Abstract
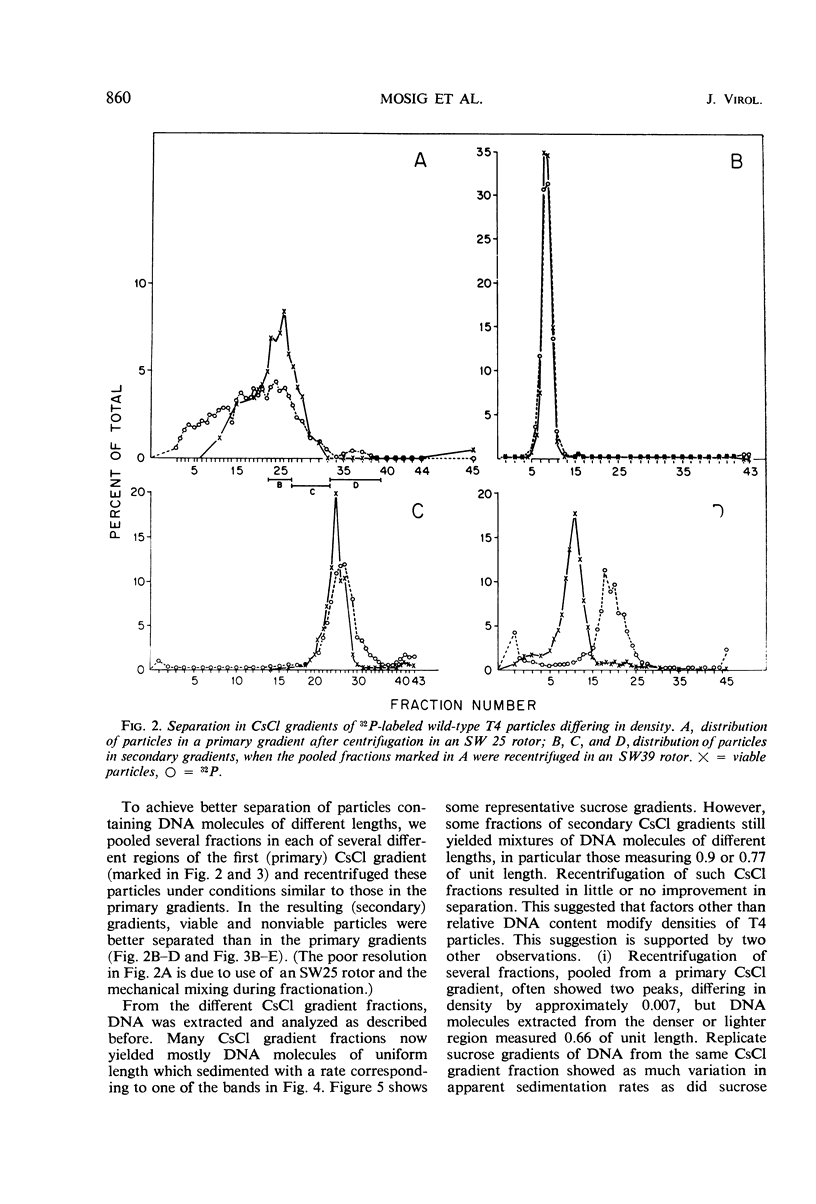
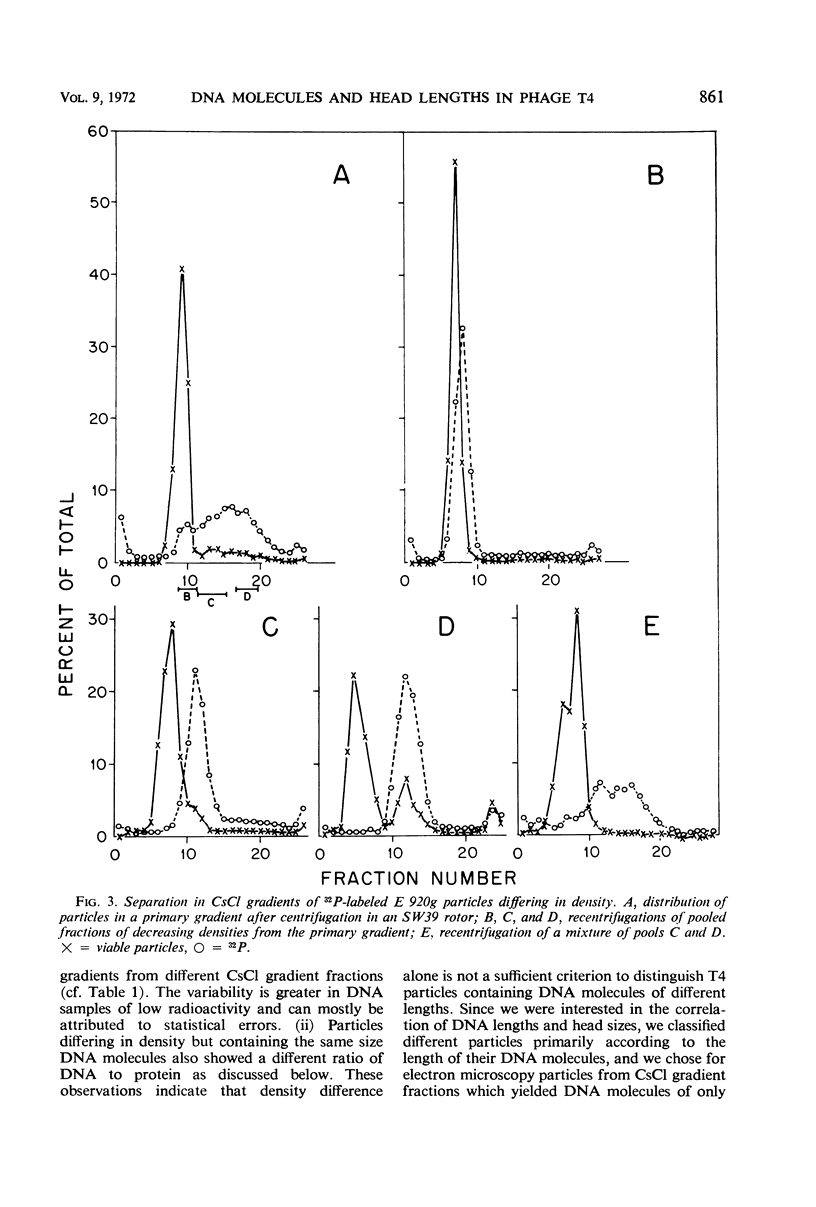
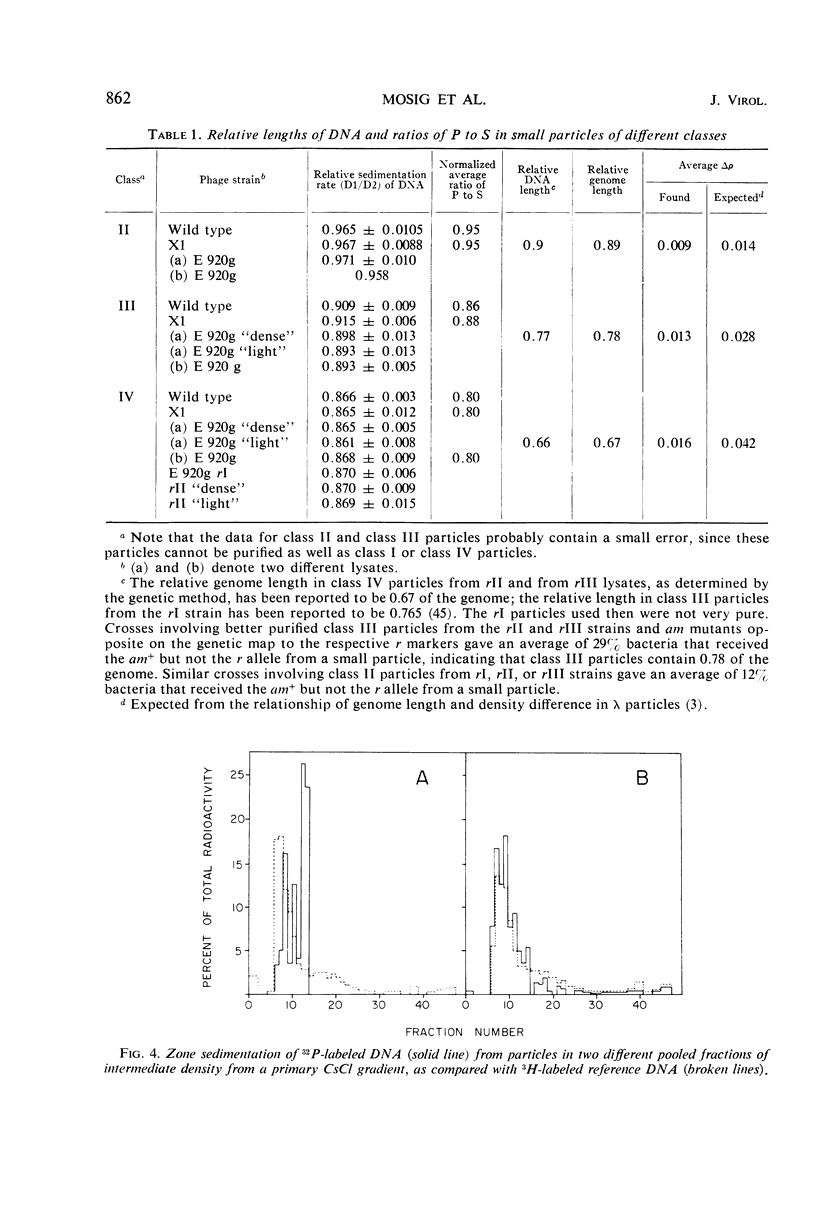
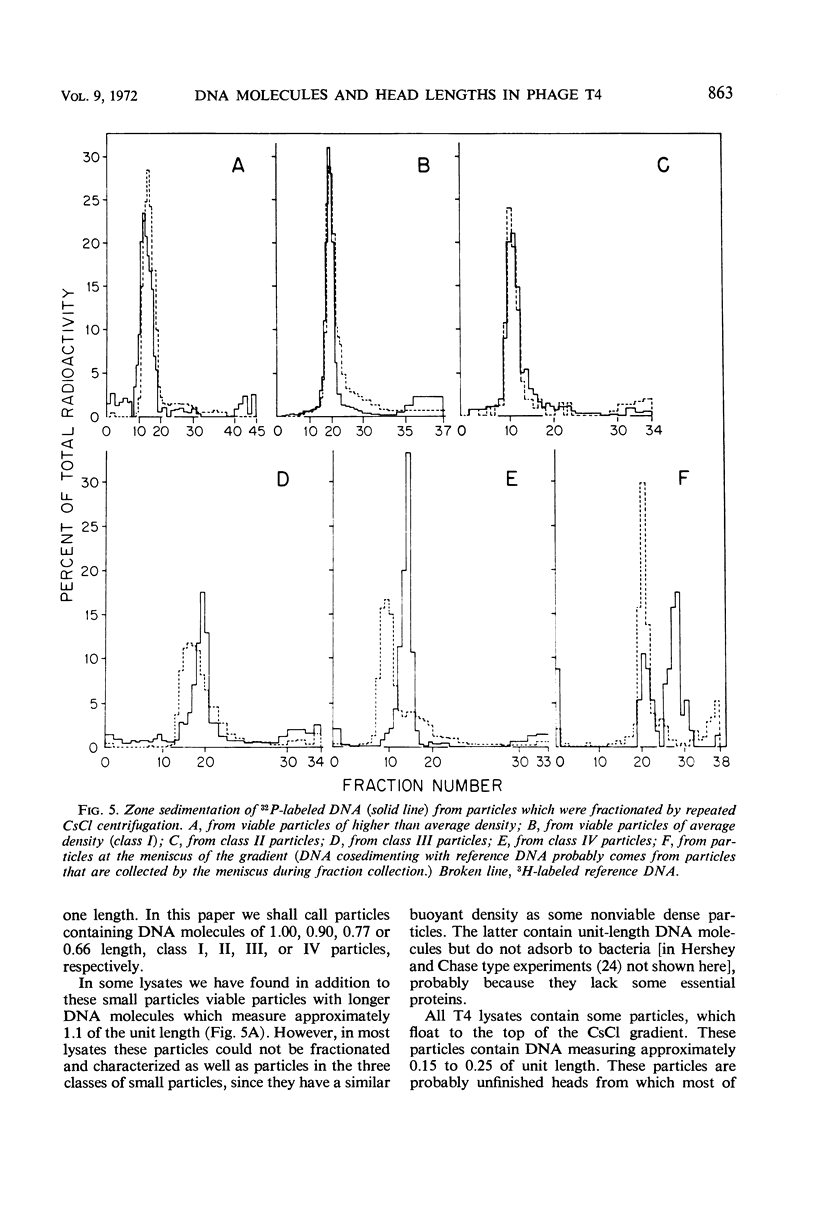
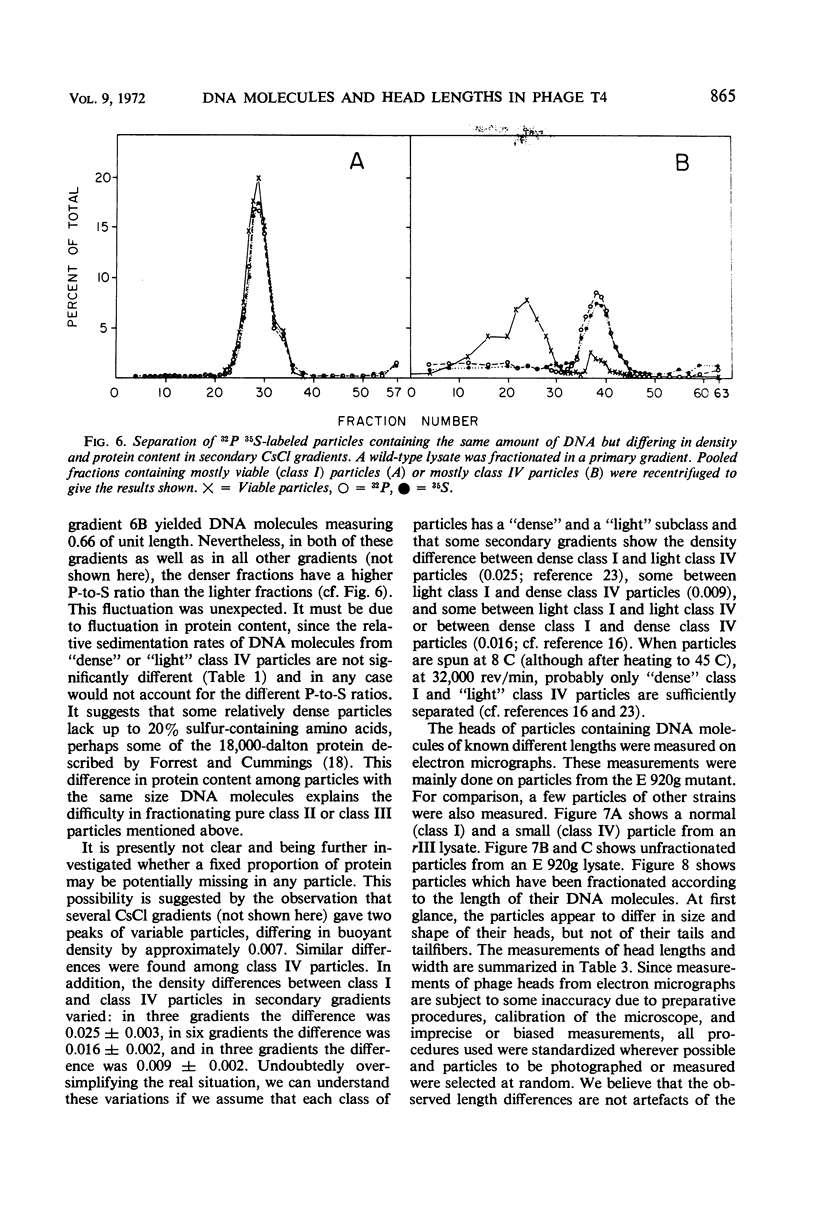
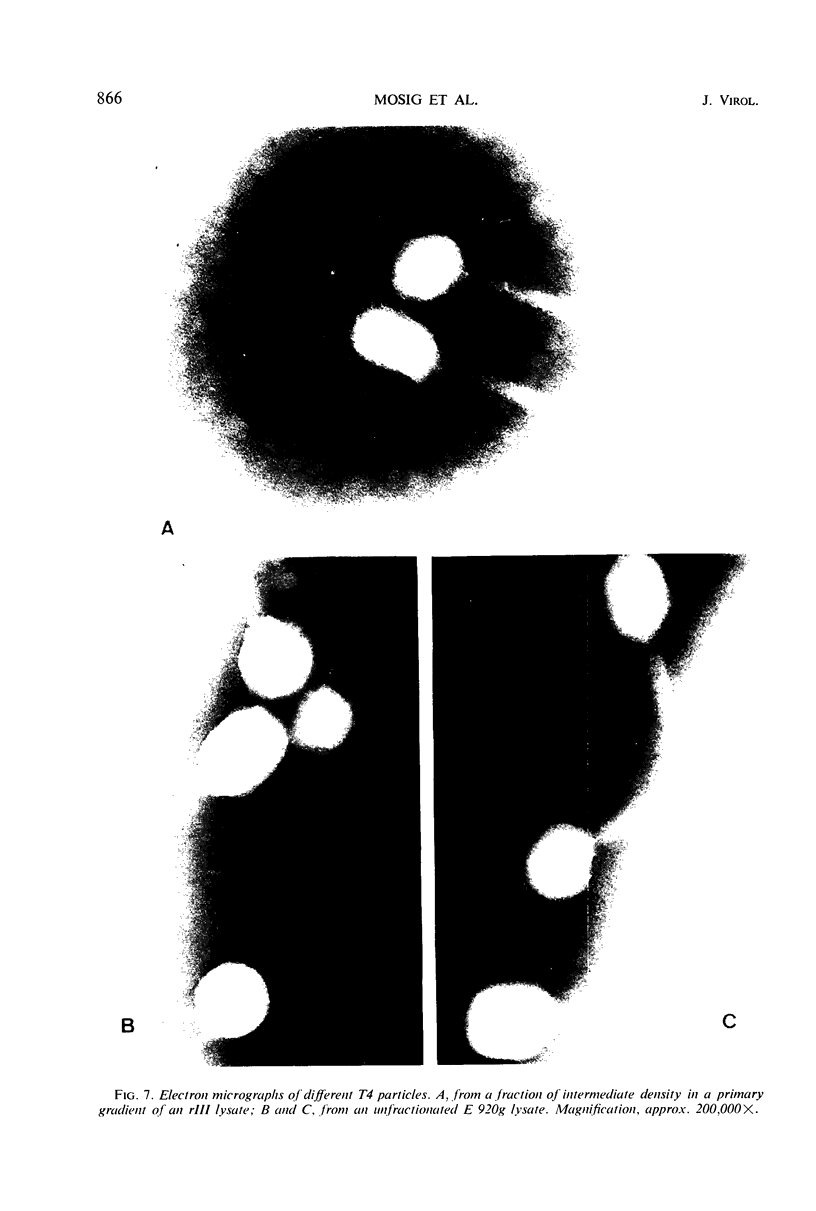
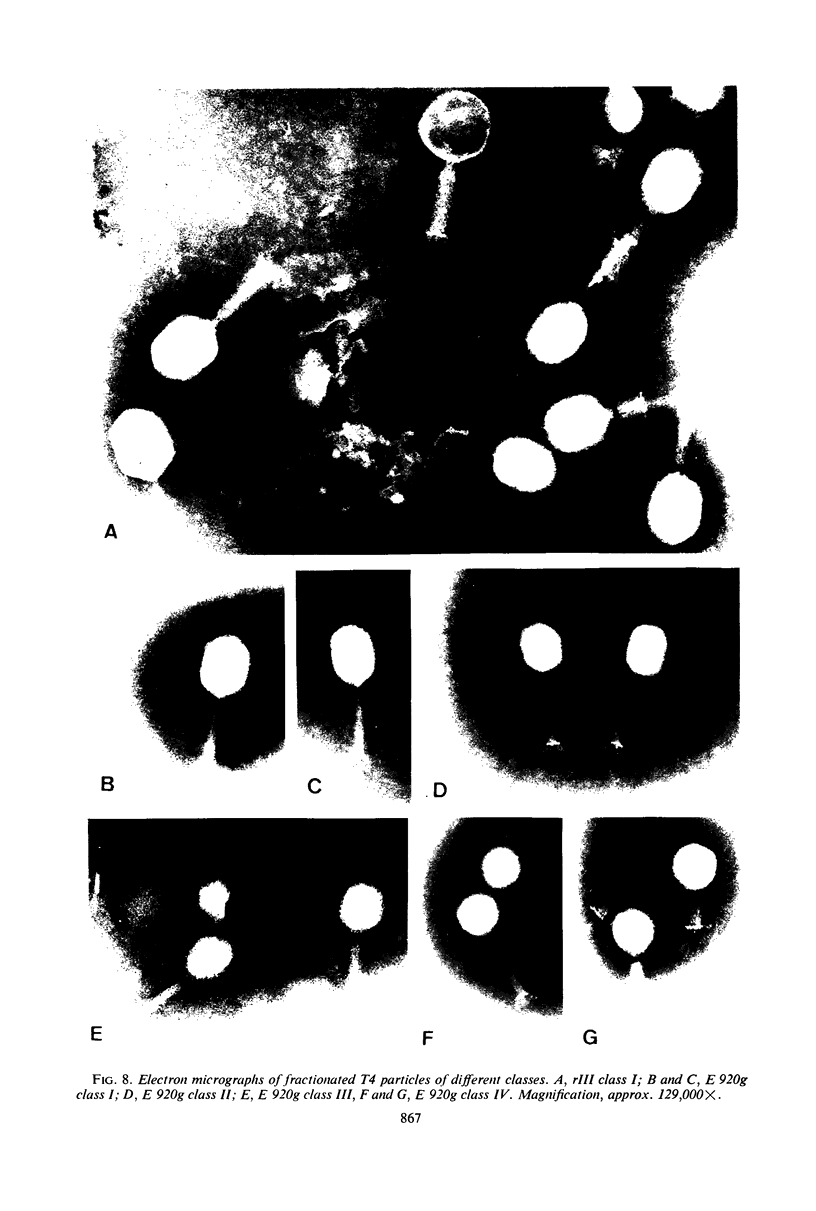
We have investigated three classes of small bacteriophage T4 particles which differ from normal T4 particles in length of their deoxyribonucleic acid (DNA), in head length, in protein content, and in density. The different particles contain DNA molecules measuring 0.90, 0.77, or 0.67, respectively, of the normal T4 length. An additional class of viable particles contains DNA molecules of 1.1 unit length. These discrete differences in DNA length correspond to discrete differences in length (but not width) of the respective heads and are roughly proportional to the resulting differences in head volumes. The measured relative dimensions of the different heads fit best the relative dimensions predicted by a quasi-icosahedral model in which the smallest T4 head corresponds to an icosahedron with a triangulation number T = 21. The mid-portion of this structure is thought to be elongated by adding successive rows of gene 23 protein hexamers, the normal T4 head having three added rows. Different mutants produce small particles of the three classes in varying proportions, but no mutant produces exclusively particles of a single class. Particles of each class, with indistinguishable DNA content, show additional minor differences in protein content, as measured by differences in buoyant density and in the relative ratio of 32P to 35S.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Bacteriophage T2 as seen with the freeze-etching technique. Virology. 1970 Mar;40(3):703–718. doi: 10.1016/0042-6822(70)90215-1. [DOI] [PubMed] [Google Scholar]
- Black L. W. Ahmad-Zadeh C,+AHMADAAZADEH C: Internal proteins of bacteriophage T4D: their characterization and relation to head structure and assembly. J Mol Biol. 1971 Apr 14;57(1):71–92. doi: 10.1016/0022-2836(71)90120-3. [DOI] [PubMed] [Google Scholar]
- Boy de la Tour E., Kellenberger E. Aberrant forms of the T-even phage head. Virology. 1965 Oct;27(2):222–225. doi: 10.1016/0042-6822(65)90163-7. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J. Subunit basis of head configurational changes in T2 bacteriophage. Biochim Biophys Acta. 1963 Mar 26;68:472–480. doi: 10.1016/0006-3002(63)90169-0. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Shaw B. D., Smith M. G. Two stages in the replication of bacteriophage lambda DNA. Biochim Biophys Acta. 1969 Dec 16;195(2):494–505. doi: 10.1016/0005-2787(69)90656-x. [DOI] [PubMed] [Google Scholar]
- Childs J. D. A map of molecular distances between mutations of bacteriophage T4D. Genetics. 1971 Apr;67(4):455–468. doi: 10.1093/genetics/67.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., Kusy A. R., DeLong S. S. Structural aberrations in T-even bacteriophage. II. Characterization of the proteins contained in aberrant heads. Virology. 1971 May;44(2):425–442. doi: 10.1016/0042-6822(71)90273-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Couse N. L., Forrest G. L. Structural defects of T-even bacteriophages. Adv Virus Res. 1970;16:1–41. doi: 10.1016/s0065-3527(08)60020-2. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Eddleman H. L., Champe S. P. Components in T4-infected cells associated with phage assembly. Virology. 1966 Nov;30(3):471–481. doi: 10.1016/0042-6822(66)90123-1. [DOI] [PubMed] [Google Scholar]
- Eiserling F. A., Geiduschek E. P., Epstein R. H., Metter E. J. Capsid size and deoxyribonucleic acid length: the petite variant of bacteriophage T4. J Virol. 1970 Dec;6(6):865–876. doi: 10.1128/jvi.6.6.865-876.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARRANT J. L. An electron microscopic study of ferritin. Biochim Biophys Acta. 1954 Apr;13(4):569–576. doi: 10.1016/0006-3002(54)90376-5. [DOI] [PubMed] [Google Scholar]
- FRANKEL F. R. An unusual DNA extracted from bacteria infected with phage T2. Proc Natl Acad Sci U S A. 1963 Mar 15;49:366–372. doi: 10.1073/pnas.49.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest G. L., Cummings D. J. Head proteins from T-even bacteriophages. II. Physical and chemical characterization. J Virol. 1971 Jul;8(1):41–55. doi: 10.1128/jvi.8.1.41-55.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D. An upper limit to the protein content of the germinal substance of bacteriophage T2. Virology. 1955 May;1(1):108–127. doi: 10.1016/0042-6822(55)90009-x. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D. Some minor components of bacteriophage T2 particles. Virology. 1957 Oct;4(2):237–264. doi: 10.1016/0042-6822(57)90061-2. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda J., Mathews E. DNA replication in vivo by polynucleotide-ligase defective mutants of T4. II. Effect of chloramphenicol and mutations in other genes. J Mol Biol. 1971 Jan 28;55(2):155–179. doi: 10.1016/0022-2836(71)90189-6. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. 3. Studies with small phage particles. J Mol Biol. 1965 Nov;14(1):120–129. doi: 10.1016/s0022-2836(65)80234-0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., BOLLE A., BOYDELATOUR E., EPSTEIN R. H., FRANKLIN N. C., JERNE N. K., REALE SCAFATI A., SECHAUD J. FUNCTIONS AND PROPERTIES RELATED TO THE TAIL FIBERS OF BACTERIOPHAGE T4. Virology. 1965 Jul;26:419–440. doi: 10.1016/0042-6822(65)90006-1. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Der Kamp C. K.-V. On a modification of the gene product P23 according to its use as subunit of either normal capsids of phage T4 or of polyheads. FEBS Lett. 1970 Jun 1;8(3):140–144. doi: 10.1016/0014-5793(70)80247-2. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. Studies on the morphopoiesis of the head of phage T-even. V. The components of the T4 capsid and of other, capsid-related structures. Virology. 1968 Mar;34(3):549–561. doi: 10.1016/0042-6822(68)90074-3. [DOI] [PubMed] [Google Scholar]
- LEVINE L., BARLOW J. L., VAN VUNAKIS H. An internal protein in T2 and T4 bacteriophages. Virology. 1958 Dec;6(3):702–717. doi: 10.1016/0042-6822(58)90116-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Beguin F., Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970 Jan 14;47(1):69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Larcom L. L., Bendet I. J., Mumma S. Subunits of T4 head structures. Virology. 1970 May;41(1):1–11. doi: 10.1016/0042-6822(70)90048-6. [DOI] [PubMed] [Google Scholar]
- Luftig R. B. Further studies on the dimensions of viral and protein structures using the catalase crystal internal marker technique. J Ultrastruct Res. 1968 Apr;23(1):178–181. doi: 10.1016/s0022-5320(68)80041-3. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Moody M. F. The shape of the T-even bacteriophage head. Virology. 1965 Aug;26(4):567–576. doi: 10.1016/0042-6822(65)90319-3. [DOI] [PubMed] [Google Scholar]
- Mosig G. A map of distances along the DNA molecule of phage T4. Genetics. 1968 Jun;59(2):137–151. doi: 10.1093/genetics/59.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. Distances separating genetic markers in T4 DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1177–1183. doi: 10.1073/pnas.56.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. Recombination in bacteriophage T4. Adv Genet. 1970;15:1–53. doi: 10.1016/s0065-2660(08)60070-x. [DOI] [PubMed] [Google Scholar]
- Mosig G., Werner R. On the replication of incomplete chromosomes of phage T4. Proc Natl Acad Sci U S A. 1969 Oct;64(2):747–754. doi: 10.1073/pnas.64.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousset S., Thomas R. Ter, a function which generates the ends of the mature lambda chromosome. Nature. 1969 Jan 18;221(5177):242–244. doi: 10.1038/221242a0. [DOI] [PubMed] [Google Scholar]
- Parma D. H. The structure of genomes of individual petit particles of the bacteriophage T4D mutant E920/96/41. Genetics. 1969 Oct;63(2):247–261. doi: 10.1093/genetics/63.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. B., Berger H. Replication of gene 46-47 amber mutants of bacteriophage T4D. J Mol Biol. 1971 Apr 14;57(1):17–34. doi: 10.1016/0022-2836(71)90117-3. [DOI] [PubMed] [Google Scholar]
- Shalitin C., Kahana S. Conversion of T4 gene 46 mutant deoxyribonucleic acid into nonviable bacteriophage particles. J Virol. 1970 Sep;6(3):353–362. doi: 10.1128/jvi.6.3.353-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L. DNA virus maturation. J Cell Physiol. 1969 Oct;74(2 Suppl):21–32. doi: 10.1002/jcp.1040740404. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Cummings D. J. Isolation and characterization of two basic internal proteins from the T-even bacteriophages. J Virol. 1970 Oct;6(4):445–454. doi: 10.1128/jvi.6.4.445-454.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. A., Jr The rule of the ring. J Cell Physiol. 1967 Oct;70(2 Suppl):13–33. doi: 10.1002/jcp.1040700404. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Jr, Anderson T. F. Morphological variants of coliphage P1. J Virol. 1970 Jun;5(6):765–782. doi: 10.1128/jvi.5.6.765-782.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Jr, Mosig G., Bayer M. E. Bacteriophage T4 head models based on icosahedral symmetry. J Virol. 1972 May;9(5):872–875. doi: 10.1128/jvi.9.5.872-875.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]