Abstract
Mast cells (MC) and basophils (Ba) share expression of the high affinity receptor for IgE (FcεRI) but can be distinguished by their divergent expression of KIT and CD49b. In BALB/c mice, MC lineage cells expressing high levels of FcεRI by flow cytometry were seen only in bone marrow (BM) while those expressing intermediate levels of FcεRI were present in BM and spleen of naïve mice and in mesenteric lymph nodes (mLN) of Trichinella spiralis-infected mice. These FcεRI+KIT+CD49b− cells had a membrane phenotype like intraperitoneal connective tissue-type MC, but were smaller and hypogranular by flow cytometry forward and side scatter profiles, respectively. Consistent with this, they lacked the prominent secretory granules identified by histochemistry and immunodetection for the MC-specific granule proteases that are readily seen in mature jejunal mucosal MC (MMC) that also are induced by the infection and present at the same time. The concentration of these MC lineage cells in mLN determined by flow cytometry was comparable to that of MC progenitors (MCp) measured by limiting dilution and clonal expansion with maturation. We observed upregulation of IL-4 mRNA levels by MCp in mLN and spleens of helminth-infected 4get mice, and demonstrated by intracellular cytokine staining production of IL-4 and IL-6 by the mLN MCp in helminth-infected mice. Furthermore, treatment of helminth-infected mice with anti-FcεRI mAb, a protocol known to deplete Ba, also depleted mLN MCp. Thus, this study identifies a hypogranular subset of MCp recruited to mLN by helminth infection that may be an important unrecognized source of cytokines.
Introduction
Mast cells (MC) and basophils (Ba) express the high affinity receptor for IgE and have been appreciated and characterized for their respective effector roles in the Th2 host response to helminth infection (1, 2). Although Ba are detected in bone marrow (BM), spleen, and lymph nodes (LN) by flow cytometry as FcεRI+CD49b+KIT− cells in various models of immune responses (2–4), the paucity of MC in these tissues has been demonstrated only by histochemical assessment. MC are long lived as connective tissue MC (CTMC) (5) but transient when induced in the intestine as mucosal MC (MMC) (6, 7). While mature MC are not detected in blood and are rare in the parenchyma of BM, LN and spleen, MC progenitors (MCp) are readily detected in cell suspensions from BM, spleen and small intestine of naïve mice when assayed by limiting dilution and clonal expansion/maturation assays (LDA) (8–11). MCp are recruited to intestine and mesenteric LN (mLN) during Th2-mediated helminth-induced inflammation (12–15) or in response to hyperimmunization (9, 16). Nonetheless, as MCp cannot be detected histochemically, their presence has not been directly assessed in many models of inflammation.
We used the LDA assay with stem cell factor (SCF) and IL-3 as growth and maturation factors to demonstrate the absence of jejunal MCp in mice lacking β7-integrin (11). We further demonstrated that MCp migration from the BM to jejunum is constitutive and requires α4β7 integrin, as revealed by the depletion of jejunal MCp in WT mice treated for a week with mAb to this integrin (11, 17). This constitutive basal homing from BM to intestine provides a dynamic reservoir of MCp in naïve mice from which MMC are induced with helminth infection (18). Such basal homing is minimal to lung, but recruitment of MCp to lung parenchyma occurs during Th2-mediated pulmonary inflammation using interdependent adhesion and migration pathways (19, 20). This recruitment precedes the induction of mucosal MC (MMC) in the trachea and large airways (21). Because MCp reside in tissues of naïve mice and can be recruited to peripheral tissues with inflammation, their predominant function has been viewed as providing a reservoir for MMC expansion and attendant secretory functions.
Numerous recent studies have been focused on the role of the Ba in the immune response (reviewed in (22)) and yet the role of the MCp in these studies is rarely considered. Furthermore, the analysis of MCp numbers by the in vitro LDA does not distinguish between the committed FcεRI+KIT+ cells and those earlier multipotential cells capable of entering into the lineage. We turned to flow cytometric analysis without sorting to study the development and distribution of MC and Ba during an inflammatory response, with the goal of identifying both progenitor and more mature populations. These studies included mice in the naïve state as well as mice injected locally with papain or infected systemically with the helminth Trichinella spiralis. MC lineage cells and mature Ba resided in BM and spleen of naïve BALB/c mice. Ba could be recruited to local LN with both papain and T. spiralis infection while only the infection elicited a LN MC lineage population. Forward and side scatter analysis by flow cytometry of these FcεRI+KIT+CD49b− MC revealed that they were small hypogranular cells. The parallel assessment of their numbers by LDA, which requires maturation, indicated that they were MCp at d 6 and d 13 of infection. The hypogranular phenotype of these local nodal MCp at d 6 of infection was dramatically different from the appearance of fully granulated intestinal MMC in the same mice at the same time. Moreover, the hypogranular MCp in LN and spleen exhibited IL-4 mRNA which increased during T. spiralis infection in 4get reporter mice. Furthermore, in infected BALB/c mice, this population of MCp could produce IL-4 and IL-6 as determined by flow cytometric detection. Our findings suggest that MCp are not simply a developmental stage, but rather may be a tissue population with independent functions in locations where granulation is not needed or desirable.
Material and Methods
Mice
7–18 wk old BALB/c, and 4get (C.129-Il4tm1Lky/J) mice were obtained from Taconic Farms or the Jackson Laboratory. IL-3−/− mice are maintained in house at the Dana Farber Cancer Institute (23). The use of mice for these studies was in accordance with institutional guidelines with review and approval by the Animal Care and Use Committee of the Dana Farber Cancer Institute.
Antibodies
Fluorescently labeled monoclonal antibodies (mAb) directed against FcεRIα (MAR-1), mouse IgE (23G3), CD49b (DX5), B220 (RA3-6B2), CD3 (145-2C11), CD19 (6D5), CD4 (RM4-5), Ly-6G/Ly-6C (Gr-1) (RB6-8C5), TCRβ (H57-597), IL-4 (11B11), IL-6 (MP5-20F3) and TNF-α (MP6-XT22) were obtained from Biolegend (San Diego, CA). Anti-FcγRII/III (2.4G2), -KIT (2B8), and -β7 integrin (M293) mAb were obtained from BD Biosciences (San Diego, CA). Monoclonal anti-mouse MC protease (mMCP)-1 was obtained from R&D Systems (Minneapolis, MN). Anti-mMCP-5 is a rabbit anti-peptide preparation made against a specific peptide (24).
Papain administration
Fifty μg of papain (Sigma-Aldrich, St Louis, MO) in HBSS was injected subcutaneously into bilateral footpads at a concentration of 1 mg/ml 3 days prior to harvest of popliteal LN (3).
Trichinella spiralis infection
Mice were infected with 450 larvae of T. spiralis by gavage as previously described (6).
Identification of MCp and Ba and intracellular cytokines by flow cytometry
Single cell suspensions of spleen, BM, and LN were obtained by grinding tissues through 70-μm cell strainers (BD Biosciences) into RPMI1640, with 10% fetal calf serum, L-glutamine, penicillin, streptomycin, gentamicin, HEPES buffer, sodium pyruvate, and 2-mercaptoethanol (Sigma-Aldrich). Peripheral blood was collected by cardiac puncture into syringes containing 100 μL 0.5M EDTA. Erythrocytes in spleen and blood were lysed (1–2 min in 0.1 mM EDTA, 2.0 g/L potassium bicarbonate, 16.6 g/L ammonium chloride). Peritoneal cells were collected by peritoneal lavage with 10 ml of RPMI. Non-specific mAb uptake was blocked with CD16/32 (2.4G2) (BD Biosciences) for 10 min, and appropriate mAb were added for 30 min. Cells were analyzed on a FACS Canto flow cytometer (BD Biosciences) using FACSDiva acquisition software. FlowJo software (Tree Star, Ashland, OR) was used for data analysis. Positive cells were defined as those having fluorescence intensities >99% of cells incubated with isotype control mAb. For exclusion of other cell types, we used mAb against CD19, CD3, CD4, B220, TCRβ, and Gr-1. In experiments with naïve mice or papain-injected mice, anti-FcεRIα was used to identify FcεRI+ cells. After T. spiralis infection, anti-IgE mAb was used for identification of FcεRI+ cells.
For isolation of MCp from the mesenteric LN in order to examine their morphology, the nodes were removed from T. spiralis infected mice on d 6 post-infection. The single cell suspensions were fractionated on a 44%/67% Percoll gradient and the interface cells harvested and incubated with biotinylated anti-CD19 and anti-CD3 (Biolegend) for 30 minutes, after which they were washed and incubated with biotin binder Dynabeads (Invitrogen) for 30 minutes according to the manufacturer’s protocols. The cells were then placed on a Dynabead magnet (Invitrogen) for 5 minutes, and the unbound cells were collected and stained with anti-FcεRI, anti-CD117, and anti-CD49b. FcεRI+ CD117+CD49b− cells were collected using a BD FACSAria II (BD), affixed to slides using a CytoSpin (Thermo Scientific) and stained with either Diff-Quick (Siemens Healthcare Diagnostics) or toluidine blue.
To assess for intracellular cytokine production by MCp, mLN cells were cultured for 1 h in culture media supplemented with IL-3 (1–10 ng/ml) to improve viability and then for 4 h with 1 μg/ml Brefeldin A in the same cell culture media. After 5 h in culture, cells were spun down, fixed and permeabilized using a BD Cytofix/Cytoperm kit according to the manufacturer’s directions and stained for flow cytometry. Production of a specific cytokine was identified as an increase in the mean fluorescence relative to that obtained from the same cells stained with an isotype control mAb in parallel. The mean fluorescence intensity (MFI) index was defined as the MFI from the anti-cytokine mAb minus the MFI from the isotype control and then divided by the MFI from the isotype control.
To develop BM derived MC (BMMC), BM cells were cultured in complete RPMI with 10 ng/ml each of SCF and IL-3. Non-adherent cells were passed weekly and the BMMC were used after 4–6 weeks in culture.
Identification of MCp by limiting dilution and clonal expansion
The limiting dilution and clonal expansion assay (LDA) was performed as previously described with inclusion of SCF with IL-3 to improve viability and maturation (9, 11). Mononuclear cells (MNC) were obtained by Percoll (Sigma-Aldrich) gradient fractionation of harvested cells by using the 44%/67% Percoll interface. After LDA, the original MC progenitor concentration is expressed as the number of MCp per 106 MNC isolated from the tissue.
Histochemical and immunohistochemical evaluation of MC
Tissues and isolated cells were evaluated for mature MC numbers by chloroacetate esterase (CAE) reactivity and immunohistochemistry for the mouse MC-specific proteases (mMCP) (21). Briefly, tissue was harvested, fixed in 4% paraformaldehyde overnight and then embedded in paraffin for evaluation of mMCP expression or in glycol methacrylate for evaluation of CAE reactivity. To quantify the number of mature MC in the lymph node preparations, cytospins of ~200,000 cells per slide were stained for CAE reactivity and counted for CAE+ cells with morphologic characteristics consistent with mature MC.
Antibody-mediated cell depletion
Injection of anti-FcεRIα (MAR-1 from eBioscience, San Diego, CA and Biolegend) was performed essentially as described by others (4, 25). Mice were injected with 10 μg of MAR-1 intraperitoneally 72, 48, and 24 hours prior to T. spiralis infection. In initial studies with naïve mice, this protocol depleted most of the Ba in spleen and BM. In studies of T. spiralis-infected mice, BM, spleen and LN were harvested at the times noted and analyzed for MC lineage cells by clonal expansion or flow cytometry and for Ba lineage cells by flow cytometry.
Statistics
Data are expressed as the mean ± SEM when derived from three or more values. Significance was determined with a two-tailed Student’s t test where three or more values were available for analysis. Significance was determined with the Mann-Whitney U test when values did not follow a normal distribution. Values of p < 0.05 were considered significant.
Results
Identification of two Ba and two MC lineage populations in BALB/c BM and a single phenotype for each lineage in peripheral tissues
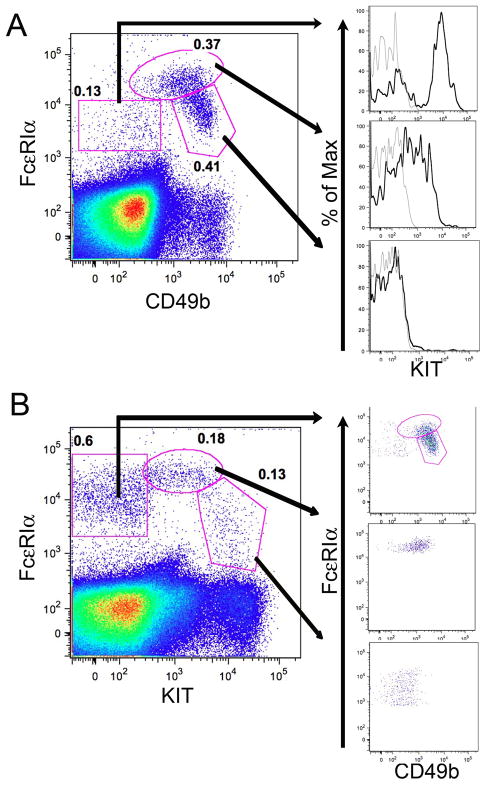
Earlier studies by our group and others with BM-derived cells cultured in vitro demonstrated that commitment to the MC and Ba lineages was closely followed by de novo expression of the high affinity IgE receptor detected either by mAb to the α chain or the ability to bind IgE (26–29). Differentiation along the MC lineage was associated with the continued expression of KIT, the receptor for stem cell factor (SCF), whereas this receptor was down regulated with Ba lineage development (29). To follow the parallel lineage development of MC and Ba in vivo, we analyzed the non-B, non-T cells (by excluding cells expressing CD19 or CD3) in BALB/c BM for expression of FcεRIα and KIT (CD117), as well as CD49b as a Ba marker (30–32). Within the non-B, non-T population there were two contiguous populations of cells positive for both FcεRI and CD49b expression (pentagonal and ovoid gates in Fig. 1A). These cells showed a 10-fold range in the fluorescence intensities of FcεRI expression, and the population of cells expressing the highest levels of FcεRI (ovoid gate in Fig. 1A) had a 10-fold range in expression of CD49b. A third population of cells was negative for CD49b expression and intermediate in FcεRI expression (rectangular gate in Fig. 1A), and was predominantly KIT+ (Fig. 1A, top histogram) suggesting a committed MC lineage identity. The CD49b+ population that expressed intermediate levels of FcεRI (pentagonal gate in Fig. 1A) uniformly lacked KIT expression (Fig. 1A, bottom histogram), suggesting a committed Ba identity. The cells expressing high levels of FcεRI (ovoid gate) with a range of CD49b expression were heterogeneous in their KIT expression (Fig. 1A, middle histogram) suggesting at least two different precursor populations. To further classify these BM cell populations, we reanalyzed the non-B, non-T cells for FcεRI and KIT expression, and then assessed CD49b expression (Fig. 1B). We identified a KIT− population (rectangle gate in Fig. 1B) that was predominantly CD49b+ (Fig. 1B, top dot plot) suggesting it is mostly composed of Ba lineage cells also identified in the oval and pentagonal gates in Fig 1A. The population of cells expressing high levels of KIT and intermediate levels of FcεRI (Fig. 1B, pentagon) was largely CD49b− (Fig. 1B, bottom dot plot) compatible with MC commitment. The population of cells with intermediate KIT and high FcεRI expression (Fig. 1B, ovoid gate) was heterogeneous for CD49b suggesting two different precursor populations (Fig. 1B, middle dot plot) similar to the cells in the oval gate identified in Fig 1A. Together, the FcεRI+ populations make up about 0.9% of non-B, non-T cells in BALB/c BM.
FIGURE 1.
BM Ba and MC lineage populations in BALB/c mice defined by flow cytometric analysis of FcεRI, CD49b and KIT expression. BALB/c BM cells were analyzed for expression of FcεRI, CD49b, and KIT after exclusion of CD3+ and CD19+ (non-T, non-B) cells. A. Three populations are defined (polygons) by FcεRI and CD49b expression. The frequencies of the populations are shown as a percent of non-T, non-B cells. Each of the three populations is further analyzed for KIT expression (black histograms) compared with isotype control (gray histograms). B. Analysis of FcεRI and KIT expression within the non-T, non-B cells as in panel A. Indicated populations are further analyzed for FcεRI and CD49b expression (right panels). Data are representative of 6–9 mice in 3 experiments. Isotype control values were less than 5% of the indicated values.
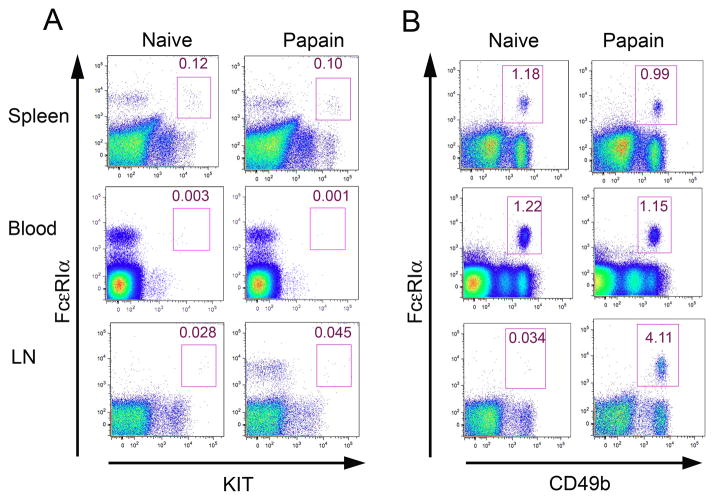
Using the same three cell surface markers, we compared the phenotypes of MC (FcεRI+KIT+CD49b−) and Ba (FcεRI+KIT−CD49b+) within the non-B, non-T cells of BALB/c spleen, blood, and popliteal lymph nodes (LN) with those in the BM, using injection of papain in the footpads to elicit Ba in popliteal LN (3). In the spleen, there was only a single population of FcεRI+KIT+ cells expressing intermediate levels of FcεRI in both naïve and papain-treated mice (Fig. 2A, top panel, rectangular gate). Few or no FcεRI+KIT+ cells were detected in blood or popliteal LN of naïve and papain-injected mice (Fig. 2A, middle and bottom panels). There also was only a single population of FcεRI+CD49b+ cells expressing intermediate levels of FcεRI in the spleen and blood of both naïve and papain injected mice (Fig. 2B, top and middle panels). This CD49b+ population corresponds to the unmarked population of FcεRI+KIT− cells seen in Fig. 2A. There were very few LN Ba in naïve mice, but papain administration induced an influx of Ba into LN (Fig. 2B, bottom panels) as previously noted (3). The KIT+FcεRIhi and KIT−FcεRIhi populations seen in BM were notably undetectable in these peripheral tissues.
FIGURE 2.
Identification of MC and Ba lineage populations in spleen, blood, and LN of naive and papain-injected BALB/c mice. A. Three d after papain or PBS injection, MC lineage cells were identified as FcεRI+KIT+ cells in spleen, blood, and popliteal LN. B. Three d after papain or PBS injection, Ba lineage cells were identified as non-T, non-B, FcεRI+CD49b+KIT− cells in spleen, blood, and popliteal LNs. Data are representative of 9–12 mice in 3 experiments. Numbers indicate the frequency of MCp and Ba as a percentage of the non-T, non-B cells isolated from the indicated tissues. Isotype control values were less than 5% of the indicated values.
Infection with T. spiralis expands MC and Ba lineage populations and recruits them to mLN
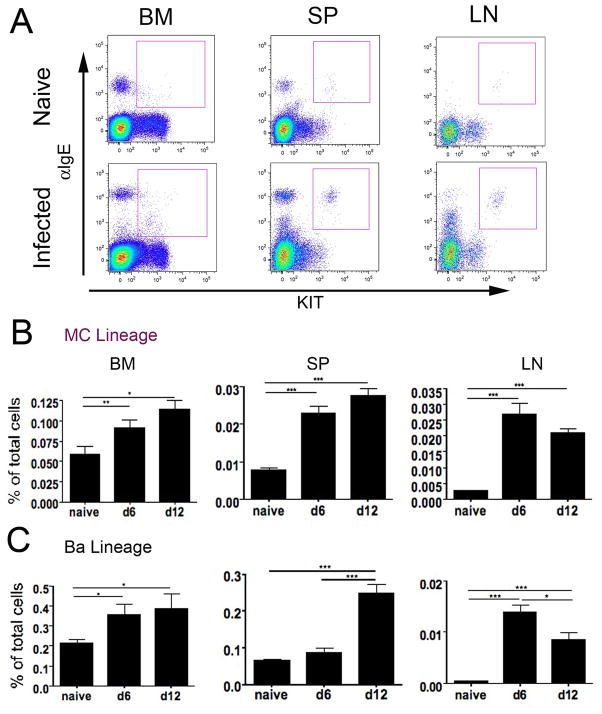
To directly study recruitment of MC lineage progenitors to LN as predicted by LDA (12–15), we employed a T. spiralis infection in which tissues were harvested in the middle of (d6–7) and toward the end of (d12–14) the acute phase of intestinal infection. In contrast to the studies with papain, we used anti-IgE rather than anti-FcεRI to monitor FcεRI expression during T. spiralis infection to avoid any competing effects of host IgE on the assessment of FcεRI+ cells (33). IgE+KIT+ MC lineage populations in naïve mice were present in BM and spleen but were rare in mLN (Fig. 3A, top). The unmarked IgE+KIT− Ba population can be seen directly to the left of the gated MCp (Fig. 3A). After T. spiralis infection, there were significant increases in the frequency of BM IgE+KIT+CD49b− MC at d6 and d12 (Fig. 3A, B). Further, distinct IgE+KIT+ MC lineage populations of similar phenotype appeared in spleen and mLN (Fig. 3A). There were concurrent significant rises in these splenic and LN populations on d6 and d12 (Fig. 3B). Ba populations identified as IgE+CD49b+ cells also significantly expanded in BM on d6 and d12 (Fig. 3C). Splenic Ba numbers lagged at d6 but had a significant increase at d12. The number of Ba in the mLN was significantly increased at d6 and d12. In the mLN, MC lineage cells constituted a larger (~2 fold) fraction of the population than Ba at each time point.
FIGURE 3.
T. spiralis- infected BALB/c mice recruit MC and Ba to mesenteric LN and expand spleen and BM lineage pools. A. Dot plots of BM, spleen, and LN cells from naïve and d12 infected BALB/c mice show the IgE+KIT+ MC lineage populations (square gate). Dot plots are representative of data from 4 experiments. B. Frequencies of MC lineage populations from naïve, d6 and d12 post-infection mice are shown as a percent of total cells isolated from BM, spleen, and LN. C. Frequencies of Ba lineage populations (IgE+CD49b+) in the BM, spleen, and LN as in panel B. Data in panels B and C represent means ± SEM, n = 13 mice per group, 4 experiments. * p<0.05, ** p<0.001, ***p<0.0001.
LN MCp assessed by flow cytometry are highly immature and correspond in number to MCp assessed by limiting dilution and clonal expansion
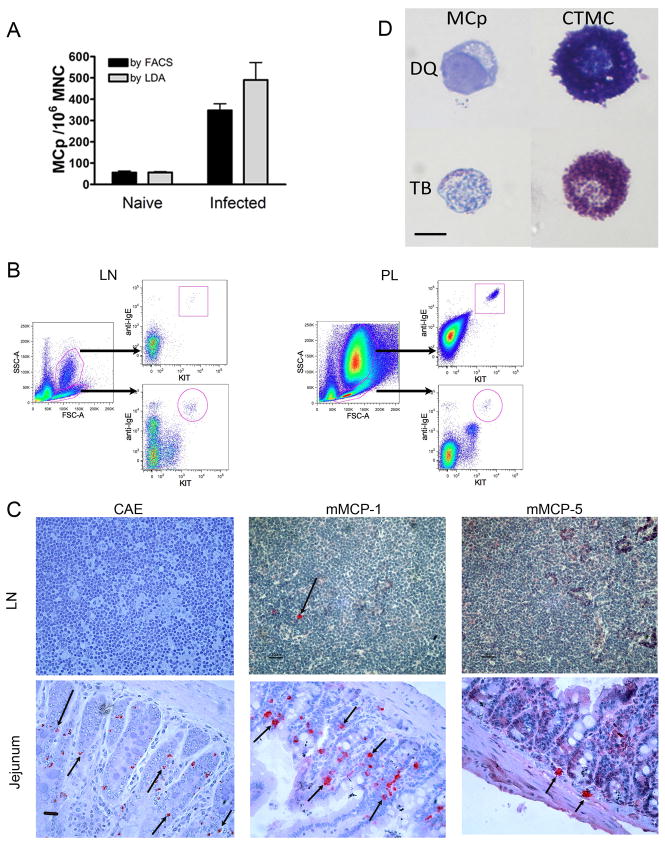
To determine if the numbers of lineage committed MCp in mLN detected directly by their distinct cell surface phenotype in flow cytometry are comparable to the numbers of MCp assayed indirectly ex vivo by LDA, we compared these numbers in naïve mice and in mice 13 d after T. spiralis infection by both assays using the MNC fraction. There were very few MCp in naïve mLN with either assay (55.8 and 56.4 MCp/106 MNC, mean from 2 experiments, 5 mice). At 13 d post infection there were significant increases in the number of MCp detected by flow cytometry (6.2 fold, p< 0.001) and by LDA (8.7 fold, p< 0.01) (Fig. 4A). There was no significant difference between the measurements of MCp per million MNC obtained by the two methods in either naïve or infected mice. The trend toward higher numbers obtained by LDA may reflect the lower specificity of this assay attributable to participation by myeloid lineage cells that differentiate into MC colonies in the presence of IL-3 and SCF (34).
FIGURE 4.
Comparison of MC and MCp populations in various tissues of naïve and T. spiralis-infected mice analyzed by flow cytometry, limiting dilution analysis, histochemical staining and immunohistochemistry. Mesenteric LN cells were isolated from BALB/c mice before and 13 d after T. spiralis infection. A. Enumeration of MCp in the MNC fraction of LN cells by flow cytometry (FACS) and by the limiting dilution/maturation assay (LDA) to quantitatively compare the 2 methods in naïve and infected mice. Data shown are means ± SEM, n = 5–7 mice per group, from 2 experiments. B. Cells from mesenteric LN and peritoneal lavage (PL) isolated 13 d after T. spiralis infection were allocated as high and low side scatter (SSC) by flow cytometry and evaluated separately for IgE binding and KIT expression (gates shown in left panel for each tissue). Data are representative of 12 mice, from 3 experiments. C. Mesenteric LN and jejunal sections from BALB/c mice obtained 13 d after T. spiralis infection were evaluated for MC by CAE reactivity, and by immunohistochemistry for mMCP-1 and mMCP-5. Arrows indicate positively stained cells. D. Comparison of the morphology of LN MCp to peritoneal CTMC from BALB/c mice. MCp (left panels) were isolated by FACS from 6 d post T. spiralis infected mice and CTMC (right panels) were obtained by peritoneal lavage. The top panels (Diff-Quik stain, DQ) shows only empty vesicles in the MCp and the bottom panels (toluidine blue stain, TB) shows only a few metachromatic granules in MCp while the CTMC has a large number of cytoplasmic granules by both stains. Data shown in A – C are representative of 5 experiments. Micrographs are taken at 400X (C), and 630X (D); black scale bars represent 50 and 10μm, respectively.
To assess the maturity of LN MCp detected by flow cytometry, we analyzed the numbers of MCp in the LN obtained by cytometry before and after density gradient fractionation and compared them to the numbers of granulated MC detected by histochemistry. It had been established previously using peritoneal cells obtained by lavage that density gradient isolation of MNC yields a cell preparation enriched for MCp and depleted of mature MC which sediment in the pellet (17). T. spiralis-infected mLN harvested at d13 post infection yielded 281 ± 57 MCp (IgE+KIT+) cells per 106 LN cells (mean ± SEM, n= 8, 2 experiments). Histochemical evaluation of granulated MC in these and one additional mLN cell preparation yielded 12.5 ± 3.1 CAE + MC per 106 LN cells (mean ± SEM, n= 11, 3 experiments). After isolation of the MNC fraction, there were 332 ± 40 MCp per 106 MNC by flow cytometry and 7.5± 1.0 CAE+ MC per 106 MNC cells by histochemistry. Thus, 96% and 98% of the LN cells identified as IgE+KIT+ were histochemically hypogranular MCp before and after isolation of the MNC fraction, respectively.
The majority of mLN MCp appeared in a low SSC gate, with only a scant few mature MC in a high SSC gate (Fig. 4B). In contrast, the majority of IgE+KIT+ cells recovered from peritoneal lavage appeared to be mature MC in the high SSC gate, with few MCp in the low SSC gate (Fig. 4B). The finding of low granularity for the MCp is consistent with the low density of helminth-induced LN MCp reported by Jarboe et al. (14). When we compared mLN and splenic MCp populations from d6 post T. spiralis infection, these induced MCp were virtually identical in size (forward scatter profile) and granularity (SSC profile) and greater than the lymphocyte population in these parameters. Of note, these induced MCp were lower in both assessments than immature BMMC derived with IL-3 and SCF emphasizing their relative agranularity (Supplemental Fig 1). Thus, although the recruited LN MCp, splenic MCp, BMMC, and peritoneal MC exhibit the same surface marker phenotype, when identified by flow cytometry, they are different in regard to size (forward scatter) and granularity (SSC).
In a histological examination of the parenchyma of mLN for MC we used CAE reactivity as well as immunohistochemistry to mMCP-1 and mMCP-5 to detect mature MC and evaluate their protease phenotype. In the intestine after T. spiralis infection, mMCP-1 and mMCP-5 identify the induced MMC and the constitutive submucosal MC, respectively (6, 7, 21). The LN parenchyma from naïve mice demonstrated virtually no CAE reactivity or immunoreactivity for mMCP-1 or mMCP-5 (data not shown). After T. spiralis infection, there was little CAE reactivity in the LN as compared to the distinct MMC staining in the jejunum. There were only rare cells immunoreactive for mMCP-1 or mMCP-5 in parenchyma of mLN (Fig. 4C, top panels) while the intraepithelial MMC from the jejunum of the same mice expressed mMCP-1 and the submucosal MC expressed mMCP-5 (Fig. 4C, bottom panels). These observations support the findings by flow cytometry that with T. spiralis infection, dispersed mLN parenchymal cells harbor very few mature MC in concert with a dominant hypogranular subset of MCp.
To directly visualize the morphology of this recruited subset, we sorted them from the mesenteric nodes of T. spiralis infected mice at d6 based on their distinct phenotype. After depleting the CD3+CD19+ cells, the sorted KIT+FcεRI+CD49b− cells were stained with Diff-Quick or toluidine blue. The LN MCp are small cells with a prominent nucleus and a cytosol which contains numerous empty vesicles after staining with Diff-Quick and reveals a few toluidine blue positive granules (Fig. 4D). By comparison, CTMC from the peritoneal cavity are large and heavily granulated cells by either Diff-Quik or toluidine blue staining.
Depletion of MCp by administration of anti-FcεRI mAb with T. spiralis infection
Depletion of Ba by systemic administration of anti-FcεRI mAb (MAR-1) to assess their role in mouse models of inflammation (3, 4, 25, 32) is based on a protocol by Denzel et al. (33) in which Ba depletion is not accompanied by a change in the number of mature peritoneal MC. To assess the effect of this treatment on MCp, we administered anti-FcεRI mAb intraperitoneally to BALB/c mice prior to infection with T. spiralis and then enumerated the numbers of MCp in mLN, spleen and BM. MCp were identified as β7-integrin+KIT+ cells, a combination that was equivalent to anti-IgE+KIT+ identification of MCp in isotype-treated infected mice (data not shown). Six days after infection, the β7-integrin+KIT+ MCp were significantly reduced by ~70% (from 471 to 141 MCp per 106 nucleated LN cells) as compared to isotype control injected mice (Fig. 5A). The fold-reduction was similar when assessed by LDA in which cell numbers were reduced from 860 to 260 MCp per 106 LN MNC (Fig. 5B). In contrast, the numbers of MCp in the BM and spleen at d6 post infection were not reduced by this treatment when assessed by flow cytometry or by LDA. The number of β7-integrin+KIT+ MCp in isotype control mAb versus MAR-1 injected mice in the BM and spleen was 2020 ± 328 versus 2058 ± 157 MCp per 106 BM cells (mean ± SEM, n=3) and 534 ± 48 versus 837 ± 227 MCp per 106 splenic MNC, respectively (mean ± SEM, n=3). By LDA, we found 624 ± 63 MCp per 106 MNC in the spleens of isotype control injected mice versus 750 ± 128 MCp per106 MNC in the spleens of MAR-1 injected mice (mean ± SEM, n=6, 2 experiments).
FIGURE 5.
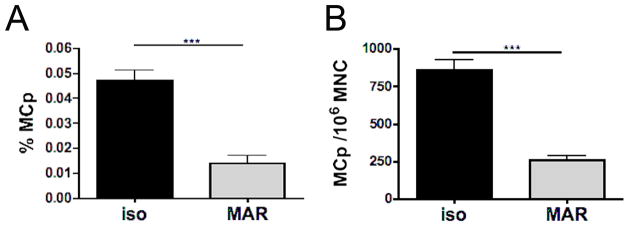
Administration of anti-FcεRI mAb depletes LN MCp. BALB/c mice were injected i.p. with anti-FcεRI mAb (MAR-1) daily for 3 d before being infected with T. spiralis. Mesenteric LN were harvested after 6 d and MCp were assayed. A. In a flow cytometric assay, MCp were identified as β7 integrin+KIT+ cells and enumerated per million live nucleated nodal cells. Data represent means ± SEM, n = 10–11 mice per group, 3 experiments. B. In the LDA, MCp were assayed after Percoll gradient isolation of MNC and expressed per million MNC. Data represent means ± SEM, n = 5–6 mice per group, 2 experiments. ***p<0.0001.
To address the possibility that the influx of Ba influenced the recruitment of MCp to draining nodes, we evaluated the response in the IL-3−/− strain which had been reported to lack Ba in their mesenteric LN by FACS analysis after N. brasiliensis infection (35). The number of LN MCp in the IL3−/− versus the BALB/c mice 6 d after infection with T spiralis was not different (0.70 ± 0.15 % versus 0.68 ± 0.05 % of non-B, non-T LN cells, respectively, n=4 mice per genotype in a single experiment) while the increase in the number of Ba in these same mice was reduced by 77 % (0.03 ± 0.01 % versus 0.15 ± 0.05 %, respectively). We obtained similar results in a second experiment indicating the independence of MCp recruitment to the DLN.
T. spiralis infection increases IL-4 mRNA in mLN MCp of 4get mice
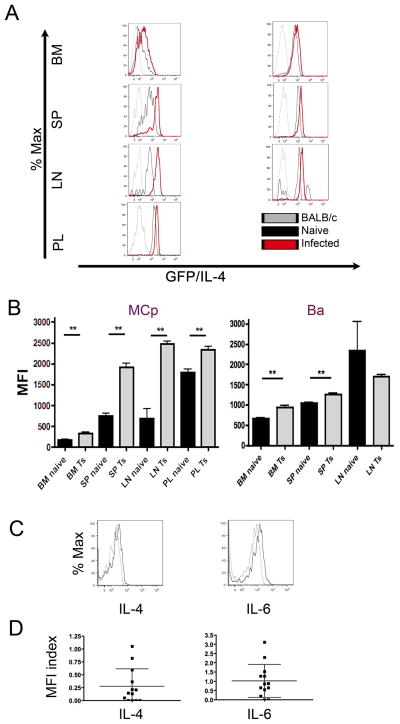
The infection-driven expansion of hypogranular MCp in the draining LN without the appearance of MMC that are so prominent in the intestine, suggests that LN MCp might have a granule-independent function. As many helminth infections generate a substantial Th2 type inflammation with an increased IgE response implicating IL-4, we examined IL-4 mRNA levels in MCp using IL-4/eGFP (4get) reporter mice. In these mice, an eGFP gene is inserted in the 3′ untranslated region of the endogenous IL-4 locus such that eGFP is transcribed in parallel with IL-4 and thus serves as a marker of IL-4 mRNA levels. In naïve 4get mice, BM MCp express very little GFP as measured by mean fluorescence intensity (MFI) (184± 10.6), consistent with the findings of Gessner et al. (36), while splenic MCp express moderate levels of GFP (761.4± 50.6), and peritoneal MC express uniformly high levels of GFP (1789± 85.3) (Fig. 6A and B, left panels). Very few naïve LN MCp were detectable but those seen expressed low GFP levels comparable to naïve splenic MCp (Fig. 6A, black histograms). Six days after T. spiralis infection, GFP expression increased in MCp in all tissues (Fig. 6A, red histograms). The increase in MFI with infection was significant for the MCp in BM, spleen and mLN as well as for peritoneal MC (Fig. 6B, left panel). The Ba lineage demonstrated initial baseline GFP expression in BM, spleen and LN, and with T. spiralis infection, it significantly increased in the BM and splenic Ba. (Fig. 6A and B, right panels). Of note, the hypogranular MCp of spleen and LN increased expression of GFP by 2.5, and 3.6 fold, respectively, while the mature granular peritoneal MC increased GFP expression by only 1.3 fold with infection.
FIGURE 6.
Increased IL-4 transcription in 4get mice and production of IL-4 and IL-6 by LN MCp after T. spiralis infection of BALB/c mice. A. BM, spleen, mesenteric LN, and peritoneal lavage (PL) fluid were harvested from naïve BALB/c and 4get mice, and from 4get mice on d6 post T. spiralis (Ts) infection. The single cell suspensions were assessed for GFP expression within the MC and Ba lineage populations. Histograms of GFP expression are shown for MC (left) and Ba (right) from each tissue as indicated. Histogram colors represent: gray, naïve BALB/c mice; black, naïve 4get mice; red, T. spiralis-infected 4get mice. Naïve LN data are based on very few events. B. Mean fluorescence intensities (MFI) of GFP in the indicated MC (left) or Ba (right) lineage populations from 4get mice represent mean ± SEM, n = 13–14 mice, 3 experiments. ** p < 0.0005, calculated by the Mann-Whitney U test. C. Intracellular cytokine staining for the indicated cytokines in mLN MCp d6 post infection in a representative BALB/c mouse. The gray lines are the isotype control, and black lines are the indicated cytokine. Histograms are from one mouse whose response was close to the mean shown in D. D. MFI index of intracellular cytokine staining for IL-4 and IL-6 in mLN MCp, showing individual values and mean values ± SEM (bars), d6 post infection, 13 mice per group, 5 experiments.
T. spiralis infection induces IL-4 and IL-6 production by mLN MCp of WT BALB/c mice
We then evaluated IL-4 and IL-6 production in LN MCp by intracellular cytokine staining in BALB/c mice on d6 after T. spiralis infection. The LN MCp from a typical infected mouse showed expression of IL-4 and IL-6 as indicated by the right shift in the histograms of intracellular cytokine staining for both cytokines (Fig. 6C). The range of expression in all mice at d6 post infection is presented in Fig. 6D and shows detectable levels of IL-4 in 9 of 13 and of IL-6 in 11 of 13 mice. Importantly, the hypogranular MCp can exhibit cytokine-generating function without being subjected to further ex vivo stimulation.
Discussion
We have addressed the parallel in vivo responses of MC and Ba from BM to blood, spleen and regional LN using flow cytometry in mice subjected to local or systemic inflammation as compared to naïve controls. Unlike other myeloid lineage cells, MC are released from BM as a progenitor which is devoid of the secretory granules that define the mature tissue resident cells. Thus, we first established that their cytometric detection and enumeration as FcεRI+KIT+CD49b− MCp corresponded in numbers with their classical assay in tissues by limiting dilution and clonal expansion with maturation to granulated immature MC (34, 37). We then observed that the size (FSC) and granularity (SSC) of MCp by cytometric assay were more than that observed for lymphocytes and demonstrably less than these parameters for culture-derived immature BMMC. These finding were also confirmed by histochemistry in which MC in the jejunum were readily observed using either CAE reactivity or immuno-histochemistry for the secretory granule proteases while those in the LN were not detectable by either measure. Turning to a positive definition of the nodal MCp, we sorted this subset by their lineage phenotype and assessed their morphology on cytospins. As expected nodal MCp are small cells with a prominent nucleus and empty cytoplasmic vesicles by Diff-Quik stain. Toluidine blue staining showed a few granules. The contrast to peritoneal MC by size and granularity was marked, again as expected from the FACS study. Thus, even though the nodal MCp had the same membrane phenotype as mature granulated MC such as those in the peritoneal cavity, they are a novel MC subset with small size and little granularity.
As the MCp recruited to parenchymal tissue of mLN or expanded in spleen with T. spiralis infection are granule deficient by cytometric presentation or direct histochemisty, they would not have been directly recognized in prior studies of MC biology. However, their phenotypic definition allows for a dynamic real time assessment of their accumulation with cytometry. While the jejunum was enriched with mMCP-1+ MMC likely derived from MCp recruited by the T. spiralis infection (12, 13), the nodal MCp were hypogranular and remained as such even as they declined in numbers by d13 when the infection is subsiding and the jejunal MMC are at their peak. Thus, we sought evidence of function in vivo for the hypogranular nodal subset. Exocytosis of preformed secretory granule mediators seemed excluded based on the lack of granules, so we turned to the production of cytokines which is a well-recognized function for BMMC in response to various agonists (38). Using 4get reporter mice, we found upregulation of IL-4 mRNA during helminth infection that increased to a greater degree in mLN MCp than in granulated mature peritoneal CTMC or in the recruited Ba. Importantly, intracellular cytokine staining demonstrated that MCp of WT BALB/c mice had cytokine generating function as shown by IL-4 and IL-6 production in most mice at d6 post T. spiralis infection. We did not need to further activate these LN ex vivo to obtain cytokine generation but did provide IL-3 to maintain their viability during their exposure to brefeldin. We have not yet sought to optimize the post infection time course for MCp cytokine production or surveyed for other cytokines. Rather, our current goal was to demonstrate function for this essentially agranular MC subset prominent in draining LN after T. spiralis infection. We appreciate that there is a longer term need to address the role of the MC lineage in afferent as well as the traditional efferent components of adaptive immunity.
Ginsburg and Lagunoff first extrapolated the appearance of MC lineage progenitors in LN of BALB/c mice, using repeated injections of horse serum or hen albumin for systemic sensitization (16). In their study, LN cells cultured on embryonic fibroblast monolayers differentiated into histochemically defined MC at higher rates from immunized mice, and the dependence of this outcome on Ag restimulation during the culture suggested cytokine dependent maturation. Subsequently, Crapper and Schrader used a conditioned media to grow immature MC from putative MCp and used a limiting dilution analysis with clonal expansion to define the levels of MCp in BM, spleen, and LN of naïve CBA mice as well to show an increase in their number following a local immunization (9). Guy-Grand et al. used such an assay to indirectly recognize MCp in both the thoracic duct lymph and in the mLN during Nippostrongylus brasiliensis infection in BALB/c mice (10). Jarboe et al. extended these findings to show that putative MCp harvested from mesenteric LN were of low density and could be expanded and matured with cytokine culture alone or on a fibroblast monolayer with the latter inducing a histochemical positivity for heparin (14). Similarly, Rennick and colleagues demonstrated that IL-4 and IL-10 promoted expansion and maturation, respectively, of MCp recruited to mesenteric LN with helminth infection and that fibroblast monolayer co-culture induced heparin biosynthesis (15). Thus, the seminal assay of LN MCp from immunized mice by Ginsburg and Lagunoff had components such as added Ag and fibroblast monolayers favoring their maturation to identifiable immature MC. A recent study has suggested that MCp identified by clonal expansion in the LN of helminth-infected mice could be due to the influx of early myeloid progenitors (34). In that study, the authors noted the recruitment of type 2 multipotential progenitors to the LN and their maturation to MC, Ba and macrophages on ex vivo culture. Notably, these prior studies have relied on in vitro culture with MC growth factors to indirectly demonstrate the presence of MC lineage progenitors and this indirect approach does not reveal their relative maturation or purity of their lineage commitment. Our studies provide a direct assay for the MCp and in concert with LDA, demonstrate that the majority of the cells of the MC lineage recruited to the draining LN with helminth infection are committed MCp. However, our findings also point out that the assays of MCp by ex vivo expansion with maturation do not define their hypogranular in vivo phenotype and apparent failure to mature to MMC within the LN parenchyma.
The number of MC subsets is of course not limited to agranular and granular. For example, within the granular subset, the MMC in the intestine induced by helminth infection express mMCP-1 while MMC induced to accumulate in the trachea by ovalbumin sensitization and challenge express not only mMCP-1, a chymase, but also another chymase, mMCP-4, an elastase, mMCP-5, two tryptases, mMCP-6 and -7 and carboxypeptidase A3 (6, 21). There are also differences in the protease profile of the innate CTMC in intestine and trachea. Taken together it begins to appear that the particular tissue and its microenvironment to which MCp are recruited can determine if there is granulation and as well as the protease profile of those that become granulated (21, 37). This heterogeneity along with the added contexts of cytokine and eicosanoid generation for a lineage with major innate and adaptive immune-induced members suggest that subset recognition is a step toward clarifying the complexities of overall MC lineage function.
As the MCp recruited to parenchymal tissue of mLN and spleen with T. spiralis infection were granule deficient by cytometric presentation or histochemistry on cytospins, they would not have been recognized in studies in which mAb to FcεRI was used to deplete and thereby recognize a Ba function. Germane to this, recently Giacomin et al (39) evaluated this treatment in C57BL/6 mice infected with T. spiralis and noted a decrease in the mucosal MC response in the small intestine which they attributed to attenuation of the Th2 response although they did not evaluate MCp. We observed an approximate 70% reduction in accumulation of LN MCp with administration of mAb to FcεRI to mice prior to T. spiralis infection. This blocking was selective as there was no significant concomitant reduction of MCp in BM or spleen assessed by either FACS or LDA. We have not yet addressed the kinetics of LN Ba and MCp depletion with mAb to FcεRI. Nonetheless, our findings with administration of anti-FcεRI are noteworthy because the contributions of IL-4 and/or IL-6 production by Ba have been implicated in host Th2 cell and B cell responses (3, 4, 25, 32, 33, 39, 40), whereas our new findings reveal a previously unrecognized possible contribution from LN MCp.
Supplementary Material
Acknowledgments
We would like to thank Dr. Wei Xing for her help in enumeration of mature MC.
This work was supported by grants from the National Institutes of Health: R01-AI083516 and T32-AI007306.
Abbreviations used in this article
- Ba
basophil
- BM
bone marrow
- LDA
limiting dilution and clonal expansion analysis
- LN
lymph node
- mLN
mesenteric LN
- MC
mast cell
- MCp
MC progenitor
- MFI
mean fluorescence intensity
- mMCP
mouse MC protease
- MMC
mucosal MC
Footnotes
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Kamiya M, Oku Y, Itayama H, Ohbayashi M. Prolonged expulsion of adult Trichinella spiralis and eosinophil infiltration in mast cell-deficient W/Wv mice. J Helminthol. 1985;59:233–239. doi: 10.1017/s0022149x00008002. [DOI] [PubMed] [Google Scholar]
- 2.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 5.Fukuzumi T, Waki N, Kanakura Y, Nagoshi J, Hirota S, Yoshikawa K, Kitamura Y. Differences in irradiation susceptibility and turnover between mucosal and connective tissue-type mast cells of mice. Exp Hematol. 1990;18:843–847. [PubMed] [Google Scholar]
- 6.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friend DS, Ghildyal N, Gurish MF, Hunt J, Hu X, Austen KF, Stevens RL. Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J Immunol. 1998;160:5537–5545. [PubMed] [Google Scholar]
- 8.Sonoda T, Kitamura Y, Haku Y, Hara H, Mori KJ. Mast-cell precursors in various haematopoietic colonies of mice produced in vivo and in vitro. Br J Haematol. 1983;53:611. doi: 10.1111/j.1365-2141.1983.tb07312.x. [DOI] [PubMed] [Google Scholar]
- 9.Crapper RM, Schrader JW. Frequency of mast cell precursors in normal tissues determined by an in vitro assay: antigen induces parallel increases in the frequency of P cell precursors and mast cells. J Immunol. 1983;131:923–928. [PubMed] [Google Scholar]
- 10.Guy-Grand D, Dy M, Luffau G, Vassalli P. Gut mucosal mast cells. Origin, traffic, and differentiation. Journal of Experimental Medicine. 1984;160:12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, Austen KF. Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. Journal of Experimental Medicine. 2001;194:1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillon SB, MacDonald TT. Limit dilution analysis of mast cell precursor frequency in the gut epithelium of normal and Trichinella spiralis infected mice. Parasite Immunol. 1986;8:503–511. doi: 10.1111/j.1365-3024.1986.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 13.Parmentier HK, Teppema JS, van Loveren H, Tas J, Ruitenberg EJ. Effect of a Trichinella spiralis infection on the distribution of mast cell precursors in tissues of thymus-bearing and non-thymus-bearing (nude) mice determined by an in vitro assay. Immunology. 1987;60:565–571. [PMC free article] [PubMed] [Google Scholar]
- 14.Jarboe DL, Marshall JS, Randolph TR, Kukolja A, Huff TF. The mast cell-committed progenitor. I. Description of a cell capable of IL-3-independent proliferation and differentiation without contact with fibroblasts. J Immunol. 1989;142:2405–2417. [PubMed] [Google Scholar]
- 15.Rennick D, Hunte B, Holland G, Thompson-Snipes L. Cofactors are essential for stem cell factor-dependent growth and maturation of mast cell progenitors: comparative effects of interleukin-3 (IL-3), IL-4, IL-10, and fibroblasts. Blood. 1995;85:57–65. [PubMed] [Google Scholar]
- 16.Ginsburg H, Lagunoff D. The in vitro differentiation of mast cells. Cultures of cells from immunized mouse lymph nodes and thoracic duct lymph on fibroblast monolayers. J Cell Biol. 1967;35:685–697. doi: 10.1083/jcb.35.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abonia JP, Austen KF, Rollins BJ, Joshi SK, Flavell RA, Kuziel WA, Koni PA, Gurish MF. Constitutive homing of mast cell progenitors to the intestine depends on autologous expression of the chemokine receptor CXCR2. Blood. 2005;105:4308–4313. doi: 10.1182/blood-2004-09-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artis D, Humphreys NE, Potten CS, Wagner N, Muller W, McDermott JR, Grencis RK, Else KJ. Beta7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur J Immunol. 2000;30:1656–1664. doi: 10.1002/1521-4141(200006)30:6<1656::AID-IMMU1656>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Abonia JP, Hallgren J, Jones T, Shi T, Xu Y, Koni P, Flavell RA, Boyce JA, Austen KF, Gurish MF. Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood. 2006;108:1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallgren J, Jones TG, Abonia JP, Xing W, Humbles A, Austen KF, Gurish MF. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA. 2007;104:20478–20483. doi: 10.1073/pnas.0709651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing W, Austen KF, Gurish MF, Jones TG. Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc Natl Acad Sci USA. 2011;108:14210–14215. doi: 10.1073/pnas.1111048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, Ishiwata K, Oboki K, Kambayashi T, Watanabe N, Karasuyama H, Nakae S, Inoue H, Kubo M. Role of Mast Cells and Basophils in IgE Responses and in Allergic Airway Hyperresponsiveness. J Immunol. 2012;188:1809–1818. doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- 23.Jones TG, Hallgren J, Humbles A, Burwell T, Finkelman FD, Alcaide P, Austen KF, Gurish MF. Antigen-Induced Increases in Pulmonary Mast Cell Progenitor Numbers Depend on IL-9 and CD1d-Restricted NKT Cells. J Immunol. 2009;183:5251–5260. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeil HP, Frenkel DP, Austen KF, Friend DS, Stevens RL. Translation and granule localization of mouse mast cell protease-5. Immunodetection with specific antipeptide Ig. J Immunol. 1992;149:2466. [PubMed] [Google Scholar]
- 25.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rottem M, Goff JP, Albert JP, Metcalfe DD. The effects of stem cell factor on the ultrastructure of Fc epsilon RI+ cells developing in IL-3-dependent murine bone marrow-derived cell cultures. J Immunol. 1993;151:4950–4963. [PubMed] [Google Scholar]
- 27.Yuan Q, Gurish MF, Friend DS, Austen KF, Boyce JA. Generation of a novel stem cell factor-dependent mast cell progenitor. J Immunol. 1998;161:5143–5146. [PubMed] [Google Scholar]
- 28.Jamur MC, Grodzki AC, Berenstein EH, Hamawy MM, Siraganian RP, Oliver C. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood. 2005;105:4282–4289. doi: 10.1182/blood-2004-02-0756. [DOI] [PubMed] [Google Scholar]
- 29.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, Tenen DG, Austen KF, Akashi K. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 31.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, Minegishi Y, Yonekawa H, Karasuyama H. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Denzel A, Maus UA, Gomez MR, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, Talke Y, Mack M. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 34.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 37.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, Dvorak AM, Galli SJ, Dorf ME. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989;170:245. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giacomin PR, Siracusa MC, Walsh KP, Grencis RK, Kubo M, Comeau MR, Artis D. Thymic Stromal Lymphopoietin-Dependent Basophils Promote Th2 Cytokine Responses following Intestinal Helminth Infection. The Journal of Immunology. 2012;189:4371–4378. doi: 10.4049/jimmunol.1200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez MR, Talke Y, Goebel N, Hermann F, Reich B, Mack M. Basophils Support the Survival of Plasma Cells in Mice. J Immunol. 2010;185:7180–7185. doi: 10.4049/jimmunol.1002319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.