc., Jena, Germany) and photomicrographs were captured by Carl Zeiss AxioCam MrC5 camera.
RESULTS
Silibinin inhibits TNFα-induced NF-κB activation in different human CRC cells
First, we assessed the effect of silibinin on the pro-inflammatory signaling pathways in CRC known to be induced by the cytokine TNFα. For this, we determined the effect on the activation of the key transcription factor NF-κB, employing Electrophoretic mobility shift assay (EMSA), in human CRC cells following a stimulus of 10 ng/mL of TNFα (Fig. 1A). In this investigative study, we employed three well-defined human CRC cell lines, namely HT29, LoVo, and SW480. The selection criterion being that HT29 cell line closely resembles normal colorectal epithelial cells in terms of biochemical and physiological properties, but SW480 cell line is moderately differentiated and invasive, and LoVo represents a more aggressive phenotype [39]. More importantly, we have extensively used HT29, LoVo, and SW480 cell lines in our completed studies with silibinin [21–23,32], wherein we observed strong preventive and/or therapeutic efficacy of silibinin against these CRC cell lines in in vitro studies as well as their xenografts in nude mice, therefore, these cell lines were the logical choice for the proposed mechanistic studies.
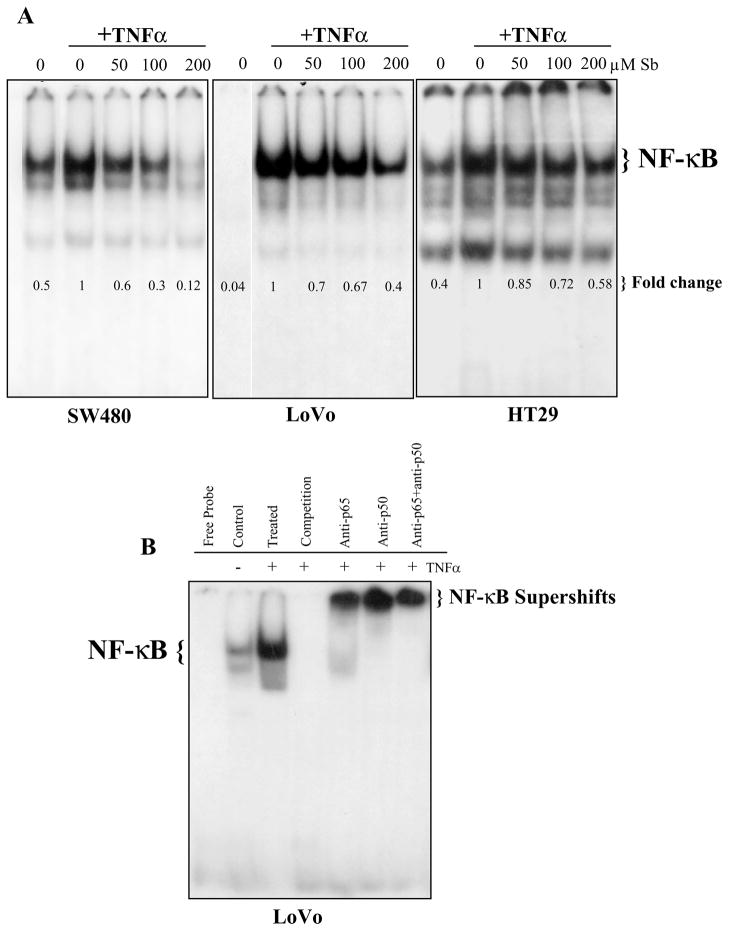
Figure 1.
Silibinin inhibits TNFα-caused NF-κB activation in human CRC cells. (A) Cells at 60% confluency were starved for 24 h, and then treated with indicated doses of silibinin (Sb) for 2 h followed by induction with TNFα (10 ng/mL) for 10 min. (B) Employing control and TNFα stimulated nuclear extracts from LoVo cells, NF-κB specificity, competition and super-shift assays were next conducted. In general nuclear extracts were prepared and EMSA was performed followed by drying of gels and autoradiography as detailed in ‘Materials and Methods’ section. Densitometric analyses of EMSA blots were done by Scion Image program (NIH, Bethesda, MD). The data shown are representative of at least two independent experiments.
The different CRC cell lines were serum starved for 24h, pretreated with different doses of silibinin (50–200 μM) for 2h and then treated with TNFα for 10 min. NF-κB activity in the respective nuclear lysates was measured using EMSA (Fig. 1A). It was observed that silibinin treatment inhibits the activation of NF-κB in a concentration dependent manner. Specifically, the level of inhibition at 50, 100 and 200 μM doses of silibinin was found to be 40%, 70% and 88% in SW480 cells; 30%, 33% and 60% in LoVo cells; whereas in HT-29 cells the inhibition was 15%, 28%, and 42%, respectively (Fig. 1A). In another EMSA study undertaken in LoVo cells for analyzing the specificity of NF-κB band, addition of 100 fold excess of unlabeled NF-κB (cold oligo) in complete reaction incubation resulted in disappearance of the NF-κB band, verifying that the band in EMSA is indeed the case (Fig. 1B). Further, in the supershift assay, nuclear extracts were first incubated with either anti-p50 or anti-p65 antibody or both and followed by EMSA. This showed a strong super shift to a higher molecular weight band in case of anti-p50 and anti-p50/65 together while a slightly moderate shift in anti-p65 alone (Fig. 1B), suggesting that the observed NF-κB band consisted of these two subunits.
Based on above findings showing that silibinin has an inhibitory effect on the activation of NF-κB: a pro-inflammatory transcription factor, following the stimulus by cytokine TNFα, we next focused on detailed mechanistic investigations. Also, since major inflammatory pathways implicated in CRC are COX-2 and iNOS, for more intrusive mechanistic studies, we chose to specifically focus on only LoVo (COX-2 positive) and SW480 (COX-2 negative) cells based on their status of COX-2: a major inflammatory player regulated by activated NF-κB pathway [11].
Silibinin inhibits TNFα-induced nuclear translocation of p50 and p65: the subunits of NF-κB complex, thereby interfering with its activation in human CRC cells
To further determine whether the observed inhibition of NF-κB activation by silibinin was due to the interference in the nuclear translocation of its subunits p50/p65, we analyzed the nuclear/cytosolic lysates by western blot analysis (Fig. 2A). These immunoblotting experiments revealed that the nuclear levels of p50 and p65 (NF-κB subunits) were also decreased by silibinin treatment in both SW480 and LoVo cells (Fig. 2A). Furthermore, the analysis of the cytosolic extracts to determine the effect on the phosphorylation of IκB (Fig. 2A), indicated that in SW480 cells there was a dose dependent decrease in the phospho IκB (pIκB) levels with a slight increase in the total IκB protein levels at 100 μM dose; in the LoVo cells there was a significant dose dependent increase in the total IκB protein levels by silibinin indicating that silibinin was able to rescue the IκB protein from proteosomal degradation which follows as a consequence of its phosphorylation by TNFα (Fig. 2A). However, there is a possibility that this is followed by a rapid turnover of the IκB protein as the phosphorylation status of IκB protein did not show a decrease in the immunoblotting experiment, but on the other hand there was an indication towards an increase in the phosphorylation of the IκB protein at both 50 and 100 μM doses (Fig. 2A).
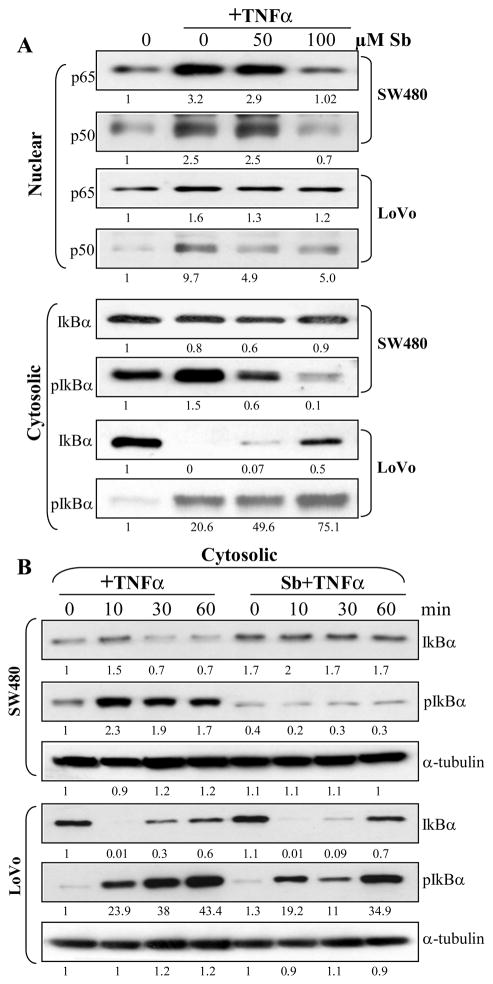
Figure 2.
Dose-and time dependent effect of silibinin on nuclear levels of p65 and p50, and cytoplasmic levels of IκBα and pIκBα in human CRC cells. (A) Cells at 60% confluency were starved for 24 h, then treated with indicated doses of silibinin (Sb) for 2 h followed by TNFα (10 ng /mL) treatment for 10 min. (B) Cells at 60% confluency were starved for 24 h, then treated for indicated time with TNFα (10 ng /mL) without or with prior treatment of silibinin (Sb) at 100 μM dose for 2 h. In general, equal amounts of protein from nuclear or cytoplasmic extracts were resolved on 12% SDS-PAGE, transferred on to a nitrocellulose membrane, probed with appropriate primary and secondary antibodies, and visualized by ECL as detailed in ‘Materials and Methods’ section. Densitometric analyses of immunoblots were done by Scion Image program (NIH, Bethesda, MD). The data shown are representative of at least two independent experiments.
In order to further confirm that the observed high turn over of IκB protein in LoVo cells was indeed the case, we proceeded to determine a time-dependent effect on cytosolic IκB protein levels (both total and phospho) upon TNFα stimulation in the absence/ presence of silibinin 100 μM dose (Fig. 2B). In this experiment, cells were serum starved for 24h and then stimulated directly by the addition of TNFα (10 ng/mL) for 10, 30 and 60 min, or they were first pre-treated with silibinin 100 μM dose for 2 h followed by stimulation with TNFα for the indicated times. Analysis of the cytosolic extracts confirmed that in SW480 cells, the increase in the phosphorylation of IκB upon TNFα stimulation was highest after 10 min compared to 30 and 60 min induction points, which was significantly inhibited at all time points by silibinin pre-treatment (Fig. 2B). There was also a significant decrease in the total IκB protein levels at 30 and 60 min induction points following TNFα stimulation; the levels of which were restored by silibinin pre-treatment (Fig. 2B). On the other hand, in LoVo cells there was a time dependent increase in the phosphorylation of IκB upon TNFα stimulation (Fig. 2B). With respect to the total IκB levels, stimulation with TNFα for 10min resulted in complete disappearance of the IκB band in the western blot, indicating complete degradation of the total protein as compared to unstimulated LoVo cells. The total protein levels of IκB started restoring back in a time dependent manner following TNFα stimulation further from 10 min to 60 min (Fig. 2B). It is this restoration of total IκB protein levels following complete degradation at earlier time point (Fig. 3B), which was predicted in the earlier experiment (Fig. 2A) as high turn over of this protein in LoVo cells, which kind of makes it difficult to specifically interpret the effect of silibinin on the fate of this molecule. Although, silibinin pre-treatment decreased the phospho IκB levels compared to their respective TNFα treated controls, the inhibition was higher in 30 min stimulated cells compared to 10 min and 60 min TNFα treated controls (Fig. 2B). These results were corroborated by comparison with total IκB levels in LoVo cells after silibinin treatment which indicated that there was indeed an increase in phospho IκB levels in coherence with increase in the total IκB protein levels (Fig. 2B). In summary, the time-kinetics study of TNFα without or with silibinin treatment showed that SW480 and LoVo cells have different time-kinetics for IκBα phosphorylation, degradation and re-appearance following TNFα treatment, and that silibinin alters all of them as its possible mechanism of inhibitory effect on NF-κB activation (Fig. 2B).
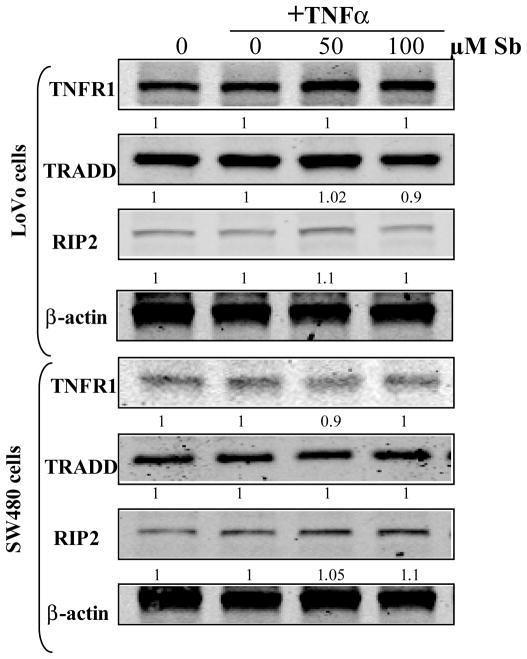
Figure 3.
Dose dependent effect of silibinin on expression levels of TNFR1, TRADD and RIP2, in human CRC cells. Cells at 60% confluency were starved for 24 h, treated with indicated doses of silibinin (Sb) for 2 h followed by TNFα (10 ng /mL) treatment for 10 min. Total cell lysates were resolved on 8% or 12% SDS-PAGE, transferred on to a nitrocellulose membrane, probed with appropriate primary and secondary antibodies, and visualized by ECL as detailed in ‘Materials and Methods’ section. Densitometric analyses of immunoblots were done by Scion Image program (NIH, Bethesda, MD).
To further determine, whether, the inhibitory effect of silibinin on NF-κB activation was due to an upstream effect caused by a decreased expression of the TNF receptor (TNFR1) and the associated adaptor proteins, which could act as a limiting factor in the transduction of TNFα induced signaling, we analyzed the expression of these molecules upon TNFα induction (Fig. 3). Results indicated that silibinin had no effect on the protein levels of these molecules (Fig. 3), further confirming, that, the NF-κB inhibitory effect was primarily due to decreased phosphorylation of IκB protein. Overall, these results indicate that silibinin inhibits TNFα-induced nuclear translocation of p50 and p65: the subunits of NF-κB complex, by interfering with the phosphorylation of the NF-κB inhibitory protein-IκB, thereby inhibiting its activation in human CRC cells.
Silibinin decreases the protein levels of the molecules known to be up-regulated via TNFα-caused NF-κB activation in human CRC cells
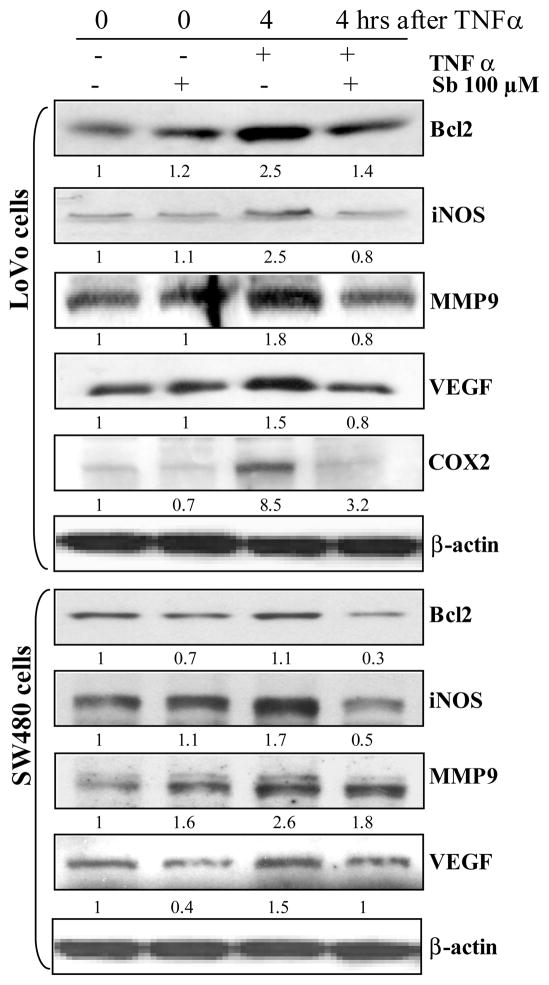
As is known, the degradation of IκB releases NF-κB which then translocates to the nucleus, wherein it mediates transcription of down stream targets which are associated with cancer initiation and progression [8,14,15,17]. In order to determine the effect of silibinin on these down stream targets of NF-κB, the two human CRC cells: LoVo and SW480, were serum starved for 24h, pre-treated with silibinin (100 μM) for 2h followed by treatment with TNFα (10 ng /mL) for 4h; total cell lysates were then prepared and subjected to western blotting assays to determine the effect on the protein levels of these downstream molecules (Fig. 4). The results indicated that, consistent with its inhibitory effect on TNFα-caused NF-κB activation, silibinin also decreased the protein levels of the molecules known to be up-regulated via TNFα-caused NF-κB activation in human CRC cells (Fig. 4). Specifically, in LoVo cells which were pretreated with silibinin for 2h prior to exposure of TNFα stimuli for 4 h, the protein levels of Bcl2, iNOS, MMP9, COX-2 and VEGF were reduced by 43%, 67%, 54%, 62%, and 45% respectively, compared to cells treated with TNFα only (Fig. 4). In case of SW480 cells, the protein levels of Bcl2, iNOS, MMP9, and VEGF under similar treatment conditions were reduced by 66%, 67%, 29%, and 34% respectively, compared to TNFα controls (Fig. 4). Overall, the results indicated that the inhibition of NF-κB activation caused by silibinin in human CRC cells eventually led to a significant inhibition in the expression of downstream targets of NF-κB, which are essentially involved in the growth and progression of this disease, thereby supporting the hypothesis that the silibinin targets CRC growth and progression by targeting the inflammatory NF-κB pathway.
Figure 4.
Silibinin decreases the protein levels of the molecules, which are up-regulated via TNFα-caused NF-κB activation in human CRC: LoVo and SW480 cells. Cells at 60% confluency were starved for 24 h, and then treated with 100 μM dose of silibinin (Sb) for 2 h followed by TNFα (10 ng /mL) for indicated times. Total cell lysates were resolved on 8%, 12% or 16% SDS-PAGE, transferred on to a nitrocellulose membrane, probed with appropriate primary and secondary antibodies, and visualized by ECL as detailed in ‘Materials and Methods’ section. Densitometric analyses of immunoblots were done by Scion Image program (NIH, Bethesda, MD). The data shown are representative of at least two independent experiments.
Oral feeding of silibinin for six weeks inhibits NF-κB activation and expression of its down stream targets in SW480 and LoVo tumor xenografts in nude mice
In our completed studies, we have earlier observed strong preventive and/or therapeutic efficacy of silibinin against CRC xenografts (HT29, LoVo, and SW480) in nude mice [22,23,32,34]. In order to further determine whether this anti cancer efficacy of silibinin was due to the targeting of the inflammatory NF-κB pathway and that silibinin had the potential to alter the expression of multiple targets of NF-κB pathway which was independent of the COX-2 status of these CRC cell lines, we proceeded to analyze these xenograft tissues.
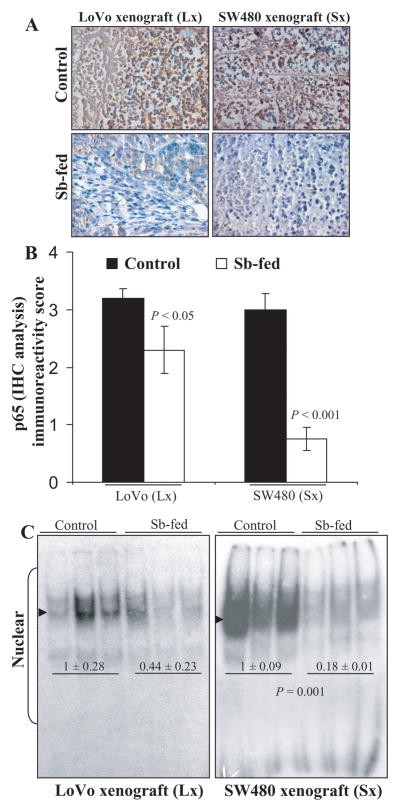
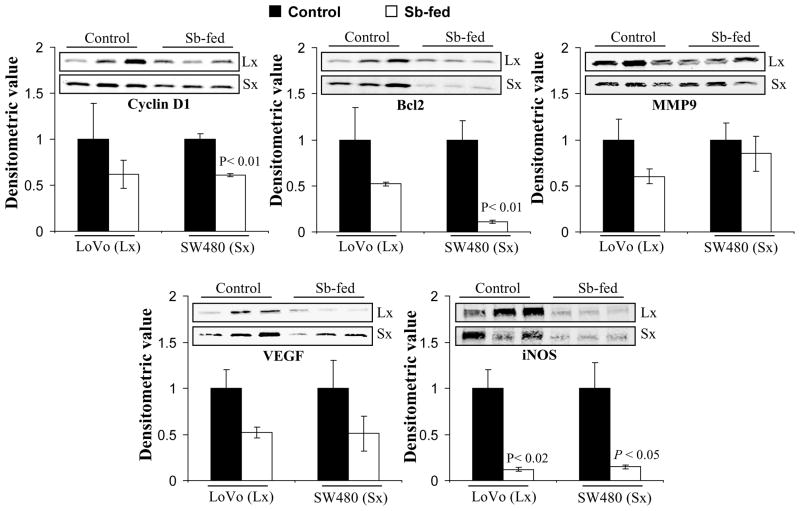
IHC analysis was done to determine the expression NF-κB/total p65 subunit in the xenograft tissues (Fig. 5A &B). Results indicated that compared to their respective controls, the immunoreactivity score of total p65 decreased by 28% in silibinin-fed LoVo xenografts (P<0.05), whereas the decrease was 75% (P<0.001) in case of SW480 (Fig. 5B). Further, the nuclear lysates of these xenografts were also subjected to EMSA to determine the effect of silibinin feeding on the activation of NF-κB (Fig. 5C). It was observed that silibinin feeding significantly inhibited the activation of NF-κB in SW480 (82% decrease, P=0.001) tumor xenografts (Fig. 5C). In case of LoVo tumor xenografts, the activation of NF-κB, though reduced by 56% was not statistically significant due to increased variability in control tissues (Fig. 5C). We also determined the effect of silibinin feeding on the expression of downstream targets of NF-κB in the xenograft tissue lysates. Western blots for Cyclin D1, Bcl2, MMP9, VEGF, and iNOS with densitometric data (adjusted with β-actin as loading control) are shown in Figure 6. Here, it should be noted that membranes were stripped and reprobed for β-actin for each blot (data not shown). Silibinin feeding significantly decreased the protein levels of Cyclin D1, Bcl-2, and iNOS by 39% (P<0.01), 89% (P<0.01), and 85% (P<0.05), respectively, in SW480 xenografts compared to the controls (Fig. 6). On the other hand, while silibinin feeding also showed a decrease in the expression of MMP9 and VEGF in SW480 tissues, the decrease was not statistically significant compared to controls. Furthermore, in LoVo xenografts, the protein level of iNOS was also strongly decreased by 88% (P<0.01) in silibinin-fed group compared to controls. While a decrease in the protein expression levels of Cyclin D1, Bcl-2, MMP9, and VEGF was also observed in LoVo xenografts by silibinin feeding, the decrease was not statistically significant (Fig. 6).
Figure 5.
Oral silibinin feeding inhibits NF-κB activation in CRC tumor xenografts in nude mice. Xenograft tissues of LoVo and SW480 cells, of athymic (nu/nu) nude male mice orally gavaged either with control [0.2 ml of 0.5% (w/v) carboxymethyl cellulose (CMC)/day] or silibinin (dose: 200 mg/kg body weight) in 0.2 ml of 0.5% CMC, respectively, 5 days/week for six weeks, were used in the present study. (A) Immunohistochemical staining of CRC xenograft tissues for NF-κB/total p65. Representative DAB-stained tissue specimens from control and silibinin-fed group (×400 magnifications) are shown. (B) Quantification of NF-κB/total p65-positive cells represented as immunoreactivity score is shown as mean ± SEM (error bars) of each group. (C) EMSA of nuclear lysates of xenograft tissues for activated NF-κB was done as detailed in “Materials and Methods. The difference between respective controls versus silibinin-fed groups was analyzed by unpaired two-tailed Student’s t-test. Two sided P values <0.05 were considered significant. Arrow heads indicate position of NF-κB band. Lx, LoVo xenograft; Sx, SW480 xenograft.
Figure 6.
Effect of oral silibinin feeding on protein expression levels of various NF-κB-regulated molecules viz., cyclin D1, Bcl-2, COX2, iNOS, VEGF and MMPs in CRC xenograft tissues. Xenograft tissues of LoVo and SW480 cells, detailed in Figure 5, were used and total tissue lysates were resolved on 8–12% SDS-PAGE, transferred on to a nitrocellulose membrane, probed with appropriate primary and secondary antibodies, and visualized by ECL as detailed in ‘Materials and Methods’ section. The difference between respective controls versus silibinin-fed groups was analyzed by unpaired two-tailed Student’s t-test. Two sided P values <0.05 were considered significant. Densitometric analyses of immunoblots (adjusted with β-actin as loading control) was done by Scion Image program (NIH, Bethesda, MD). Lx, LoVo xenograft; Sx, SW480 xenograft.
DISCUSSION
For years, inflammation has been recognized as a localized protective function initiated within the tissues as a result of external/internal injury, irritation or an infection [8,11]. However, it is now established that while acute inflammation can result as a part of body’s defense mechanism, it is chronic inflammation which acts as a trigger that leads to various diseases viz., cancer, pulmonary, neurological, and cardiovascular [8,11]. However, this triggering potential can be bidirectional in cancer, maturing either via an extrinsic pathway wherein inflammatory or infectious conditions augment the risk of developing cancer at the anatomical sites (e.g., colon, prostate, and pancreas) or an intrinsic pathway, wherein genetic events leading to neoplasia cause tumor cells to produce inflammatory mediators which then generate an inflammatory environment in tumors, for which, there is no underlying inflammatory condition [7,8]. Both these pathways converge, resulting in the activation of transcription factors (NF-κB, STAT3, and HIF1α) in tumor cells; which in turn coordinate the production of inflammatory mediators, like cytokine and chemokines as well as COX-2 (involved in prostaglandin production) [7,8]. These factors recruit and activate various inflammatory cells and also activate the same key transcription factors in inflammatory cells, stromal cells, and tumor cells resulting in a vicious cycle of uninhibited rise in the production of these mediators, and a pro-cancer, pro-inflammatory microenvironment being generated which further fuels the growth and progression of malignant disease [8,13,40].
With regards to CRC, chronic inflammation has been recognized as one of the primary causes of its occurrence owing to epidemiological reports indicating an increased CRC riskwith longer duration of inflammatory conditions [3,7]. While anti-inflammatory drugs esp., NSAIDs have been identified to be effective against CRC; their use is marred by complications arising due to their various side effects [4–6]. This has resulted in various efforts being made world-wide to identify non –toxic, natural agents from dietary/non-dietary sources that have cancer chemopreventive potential against CRC [5,25,41]. One such agent is silibinin, the major active constituent in a widely consumed dietary supplement: silymarin (milk thistle extract) [25]. Silibinin is used clinically as a hepatoprotective agent, and is devoid of any toxicity in animal studies and in humans [25,30,42,43]. Further, silibinin has shown anti-cancer and cancer chemopreventive efficacy in several in vitro and in vivo pre-clinical cancer models [21–36]. Regarding CRC, in our earlier studies, we have reported a strong preventive and/or therapeutic efficacy of silibinin against CRC xenografts (HT29, LoVo and SW480) in nude mice [22,23,32,34], AOM-induced colon tumorigenesis in A/J mice[28], and spontaneous tumorigenesis in APCmin/+ models [26,27]. However, with regards to CRC, the evaluation of the anticancer efficacy of an agent is incomplete without determining the effect on the inflammatory pathways known to be associated with CRC [3,7]. In this regard, we have already demonstrated the efficacy of silibinin to inhibit the activation of NF-κB pathway in various cancers, such as skin, lung, and prostate [29,30,44] wherein, it was observed that silibinin had the potential to inhibit both constitutive as well as cytokine-induced activation of NF-κB. These results also demonstrated the potential of silibinin to cause a direct inhibitory effect on IκB kinase α (IKKα) activity (as seen in in vitro kinase assays) suggesting that silibinin does not necessarily need an upstream event to cause an inhibitory effect on IKKα or related downstream effectors [45]. It is with this view; we undertook the present study to further evaluate the effect of silibinin on inflammatory mechanisms that are associated with CRC.
The results of the present study showed that silibinin strongly inhibited TNFα-induced NF-κB activation together with decreased nuclear levels of both p65 and p50 sub-units concomitant in various human CRC cell lines. Additional mechanistic studies in LoVo and SW480 cells showed that silibinin significantly increases IκBα level with a concomitant decrease in phospho-IκBα, indicating its inhibitory effect on IKKα kinase activity, which was further validated by the fact that, silibinin treatment had no effect on the expression of either the receptor TNFR1 or the associated adaptor proteins TRADD and RIP2. One highly noteworthy part of the study was that, following our initial study using 50–200 μM doses of silibinin, we selected to use only 100μM dose of silibinin in our detailed mechanistic studies. This selection was based on the completed pilot study with silibinin in CRC patients by Ho et al [46] who demonstrated that silibinin feeding (720 mg/day) results in 121 nmol/g of silibinin in colorectal tissue (which is equivalent to 121 μM silibinin in terms of concentration). Thus, in the present study, our in vitro observations related to the anti-inflammatory effect of silibinin in CRC using its pharmacologically achievable concentration are clinically relevant.
Next we assessed the effect of 6 weeks of oral silibinin feeding on NF-κB pathway in SW480 (COX-2 negative) and LoVo (COX-2 positive) tumor xenografts in nude mice. Results indicated that the inhibitory effect of silibinin on CRC tumor growth and progression, as observed earlier [22,23], was accompanied by inhibition of NF-κB activation in xenografts from both the cell lines. This result is highly significant in terms of identifying that the anti-inflammatory effect of silibinin in CRC cells involves interference in the activation of NF-κB; and that the growth inhibitory effects, as a consequence of this mechanism, are not only via COX-2 inhibition. Since NF-κB is an inducible transcription factor regulating transcription of diverse array of genes involved in proliferation, apoptosis, inflammation, angiogenesis and metastasis [8,9,19]; we also assessed silibinin effect on various NF-κB-regulated molecules involved in these processes. Results confirmed that the in vivo inhibition of CRC growth by oral feeding of silibinin in CRC xenograft models was also partly associated with anti-inflammatory mechanisms involving decreased expression of COX2 (LoVo cells) and iNOS levels (both cell lines). The protein levels of Bcl-2, VEGF, and MMPs were also decreased by silibinin in both cell culture studies and xenograft analyses, further suggesting its inhibitory effect on NF-κB transcriptional activity.
While the results from the present study strongly suggest a link between the chemopreventive efficacy of silibinin in CRC and its anti-inflammatory effects therein; however from a broader view point, before we extrapolate these results to a clinical scenario, one limitation could be that the present study does not account for the inflammatory milieu of colonic tissue, which contributes significantly to initiation and progression of CRC lesions [3,8,13,40]. To overcome this limitation, we intend to use in future a colitis-related AOM/DSS-induced colon tumorigenesis model to assess the modulatory role of silibinin on inflammatory conditions in colon [47,48]. In this model, exposure of mice to colonic genotoxic carcinogen AOM and colitis inducing synthetic sulfate polysaccharide, DSS, results in severe colonic inflammation, followed by cryptic dysplasia and adenomatous growth [47,48], and thus it would be a more clinically relevant model to identify the effect of silibinin on inflammatory conditions in colon associated with CRC. Nevertheless, the findings of the present study support all such future studies, and are highly significant in establishing, for the first time, that silibinin could suppress CRC growth and progression, also by targeting the inflammatory NF-κB pathway and thus has potential against human CRC.
Acknowledgments
Grant support: This work was supported NCI RO1 grant CA112304.
Abbreviations
- CRC
colorectal cancer
- Sb
silibinin
- IBD
inflammatory bowel diseases
- NSAIDs
nonsteroidal anti-inflammatory drugs
- COX-2
cyclooxygenase-2
- NF-κB
nuclear factor-kappa B
- IκBs
inhibitors of NF-κB
- EMSA
Electrophoretic Mobility Shift Assay
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TNFα
tumor necrosis factor α
- AOM
azoxymethane
- CMC
carboxymethyl cellulose
- ECL
enhanced chemiluminescence
- IHC
Immunohistochemistry
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313–3323. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]
- 4.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 5.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–219. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 6.Lanas A, Ferrandez A. NSAIDs and the colon. Curr Opin Gastroenterol. 2009;25:44–49. doi: 10.1097/MOG.0b013e3283157c4d. [DOI] [PubMed] [Google Scholar]
- 7.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42:161–170. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 13.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 14.Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–199. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Herrmann A, Deng JH, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 18.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 20.Zhou A, Scoggin S, Gaynor RB, Williams NS. Identification of NF-kappa B-regulated genes induced by TNFalpha utilizing expression profiling and RNA interference. Oncogene. 2003;22:2054–2064. doi: 10.1038/sj.onc.1206262. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal C, Singh RP, Dhanalakshmi S, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 22.Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling. Neoplasia. 2010;12:415–424. doi: 10.1593/neo.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur M, Velmurugan B, Tyagi A, et al. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol Cancer Ther. 2009;8:2366–2374. doi: 10.1158/1535-7163.MCT-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajamanickam S, Agarwal R. Natural products and colon cancer: current status and future prospects. Drug Dev Res. 2008;69:460–471. doi: 10.1002/ddr.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajamanickam S, Kaur M, Velmurugan B, Singh RP, Agarwal R. Silibinin suppresses spontaneous tumorigenesis in APC min/+ mouse model by modulating beta-catenin pathway. Pharm Res. 2009;26:2558–2567. doi: 10.1007/s11095-009-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R. Chemoprevention of Intestinal Tumorigenesis in APCmin/+ Mice by Silibinin. Cancer Res. 2010;70:2368–2378. doi: 10.1158/0008-5472.CAN-09-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravichandran K, Velmurugan B, Gu M, Singh RP, Agarwal R. Inhibitory effect of silibinin against azoxymethane-induced colon tumorigenesis in A/J mice. Clin Cancer Res. 2010;16:4595–4606. doi: 10.1158/1078-0432.CCR-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45:436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 31.Singh RP, Deep G, Chittezhath M, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 32.Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–2050. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 33.Tyagi A, Raina K, Singh RP, et al. Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Mol Cancer Ther. 2007;6:3248–3255. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]
- 34.Velmurugan B, Gangar SC, Kaur M, Tyagi A, Deep G, Agarwal R. Silibinin exerts sustained growth suppressive effect against human colon carcinoma SW480 xenograft by targeting multiple signaling molecules. Pharm Res. 2010;27:2085–2097. doi: 10.1007/s11095-010-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velmurugan B, Singh RP, Tyagi A, Agarwal R. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats. Cancer Prev Res (Phila) 2008;1:376–384. doi: 10.1158/1940-6207.CAPR-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verschoyle RD, Greaves P, Patel K, et al. Evaluation of the cancer chemopreventive efficacy of silibinin in genetic mouse models of prostate and intestinal carcinogenesis: relationship with silibinin levels. Eur J Cancer. 2008;44:898–906. doi: 10.1016/j.ejca.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Singh RP, Dhanalakshmi S, Mohan S, Agarwal C, Agarwal R. Silibinin inhibits UVB-and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol Cancer Ther. 2006;5:1145–1153. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 38.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67:3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 39.Kaur M, Mandair R, Agarwal R, Agarwal C. Grape seed extract induces cell cycle arrest and apoptosis in human colon carcinoma cells. Nutr Cancer. 2008;60 (Suppl 1):2–11. doi: 10.1080/01635580802381295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A. Role of inflammatory cells and mediators in tumor invasion and metastasis. Cancer Metastasis Rev. 2010;29:241. doi: 10.1007/s10555-010-9228-1. [DOI] [PubMed] [Google Scholar]
- 41.Marshall JR. Prevention of colorectal cancer: diet, chemoprevention, and lifestyle. Gastroenterol Clin North Am. 2008;37:73–82. vi. doi: 10.1016/j.gtc.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broussard CN, Aggarwal A, Lacey SR, et al. Mushroom poisoning--from diarrhea to liver transplantation. Am J Gastroenterol. 2001;96:3195–3198. doi: 10.1111/j.1572-0241.2001.05283.x. [DOI] [PubMed] [Google Scholar]
- 43.McPartland JM, Vilgalys RJ, Cubeta MA. Mushroom poisoning. Am Fam Physician. 1997;55:1797–1800. 1805–1799, 1811–1792. [PubMed] [Google Scholar]
- 44.Chittezhath M, Deep G, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits cytokine-induced signaling cascades and down-regulates inducible nitric oxide synthase in human lung carcinoma A549 cells. Mol Cancer Ther. 2008;7:1817–1826. doi: 10.1158/1535-7163.MCT-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits constitutive and TNFalpha-induced activation of NF-kappaB and sensitizes human prostate carcinoma DU145 cells to TNFalpha-induced apoptosis. Oncogene. 2002;21:1759–1767. doi: 10.1038/sj.onc.1205240. [DOI] [PubMed] [Google Scholar]
- 46.Hoh C, Boocock D, Marczylo T, et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res. 2006;12:2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki R, Kohno H, Sugie S, Tanaka T. Dose-dependent promoting effect of dextran sodium sulfate on mouse colon carcinogenesis initiated with azoxymethane. Histol Histopathol. 2005;20:483–492. doi: 10.14670/HH-20.483. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]