Abstract
The self-perceived physical health of mothers raising children with developmental delay (DD; n = 116) or typical development (TD; n = 129) was examined across child ages 3–9 years, revealing three main findings. First, mothers of children with DD experienced poorer self-rated physical health than mothers of children with TD at each age. Latent growth curve analyses indicated that mothers in the DD group experienced poorer health from age 3 but that the two groups showed similar growth across ages 3–9 years. Second, cross-lagged panel analyses supported a child-driven pathway in early childhood (ages 3–5) by which early mother-reported child behavior problems predicted poorer maternal health over time, while the reversed, health-driven path was not supported. Third, this cross-lagged path was significantly stronger in the DD group, indicating that behavior problems more strongly impact mothers’ health when children have developmental delay than when children have typical development. The health disparity between mothers of children with DD versus TD stabilizes by child age 5 and persists across early and middle childhood. Early interventions ought to focus on mothers’ well-being, both psychological and physical, in addition to child functioning.
Keywords: Children with developmental delays, maternal health, early childhood
1. Introduction
Mothers of children with developmental delay (DD) experience poorer psychological well-being compared to other mothers (Baker et al., 2003; Hauser-Cram, Warfield, Shonkoff, & Krauss, 2001); however, little is known about these mothers’ physical well-being. The present study examines associations between child DD, child behavior problems, and mothers’ perceived physical health across early and middle childhood.
1.1 Psychological Well-Being Among Mothers of Children with DD
Parents of children with DD face increased stress, depression, and other negative psychological outcomes relative to parents of children with typical development (TD; e.g. Baker, Blacher, Crnic, & Edelbrock, 2002; Olsson & Hwang, 2001). At the same time, individual and contextual factors, such as child adaptive functioning (Boyce, Behl, Mortensen, & Akers, 1991), parents’ social support (Manuel, Naughton, Balkrishnan, Smith, & Koman, 2003), and quality of support services (Mitchell & Hauser-Cram, 2008), contribute to variability in parent well-being, and many parents experience marked resilience in the face of child DD or behavior problems (Broberg, Blacher, & Emerson, 2009; Gerstein, Crnic, Blacher, & Baker, 2009).
Children and adults with intellectual disability (ID) have three to four times the rates of psychological disorder as their peers with typical cognitive development (e.g. Dekker, Koot, van der Ende, & Verhulst, 2002), a discrepancy that emerges as early as age 3 in the form of elevated rates of behavior problems (Baker et al., 2002). Researchers have repeatedly found that the presence of child DD itself does not account for poorer maternal well-being; rather, it is this increased child psychopathology that negatively affects maternal well-being (e.g. Baker et al., 2003; Peters-Scheffer, Didden, & Korzilius, 2012). Lower socio-economic resources and social capital also exacerbate the poorer well-being of parents of children with DD (Emerson, 2003) in ways that may worsen over time as child-related stressors and limitations take a cumulative toll on family resources (Emerson, Hatton, Llewellyn, Blacher, & Graham, 2006).
1.2 Physical Health among Mothers of Children with DD
In the limited research that has focused on the impact of raising a child with DD on mothers’ physical health, mothers of children with DD have reported lower physical health relative to fathers of children with DD and mothers and fathers of children with TD (Olsson & Hwang, 2008). More broadly, raising a child with a chronic health condition or disability has been associated with cellular aging (Epel et al., 2004) and cortisol dysregulation (Seltzer et al., 2010), factors that are in turn linked to immune suppression and cardiovascular disease (Djuric et al., 2008). Among older mothers of adult children with DD, the physical health risks are well-documented, and include greater reported somatic symptoms (e.g. headaches, backaches, trouble sleeping, joint stiffness) (Ha, Hong, Seltzer, & Greenberg, 2008) and arthritis-related limitations (Magaña & Smith, 2006) than parents of offspring with TD. In fact, while differences in psychological health attenuate over time, health differences persist at later ages (Ha et al., 2008).
Stressors associated with child disability and behavior problems may negatively affect mothers’ physical well-being in a delayed, cumulative fashion (see Taylor, Repetti, & Seeman, 1997 for a review of chronic stress and health), as the effects of stress on physical health often emerge approximately two years after the stressor itself (McEwen, 2000). Indeed, in previous work with the present sample, child DD at age three predicted poorer perceived maternal health two years later (Eisenhower, Baker, & Blacher, 2009). As with psychological well-being, the effects on physical well-being may be accounted for by the heightened child behavior problems among children with DD, rather than by the DD itself (Eisenhower et al., 2009). In the present study we examine contributions of child developmental status (DD vs. TD), behavior problems, and their interaction in predicting maternal health over time from child age 3 to 9 years.
1.3 Health Changes Over Time for Mothers of Children With and Without DD
We examine trajectories of mothers’ health as children enter formal schooling and continue through elementary school. Challenges facing families during these years include new school demands, emerging relationships with teachers and peers, and children’s increased independence from parents (Rimm-Kaufman & Pianta, 2000). The transition to school may be particularly stressful for families of children with DD as they seek and manage services to address children’s special education needs (McIntyre, Blacher, & Baker, 2006).
Reciprocal associations between child characteristics and parental well-being over time are well-established (Gross, Shaw, Moilanen, Dishion, & Wilson, 2008; Sameroff, 2009), including links between child behavior problems and parenting stress across early childhood (Baker et al., 2002) and middle childhood (Neece, Green, & Baker, 2012). However, unlike the association with parent stress, the association with parent health may be more unidirectional and driven by child characteristics. In the current paper, we examine trajectories of maternal health to determine whether mothers of preschool- and school-age children with DD experience poorer physical health across child ages 3 to 9 years than mothers of children with TD. We also examine whether child behavior problems predict maternal health, both independently and in interaction with child developmental delay, and whether this association is transactional over time versus unidirectional. Given that effects of chronic stress on health are often delayed and cumulative, we examine these relations longitudinally, applying cross-lagged panel analysis in order to shed light on causal processes (Kline, 2010).
2. Material and Methods
2.1 Participants
Participants were 245 children (59.2% boys) and families who enrolled in the Collaborative Family Study at either age 3 (N = 225) or age 5 (N = 20). Based at three universities (Pennsylvania State University, University of California Los Angeles, and University of California Riverside), the study included samples drawn from Central Pennsylvania (24%) and Southern California (76%). Families were assessed annually at child ages 3 through 9 years. Children were classified as having developmental delay (DD; N = 116) or typical development (TD; N = 129). Families of children with DD were recruited through regional agencies that provided diagnostic and intervention services for individuals with developmental disabilities. Selection criteria for the DD group were that the child (a) score 40–84 on a test of developmental functioning; (b) be ambulatory; and (c) not be diagnosed with autism. Families of children with typical development (TD) were recruited through preschools and daycare programs; selection criteria were that the child: (a) score 85 or above on a test of developmental functioning; and (b) not be born prematurely or have a developmental disability.
Table 1 shows demographics. Children recruited at age 5 did not significantly differ from those recruited at age 3 with DD on any of these demographic factors. Drawn from an original sample of 258, only families with behavior problems and maternal health data together at a minimum of two time points (N = 245) were analyzed. These families did not differ from excluded families on any demographics shown in Table 1 or on age 3 behavior problems, maternal health, or developmental status. Parents reported child race on a closed-ended, five-option question as Black or African-American (7.8%), Asian (2.4%), Hispanic/Latino(a) (15.5%), White non-Hispanic (60.8%), or other or multiple races (13.5%). Recruitment initially focused on intact families, so most parents (84.9%) were married; 46.9% of mothers and 50.6% of fathers had graduated from college, and 50.6% of families had an annual income of $50,000 or more. Most mothers (54.7%) were employed.
Table 1.
Demographics by Developmental Status at Time of Enrollment (n=245).
| Typical Development (n = 129) |
Developmental Delay (n = 116) |
DD vs. TD difference |
|
|---|---|---|---|
| Child variables: | |||
| Gender (% girls) | 47.7 | 33.3 | χ2 = 5.04* |
| Race (% White) | 60.5 | 56.9 | χ2 = 0.32 |
| Mean (SD) BSID, age 3 | 104.1 (12.2) | 60.3 (13.0) | t = 25.95*** |
| Mean (SD) Stanford Binet IQ, age 5 | 104.0 (12.0) | 65.7 (19.0) | t =18.32*** |
| Family variables: | |||
| % with siblings at age 5 | 72.1 | 69.8 | χ2 = 0.14 |
| Mother age at child age 5, M (SD) | 36.1 (5.6) | 35.0 (6.3) | t = 1.42 |
| Mother education (% college degree) | 61.2 | 31.0 | χ2 = 22.38*** |
| Father education (% college degree) | 62.0 | 37.9 | χ2 = 14.17*** |
| Maternal employment (% employed) | 64.8 | 47.7 | χ2 = 4.71* |
| Marital status (% married) | 89.1 | 80.2 | χ2 = 3.84† |
| Family income (% earning $50,000+) | 62.1 | 37.9 | χ2 = 8.98** |
p < 0.10,
p < 0.05,
p < 0.01,
p < 0.001
2.2 Procedures
All procedures were approved by the IRBs of the three participating universities (UCLA, UC Riverside, Penn State University). Informed consent was obtained at the start of the first assessment. Developmental status was determined through home-based child assessments, and mother-report questionnaires assessing behavior problems and maternal health were completed during annual visits held alternately at the lab or the home.
2.2.1. Child Developmental Status
For children enrolled at age 3, developmental status was evaluated with the Bayley Scales of Infant Development-II (BSID-II; Bayley, 1993), an assessment of mental and motor development in children aged 1 to 42 months. These were administered by Clinical Psychology graduate students and Master’s-level clinicians who were supervised by a licensed psychologist; there were 1–3 assessors per site. Only mental development items were given. The Mental Development Index (MDI) is normed with a mean of 100 and standard deviation of 15. Bayley (1993) reported high short-term test-retest reliability for the mental index (r = .91). For children enrolled at age 5, developmental status was assessed with the Stanford-Binet Intelligence Scale-Fourth Edition (SB-IV; Thorndike, Hagen, & Sattler, 1986), an assessment tool that has high internal consistency (Glutting, 1989) and good evidence of validity (Thorndike et al., 1986). It is well-suited to the evaluation of children with DD because the examiner adapts starting points to the child’s developmental level. The SB-IV yields a Composite IQ score (normative mean = 100, SD = 15). Children’s scores on either test were classified in the DD (scores of 40–84) or TD (85 and above) group.
2.2.2. Child Behavior Problems
The Child Behavior Checklist was completed by mothers annually, including the preschool version (ages 1.5–5; 99 items; Achenbach, 2000) at ages 3–5 and the older version (ages 6–18; 113 items; Achenbach & Rescorla, 2001) at ages 6–9. Each item describes a child problem; parents indicate whether the item is (0) not true, (1) somewhat or sometimes true, or (2) very true or often true, now or in the past two months. Only total problem scores were utilized; these were converted to T scores (normative mean = 50, SD = 10). T scores of 60 and above fell in the borderline or clinical range. Total score alphas for ages 3–9 ranged from 0.94 to 0.96.
2.2.3. Maternal Health
At annual assessments, mothers responded to a single-item measure evaluating global physical health: “How is your physical health in general?” The response scale is: 1=Poor; 2=Fair; 3=Good; 4=Excellent. This measure of self-perceived physical health has been shown to predict morbidity and mortality across a range of diseases and populations (see Bailis, Segall, & Chipperfield, 2003 and Idler & Benyamini, 1997 for reviews), is strongly correlated with other health indicators and health behaviors (Franzini & Fernandez-Esquer, 2004; Manderbacka, Lundberg, & Martikainen, 1999), and has correlated 0.70 with a physical examination (Multidimensional Functional Assessment, 1978). It and similar 3-point and 5-point measures are widely used as indicators of self-perceived health; high construct validity has been demonstrated among ethnically diverse individuals (e.g. Bzostek, Goldman, & Pebley, 2007; Mulvaney-Day, Alegria, & Sribney, 2007), parents of young children (e.g. Belzeval, 1998; Neises & Gruneberg, 2005; Waters et al., 2000), and parents of individuals with ID (e.g. Chen, Ryan-Henry, Heller, & Chen, 2001; Seltzer & Krauss, 1989).
3. Results
All analyses were conducted with MPlus using full information maximum likelihood (FIML) to estimate missing data; FIML has been demonstrated to be a robust estimator in SEM that performs better than pairwise deletion, listwise deletion, or similar-response-pattern imputation (Enders & Bandalos, 2001; Schlomer, Bauman, & Card, 2010). Results utilize maximum likelihood estimates (MLE). All models were also run using maximum likelihood with robust errors (MLR) revealing virtually identical results; thus, only MLE results are reported. Outliers (scores > 3 SD from the sample mean) were included in all reported analyses; results were virtually identical when outliers were set to +/− 3 SD from the mean.
Across families, an average of 15.8% of maternal health data and 18.5% of child behavior problems data was missing at each time point, ranging from 2.4% to 29.0% of health data and 4.5% to 31.4% of behavior problems data across ages.
DD and TD groups differed on key demographic variables (mother’s education, fathers’ education, family income, maternal employment) shown in Table 1. Of these variables, maternal education, paternal education, and family income also related to maternal health at one or more ages. These three variables were highly correlated with one another and, after controlling for maternal education, family income and paternal education were no longer correlated with maternal health; thus, only maternal education, for which the most complete data were available, was converted to a z score and covaried when noted. The maternal education variable was based on education data at child age 3 or, when age 3 education data were unavailable, at child age 5.
3.1 Differences in Maternal Health by Child Developmental Delay and Behavior Problems
Our first question asked whether mothers of preschool- and school-age children with DD experience poorer physical health across child ages 3 through 9 than mothers of children with TD. Independent samples t-tests indicated significant differences in maternal health between DD and TD groups across ages 3–9, although significance was only marginal at age 8. At all ages, mothers of children with DD reported poorer health than mothers of children with TD. Hierarchical linear regressions indicated that significant associations between developmental status and maternal health persisted at ages 3, 4, and 7 in the presence of the maternal education covariate with standardized betas of −0.15 (p = 0.021), −0.15 (p = 0.028), and −0.18 (p = 0.012), respectively, but were no longer significant at ages 5, 6, 8, and 9.
We also asked whether mothers whose children had elevated behavior problems (BP) showed poorer physical health than mothers of children with lower BP at these ages. Correlations between BP and maternal health were significant at each age (rs ranged from −0.21 to −0.36, ps = 0.004 to < 0.001). Hierarchical linear regressions showed that mothers of children with elevated BP continued to report significantly poorer health at each age after covarying maternal education; in the final model, standardized betas ranged from −0.20 to −0.35 for behavior problems (ps = 0.005 to < 0.001).
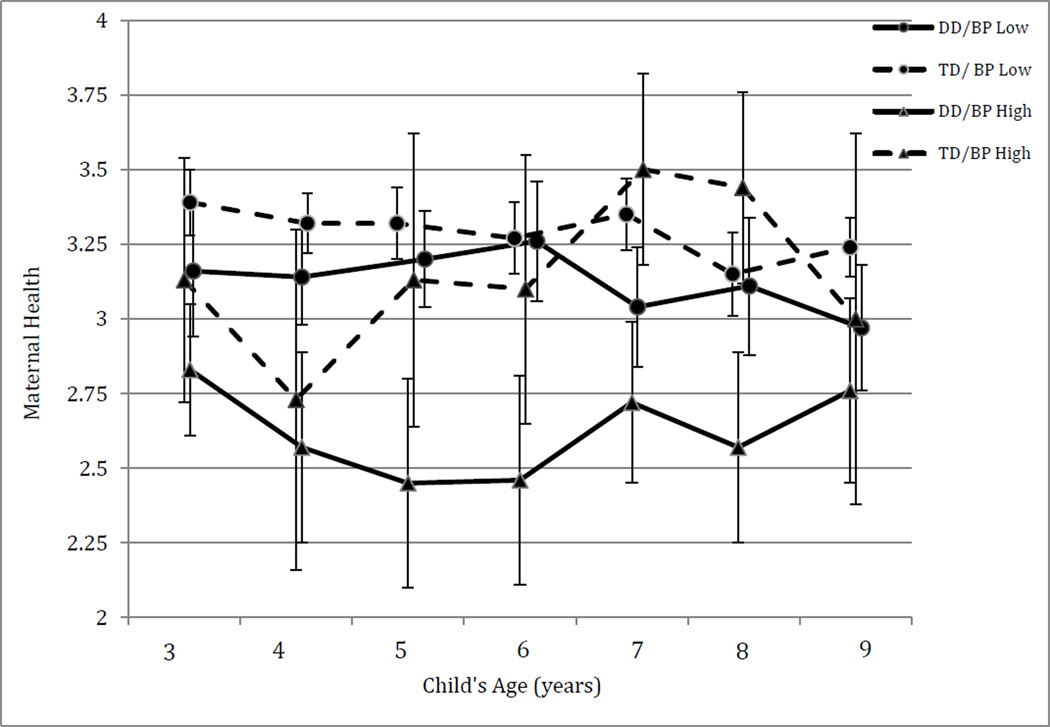
An ANOVA with four groups based on BP group (non-clinical vs. borderline or clinical) and developmental status (DD vs. TD) was conducted on maternal health at each age; see Table 2. To correct for multiple ANOVAs we applied a more conservative threshold of p < .01 for significance. BP group had significant effects on maternal health at ages 3 and 4, while developmental status had significant effects on maternal health at ages 5, 7, and 8. The interaction of developmental status and BP group on maternal health showed a trend toward significance at ages 5 and 8. At these ages, the group with both DD and high BP showed poorer maternal health than one or more of the other three groups. Figure 1 shows means for the four groups over time.
Table 2.
Mean Maternal Health Ratings Across Developmental Delay - Behavior Problem Profiles at Child Ages 3–9 Years.
| Typical Development | Developmental Delays | ||||||
|---|---|---|---|---|---|---|---|
| Low BP | High BP | Low BP | High BP |
F (Status) |
F (BP) |
F (StxBP) |
|
| age 3 | 3.39a | 3.06ab | 3.15ab | 2.83b | 4.19* | 8.13** | 0.00 |
| age 4 | 3.26a | 2.94ab | 3.09a | 2.58b | 5.38* | 13.04*** | 0.74 |
| age 5 | 3.19a | 3.19a | 3.15a | 2.55b | 7.04** | 5.63* | 5.72* |
| age 6 | 3.27a | 3.11ab | 3.17a | 2.74b | 3.32† | 5.43* | 1.09 |
| age 7 | 3.37a | 3.18ab | 3.02b | 2.83b | 10.50** | 3.10† | 0.00 |
| age 8 | 3.16a | 3.35a | 3.09ab | 2.71b | 6.97** | 0.48 | 4.30* |
| age 9 | 3.28a | 3.05ab | 3.02ab | 2.74b | 5.46* | 4.35* | 0.06 |
Note. Different superscripts indicate significant mean differences between groups at p < .05 on Tukey HSD post-hoc tests.
p < 0.10,
p < 0.05,
p < 0.01,
p < 0.001.
Figure 1.
Mean scores for maternal health across child ages 3–9 by developmental status (DD versus TD) and behavior problems (BP) level. Health scale ranges from 1 (poor) to 4 (excellent).
3.2 Trajectories of Maternal Health Over Time
Multiple-group latent growth curve (LGC) analysis was undertaken to examine the differences in initial level of maternal health and trajectories of change in health for mothers of children with DD versus TD. Models were tested for goodness of fit using the comparative fit index (CFI) and the root mean-square error of approximation (RMSEA). Values greater than .90 for the CFI and values less than or equal to .08 for the RMSEA are generally considered to indicate acceptable model fit. We also report the χ2 fit statistic for all models and change in χ2 (Δχ2) in order to compare nested models. Although the χ2 statistic can be an inadequate measure of model fit in larger samples, it is an effective marker of change in goodness of fit across nested models. Throughout the paper, beta values reported in the text represent standardized estimates.
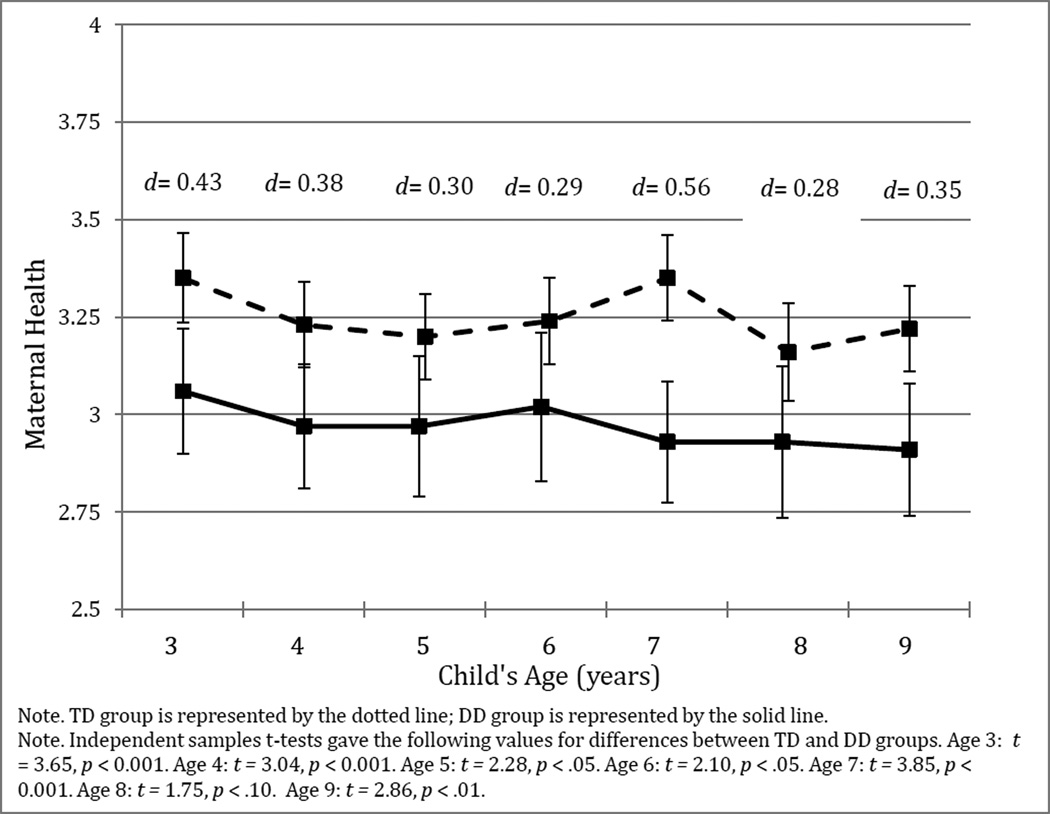
In our first model, all parameters were constrained to be equal across the two groups in order to test equality in a strict sense. The slope of maternal health was fixed at 0 for age 3, at 1 for age 4, age 2 for age 5, at 3 for age 6, at 4 for age 7, at 5 for age 8, and at 6 for age 9, and the intercept was fixed at 1 for each age [see Bollen & Curran (2006) and Duncan, Duncan, Stryker, Li, & Alpert (1999) for introductions to this approach]. This level and shape model allows us to test for linearity of the model; the intercept (or level) is initial maternal health at age 3, while the slope (or shape) represents change in maternal health across time. Maternal education was covaried with maternal health at each age. Model fit was adequate on both indices [CFI = .953; RMSEA = .066 (.038; .091)], and χ2(65, N of TD group = 129, N of DD group = 116) = 99.70, p = 0.004, suggesting that fit may be improved by unconstraining parameters between groups. We next unconstrained all paths across the two groups. The unconstrained model fit was good [CFI = .982; RMSEA = .048 (.000; .081); χ2(46, N of TD group = 129, N of DD group = 116) = 58.85, p = 0.097], indicating that the intercept and slope of maternal health were adequately explained by a linear trajectory. A Δχ2 test showed that unconstraining all paths significantly improved fit over the constrained model [Δχ2(19) = −40.85, p = 0.01]. This significant difference indicates that the intercepts or slopes for the DD and TD groups significantly differ from one another. Follow-up tests, in which only the intercept or slope were unconstrained, indicated that the TD and DD groups differed significantly in their intercept [Δχ2(1) = 92.56 – 99.70 = −7.14, p < 0.01], with the DD group having significantly lower means than the TD group, but the two groups did not differ in the slope of their growth in maternal health [Δχ2(1) = 98.93 – 99.70 = −0.77, p = ns]. As shown in Figure 2, this pattern indicates that the maternal health deficit in the DD group is present at age 3 and that changes thereafter are essentially parallel for the two groups.
Figure 2.
Mean scores for maternal health across child ages 3–9 by developmental status (DD vs. TD).. Health scale ranges from 1 (poor) to 4 (excellent). Cohen’s d values represent effect sizes for developmental status on maternal health.
The intercept and slope were unrelated to one another in both the DD and TD groups. Consistent with the 4-point scale of maternal health, the intercept was greater than zero in both the TD (B = 2.50, SE = .31, β = 5.47, p < 0.001) and DD (B = 1.56, SE = .38, β = 3.04, p < 0.001) groups, while the slope did not differ from zero in either the TD (B = −0.05, SE=0.06, β = −1.15, p = 0.37) or DD (B= 0.11, SE = 0.09, β = 1.30 p = 0.22) group. The intercept by slope interaction was not significant for either the TD (B = 0.001, SE = .005, β = 0.06, p = 0.83) or the DD (B = −0.004, SE = .009, β = −0.10, p = 0.67) group.
3.3 Behavior Problems and Maternal Health Over Time
We next examined relationships over time between child behavior problems T scores and maternal health. We utilized multi-group, cross-lagged panel analyses to examine potential bi-directional effects of maternal health and behavior problems over time. Models were designed to answer two questions. First, for both the DD group and the TD group, do early child behavior problems predict changes in subsequent maternal health (a child-driven relationship), or is the opposite path, in which early maternal health predicts changes in child behavior problems, better supported (a health-driven relationship)? Second, is either pathway stronger for the DD group than for the TD group? For instance, do child behavior problems predict changes in health for mothers of children with DD more strongly than for mothers of children with TD?
To test the first question, we included two sets of cross-lagged paths: The path from behavior problems at the first age (e.g. 3) to maternal health at the second age (e.g. 5), and the path from maternal health at the first age to behavior problems at the second age. To test the second question, we ran the model first with the cross-lagged paths constrained across the two groups and next with the cross-lagged paths unconstrained. A significant decrease in χ2 coefficients between models would indicate an improvement in model fit when the cross-lagged paths are allowed to vary freely between groups, suggesting that the cross-lagged paths significantly differ between groups. We tested a multiple-wave, cross-lagged model across ages 3–9; in line with findings that stressors have a two-year delayed, cumulative impact on health, we included two-year cross-lagged paths between child behavior problems and maternal health. In each model, we estimated correlations between measures taken at the same age, as well as one- and two-year autoregressions for each variable across time. The maternal education covariate was allowed to correlate with both maternal health and child behavior problems at each age.
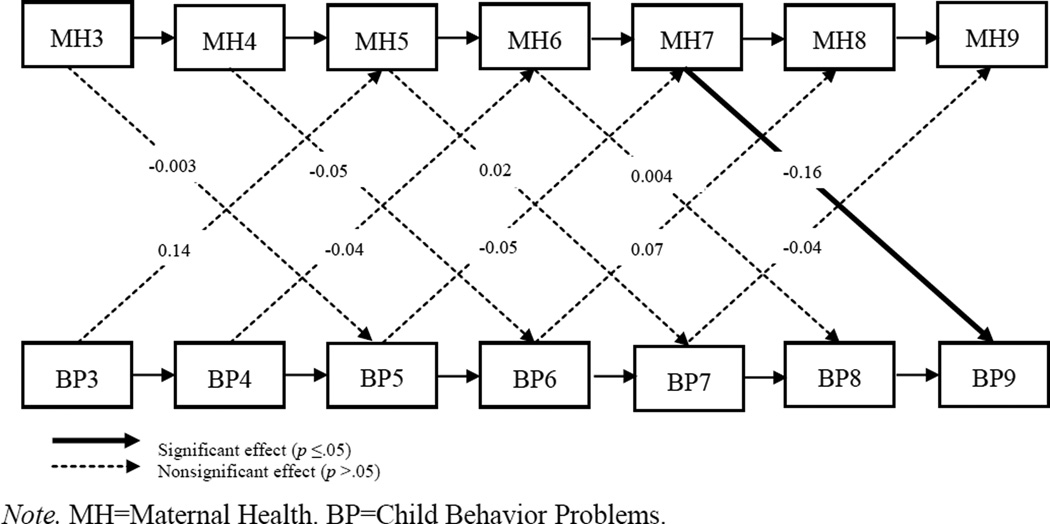
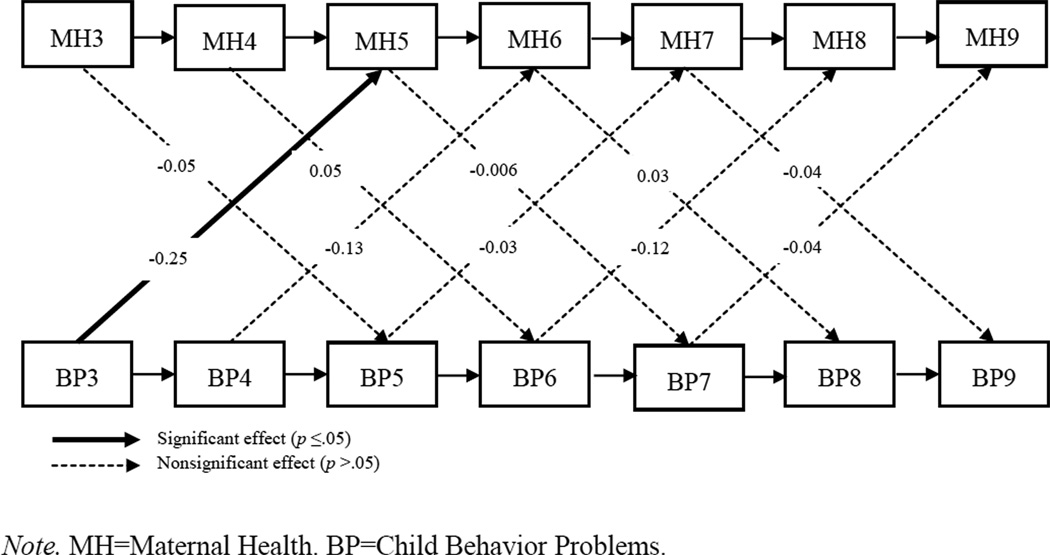
In the constrained model, fit was adequate on both indices [CFI = 0.941; RMSEA = 0.077 (C.I. 0.062 – 0.093)], and χ2(157, N = 245) = 272.47, p < 0.001. None of the cross-lagged paths from child behavior problems to maternal health were significant in the combined sample. Only one cross-lagged path from maternal health to child behavior problems was significant in the combined sample: maternal health at age 7 predicted child behavior problems at age 9 (β = −1.34, p = 0.032). We next tested whether the paths were equivalent across the two groups by unconstraining the cross-lagged paths. Model fit was again adequate on both indices [CFI = 0.948; RMSEA = 0.075 (C.I. 0.059 – 0.008)], and χ2(147, N = 129 for TD group, N = 116 for DD group) = 248.44, p < 0.001. In the TD group, behavior problems did not predict maternal health at any ages, although the path from behavior problems to maternal health was marginally significant at ages 3 to 5 (B = 0.010, SE = 0.005, β = 0.14, p = 0.052) in the unexpected direction. Meanwhile, maternal health at age 7 predicted child behavior problems at age 9 (B = −2.83, SE = 1.00, β = −0.16, p = 0.005) but not at any other ages. In the DD group, the child-driven hypothesis was supported only at ages 3–5: age 3 behavior problems significantly predicted age 5 maternal health (B = −0.02, SE = 0.006, β = −3.45, p = 0.001) in the hypothesized direction; this association dropped to marginal significance by age 4, with age 4 behavior problems marginally predicting age 6 maternal health (B = −0.01, SE = 0.005, β = −0.13, p = 0.083), and was non-significant at subsequent ages. Maternal health did not predict child behavior problems at any of the ages examined for the DD group. Thus, the hypothesized child-driven path was supported in the DD group across ages 3 to 5 but was not supported in the TD group; the health-driven path was supported only in the TD group across ages 7 to 9. Figures 3 and 4 show the cross-lagged models for both groups.
Figure 3.
Standardized estimates of cross-lagged panel model predicting maternal health and child behavior problems at ages 3–9: typically development group. Maternal education, not shown, was covaried at each age.
Figure 4.
Standardized estimates of cross-lagged panel model predicting maternal health and child behavior problems at ages 3–9: developmental delay group. Maternal education, not shown, was covaried at each age.
Next, a χ2 difference test comparing the constrained and unconstrained model was significant [Δχ2(10) = 24.02, p < 0.001], indicating that the unconstrained model is a better fit for the data than the constrained model. This signifies that the cross-lagged paths were significantly different across the DD and TD groups. In other words, the association between child behavior problems and maternal health over time was significantly moderated by children’s developmental status. Follow-up tests, in which each significant cross-lagged path was unconstrained one at a time, confirmed that the BP3 → MH5 path significantly differed between the DD and TD groups, as shown by the significant reduction in χ2 value when this path was unconstrained [Δχ2(1) = 14.64, p < 0.001]; meanwhile, the MH7 → BP9 cross-lagged path did not significantly differ between groups [Δχ2(1) = 3.47, p = ns]. Thus, it is the BP3→ MH5 path (the child-driven path) that accounts for the improvement in fit observed between constrained and unconstrained models. Thus, the association between early behavior problems and later maternal health was moderated by the presence of DD, such that the combination of both DD and elevated behavior problems at age 3 was associated with particularly poor maternal health at child age 5.
Both the constrained and unconstrained models were also run with CBCL behavior problems as a binary variable, comparing scores of 60 or higher (borderline or clinical range) and scores below 60 (non-clinical range). Model fit was very similar, as was the difference in χ2 values between constrained and unconstrained models.
4. Discussion
Across early and middle childhood, the self-perceived physical health of mothers raising children with developmental delay (DD) was poorer than that of mothers raising children with typical development (TD). Latent growth curve analyses indicated that both groups showed similar, linear health trajectories over time. Just as differences in mothers’ psychological well-being emerge as early as age 3 (Baker et al., 2003), the disparity in mothers’ physical health was also evident at age 3 and persisted throughout early and middle childhood.
Behavior problems were a stronger predictor of health for mothers of children with DD than for mothers of children with TD. Thus, the physical toll of child behavior problems appears to be greater for mothers in the context of child DD than when these behavior problems occur in the context of otherwise typical development. For children with DD and their mothers, cross-lagged models revealed that early behavior problems drove changes in maternal health over time, suggesting a causal impact of child behavior on maternal health, though an earlier-occurring factor or unexamined variable may also be a causal agent (Little, Preacher, Selig, & Card, 2007). These effects emerged early: the path between behavior problems and subsequent maternal health was significant during the early childhood years (3 to 5) but not during middle childhood.
4.1 Clinical implications
Our findings highlight the importance of intervening early with families affected by child DD. Interventions should address the caregiver’s needs as well as the child’s; in addition, family-level intervention should consider physical well-being, not only psychological well-being, of parents. Further, such intervention should happen early, before or during the preschool years; waiting until children are school-age appears to be too late, as the impact of child behavior on maternal health emerges early. Numerous evidence-based health promotion programs used with other chronically stressed groups are brief and cost-effective (Goldgruber & Ahrens, 2010; Hope, Kelleher, & O’Connor, 1999), but few studies have examined such programs’ effectiveness for parents of children with DD, suggesting this as a valuable area for future study. Intervention directed jointly at child behavior problems and maternal stress is also vital, as these two factors interact in families affected by DD (Neece et al., 2012) and are likely related to maternal health.
Results suggest that maternal health is a worthwhile target of screening as well as intervention. Providers should monitor both psychological and physical well-being of parents, especially when child behavior problems are present, to gauge concurrent distress and to prevent exacerbation of health problems. Such health problems may become particularly acute as the child with DD becomes older and the extension of parental caregiving activities into midlife becomes non-normative (Seltzer, Krauss, Choi & Hong, 1996). Assessing self-perceived health is brief and inexpensive relative to the potential benefits of early intervention to the mother, the child, and the family.
Child behavior problems most strongly predicted maternal health at ages 3–5, coinciding with children’s entry into formal schooling, perhaps due to heightened demands on parents during this period. Indeed, the transition to kindergarten, when accompanied by disruptions in routines, predicts mothers’ cortisol dysregulation, a biomarker of health risk (DeCaro & Worthman, 2011). Families face uncertainties and daily stressors in adjusting to a new school setting (Rimm-Kaufman & Pianta, 2000) and in identifying and advocating for services to meet their children’s educational needs (Stoner & Angell, 2006). Later transitions, such as the transition out of school in young adulthood, are also periods of added stress (Kraemer & Blacher, 2008); monitoring mothers’ symptoms may be especially critical during such transitions.
4.2 Limitations and Future Directions
This study’s reliance on a brief, self-report measure of physical health is both a strength and a limitation. To understand self-perceptions, a self-report measure is crucial; the strength of our findings also suggests that such screening can be brief and cost-effective. Yet a more detailed tool would reveal the domains in which health is most compromised. Moreover, comparison with an objective indicator of health would clarify the extent to which self-reports reflect the negative cognitions that co-occur with psychosocial distress. Future research should ideally incorporate data from primary care providers, physiological markers, or a behaviorally-anchored self-report measure. Additional progress could be made by considering mechanisms by which child behavior and DD may relate to maternal health, such as behavioral, psychosocial, or lifestyle factors. For instance, mothers of children with DD are susceptible to frequent, chronic sleep disruption (Gallagher, Phillips, & Carroll, 2010); they may also experience the endocrine dysregulation and immune suppression linked to chronic and acute stress (Seltzer et al., 2010). Child DD or behavior problems may also interfere with maternal health behaviors such as exercise (Burton, Newsom, Schulz, Hirsch, & Garmen, 1997), healthy eating (Lee, Colditz, Berkman, & Kawachi, 2003), obtaining routine and non-routine medical care (Magaña & Smith, 2008), and the ability to obtain social support (Gallagher, Phillips, Oliver, & Carroll, 2008), engage in self-care, or hold other fulfilling social roles (Eisenhower & Blacher, 2006).
4.3 Conclusions
Findings indicate that mothers of children with DD experience poorer physical health than mothers of typically-developing children. This health disparity is present by child age 3 and persists across early and middle childhood; the comorbid behavior problems common among children with DD may exacerbate this difference. These elevated behavior problems among children with DD, when present in early childhood, appear to contribute to subsequent maternal health decrements. In all, mothers of children with DD showed poorer initial health than other mothers, reported greater impact of behavior problems on subsequent health, and failed to catch up to other mothers over time. Findings suggest that intervention for children with DD ought to focus on parent well-being, including physical well-being; in addition, treatment to mitigate child behavior problems may carry over to benefit mothers’ well-being and physical health.
Highlights.
-
▪
Mothers of children with developmental delay (DD) have poorer mental health.
-
▪
We found that mothers of children with DD also have poorer physical health.
-
▪
Mothers whose children have typical development (TD) had better health.
-
▪
This difference in health was present when children were 3 to 9 years old.
-
▪
The higher behavior problems in children with DD may explain mothers’ poorer health.
Acknowledgements
This paper was based on the activities of the Collaborative Family Study, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant number: 34879-1459 (Drs. Keith Crnic, Bruce L. Baker, and Jan Blacher, PIs). We are indebted to our staff, to the doctoral students who worked on this study, and to the families who participated in this longitudinal research. We also thank Nathan Dieckmann for statistical consultation and Esror Tamim Mohammad for assistance with figures and tables.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Manual for the Child Behavior Checklist 1.5-5. Burlington, VT: University of Vermont, Department of Psychiatry; 2000. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age forms and profiles. Research Center for Children, Youth, and Families, University of Vermont; 2001. [Google Scholar]
- Bailis DS, Segall A, Chipperfield JG. Two views of self-rated general health status. Social Science and Medicine. 2003;56(2):203–217. doi: 10.1016/s0277-9536(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Baker B, Blacher J, Crnic KA, Edelbrock C. Behavior problems and parenting stress in families of three-year-old children with and without developmental delays. American Journal on Mental Retardation. 2002;107(6):433–444. doi: 10.1352/0895-8017(2002)107<0433:BPAPSI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Baker BL, McIntyre LL, Blacher J, Crnic K, Edelbrock C, Low C. Pre-school children with and without developmental delay: Behaviour problems and parenting stress over time. Journal of Intellectual Disability Research. 2003;47:217–230. doi: 10.1046/j.1365-2788.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development, Second Edition: Manual. San Antonio: Psychological Corp; 1993. [Google Scholar]
- Belzeval M. The self-reported health status of lone parents. Social Science and Medicine. 1998;46(10):1337–1353. doi: 10.1016/s0277-9536(97)10083-1. [DOI] [PubMed] [Google Scholar]
- Bollen KA, Curran PJ. Latent curve models: A structural equation perspective. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- Boyce GC, Behl D, Mortensen L, Akers J. Child characteristics, family demographics and family processes: Their effects on the stress experienced by families of children with disabilities. Counseling Psychology Quarterly. 1991;4(4):273–288. [Google Scholar]
- Broberg M, Blacher J, Emerson E. Editorial: Resilience. Journal of Intellectual Disability Research. 2009;53:955–956. doi: 10.1111/j.1365-2788.2009.001225.x. [DOI] [PubMed] [Google Scholar]
- Burton LC, Newsom JT, Schulz R, Hirsch CH, German PS. Preventive health behaviors among spousal caregivers. Preventative Medicine. 1997;26:162–169. doi: 10.1006/pmed.1996.0129. [DOI] [PubMed] [Google Scholar]
- Bzostek S, Goldman N, Pebley A. Why do Hispanics in the USA report poor health? Social Science & Medicine. 2007;65(5):990–1003. doi: 10.1016/j.socscimed.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Chen SC, Ryan-Henry S, Heller T, Chen EH. Health status of mothers of adults with intellectual disability. Journal of Intellectual Disability Research. 2001;45(5):439–449. doi: 10.1046/j.1365-2788.2001.00352.x. [DOI] [PubMed] [Google Scholar]
- DeCaro JA, Worthman CM. Changing family routines at kindergarten entry predict biomarkers of parental stress. International Journal of Behavioral Development. 2011;35(5):441–448. [Google Scholar]
- Dekker MC, Koot HM, van der Ende J, Verhulst FC. Emotional and behavioral problems in children and adolescents with and without intellectual disability. Journal of Child Psychology and Psychiatry. 2002;43(8):1087–1098. doi: 10.1111/1469-7610.00235. [DOI] [PubMed] [Google Scholar]
- Djuric Z, Bird CE, Furumoto-Dawson A, Rauscher GH, Ruffin MT, Stowe RP, Tucker KL, Masi CM. Biomarkers of psychological stress in health disparities research. Open Biomark Journal. 2008;1:7–19. doi: 10.2174/1875318300801010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Strycker LA, Li F, Alpert A. An introduction to latent variable growth curve modeling: Concepts, issues, and applications. 2nd ed. Mahwah, NJ: Erlbaum; 1999. [Google Scholar]
- Eisenhower A, Baker BL, Blacher J. Children's delayed development and behavior problems: Impact on mothers' perceived physical health across early childhood. Social Science and Medicine. 2009;68 (1):89–99. doi: 10.1016/j.socscimed.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhower A, Blacher J. Mothers of young adults with intellectual disability: Multiple roles, ethnicity and well-being. Journal of Intellectual Disability Research. 2006;50(12):905–916. doi: 10.1111/j.1365-2788.2006.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson E. Mothers of children and adolescents with intellectual disability: Social and economic situation, mental health status, and the self-assessed social and psychological impact of the child's difficulties. Journal of Intellectual Disability Research. 2003;47(4–5):385–399. doi: 10.1046/j.1365-2788.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- Emerson E, Hatton C, Llewellyn G, Blacher J, Graham H. Socio-economic position, household composition, health status and indicators of the well-being of mothers of children with and without intellectual disabilities. Journal of Intellectual Disability Research. 2006;50(12):862–873. doi: 10.1111/j.1365-2788.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8(3):430–457. [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S, Phillips AC, Carroll D. Parental stress is associated with poor sleep quality in parents caring for children with developmental disabilities. Journal of Pediatric Psychology. 2010;35(7):728–737. doi: 10.1093/jpepsy/jsp093. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Phillips AC, Oliver C, Carroll D. Predictors of psychological morbidity in parents of children with intellectual disabilities. Journal of Pediatric Psychology. 2008;33:1129–1136. doi: 10.1093/jpepsy/jsn040. [DOI] [PubMed] [Google Scholar]
- Gerstein ED, Crnic KA, Blacher J, Baker BL. Resilience and the course of daily parenting stress in families of young children with intellectual disabilities. Journal of Intellectual Disability Research. 2009;53(12):981–997. doi: 10.1111/j.1365-2788.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glutting JJ. Introduction to the structure and application of the Stanford-Binet Intelligence Scale - Fourth Edition. Journal of School Psychology. 1989;27(1):69–80. [Google Scholar]
- Goldgruber J, Ahrens D. Effectiveness of workplace health promotion and primary preventive interventions: A review. Journal of Public Health. 2010;18(1):75–88. [Google Scholar]
- Gross HE, Shaw DS, Moilanen KL, Dishion TJ, Wilson MN. Reciprocal models of child behavior and depressive symptoms in mothers and fathers in a sample of children at risk for early conduct problems. Journal of Family Psychology. 2008;22(5):742–751. doi: 10.1037/a0013514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Hong J, Seltzer M, Greenberg J. Age and gender differences in the well-being of midlife and aging parents with children with mental health or developmental problems: Report of a national study. Journal of Health and Social Behavior. 2008;49(3):301–316. doi: 10.1177/002214650804900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser-Cram P, Warfield ME, Shonkoff JP, Krauss MW. Children with disabilities: A longitudinal study of child development and parent well-being. Monographs of the Society of for Research in Child Development. 2001;66:1–131. [PubMed] [Google Scholar]
- Hope A, Kelleher C, O'Connor M. Lifestyle and cancer: The relative effects of a workplace health promotion program across gender and social class. American Journal of Health Promotion. 1999;13(6):315–318. doi: 10.4278/0890-1171-13.6.315. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38(1):21–37. [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3rd ed. New York: Guilford Press; 2010. [Google Scholar]
- Kraemer BR, Blacher J. Transition for Hispanic and Anglo young adults with severe intellectual disability: Parent perspectives over time. Journal on Developmental Disabilities. 2008;14(1):59–72. doi: 10.1352/2009.47:31-43. [DOI] [PubMed] [Google Scholar]
- Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: A prospective study. American Journal of Preventative Medicine. 2003;24:113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- Little TD, Preacher KJ, Selig JP, Card NA. New developments in latent variable panel analyses of longitudinal data. International Journal of Behavioral Development. 2007;31(4):357–365. [Google Scholar]
- Magaña S, Smith MJ. Health outcomes of midlife and older Latina and black American mothers of children with developmental disabilities. Mental Retardation. 2006;44(3):224–234. doi: 10.1352/0047-6765(2006)44[224:HOOMAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Magaña S, Smith MJ. Health behaviors, service utilization, and access to care among older mothers of color who have children with developmental disabilities. Intellectual and Developmental Disabilities. 2008;46(4):267–280. doi: 10.1352/1934-9556(2008)46[267:HBSUAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Manuel J, Naughton MJ, Balkrishnan R, Smith BP, Koman LA. Stress and adaptation in mothers of children with cerebral palsy. Journal of Pediatric Psychology. 2003;28(3):197–201. doi: 10.1093/jpepsy/jsg007. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McIntyre LL, Blacher J, Baker BL. The transition to school: Adaptation in young children with and without developmental disability. The Journal of Intellectual Disability Research. 2006;50:349–361. doi: 10.1111/j.1365-2788.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Hauser-Cram P. The well-being of mothers of adolescents with developmental disabilities in relation to medical care utilization and satisfaction with health care. Research in Developmental Disabilities. 2008;29(2):97–112. doi: 10.1016/j.ridd.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Multidimensional functional assessment. The OARS methodology: A manual. 2nd ed. Durham, NC: Duke University, Center for the Study of Aging and Human Development; 1978. [Google Scholar]
- Mulvaney-Day NE, Alegria M, Sribney W. Social cohesion, social support, and health among Latinos in the United States. Social Science and Medicine. 2007;64:477–495. doi: 10.1016/j.socscimed.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neece CL, Green SA, Baker BL. Parenting stress and child behavior problems: A transactional relationship across time. American Journal on Intellectual and Developmental Disabilities. 2012;117(1):48–66. doi: 10.1352/1944-7558-117.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson MB, Hwang CP. Depression in mothers and fathers of children with intellectual disability. Journal of Intellectual Disability Research. 2001;45(6):535–543. doi: 10.1046/j.1365-2788.2001.00372.x. [DOI] [PubMed] [Google Scholar]
- Olsson MB, Hwang CP. Socioeconomic and psychological variables as risk and protective factors for parental well-being in families of children with intellectual disabilities. Journal of Intellectual Disability Research. 2008;52(12):1102–1113. doi: 10.1111/j.1365-2788.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- Peters-Scheffer N, Didden R, Korzilius H. Maternal stress predicted by characteritics of children with autism spectrum disorder and intellectual disability. Research in Autism Spectrum Disorders. 2012;6(2):696–706. [Google Scholar]
- Rimm-Kaufman S, Pianta R. An ecological perspective on the transition to kindergarten: A theoretical framework to guide empirical research. Journal of Applied Developmental Psychology. 2000;21:491–511. [Google Scholar]
- Sameroff A. The transactional model of development: How children and contexts shape each other. Washington, DC, US: American Psychological Association; 2009. [Google Scholar]
- Schlomer GL, Bauman S, Card NA. Best practices for missing data management in counseling psychology. Journal of Counseling Psychology. 2010;57(1):1–10. doi: 10.1037/a0018082. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, Stawski RS. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40(4):457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Krauss MW. Aging parents with adult mentally retarded children: Family risk factors and sources of support. American Journal on Mental Retardation. 1989;94(3):303–312. [PubMed] [Google Scholar]
- Seltzer MM, Krauss MW, Choi SC, Hong J. Midlife and later-life parenting of adult children with mental retardation. In: Ryff CD, Seltzer MM, editors. the parental experience in midlife. Chicago: University of Chicago Press; 1996. pp. 459–489. [Google Scholar]
- Stoner JB, Angell ME. Parent perspectives on role engagement: An investigation of parents and children with ASD and their self-reported roles with education professionals. Focus on Autism and other Developmental Disabilities. 2006;21(3):177–189. [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health psychology: What is an unhealthy environment and how does it get under the skin? Annual Review of Psychology. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen ED, Sattler JM. The Stanford-Binet Intelligence Scale: Fourth Edition. (Technical Manual) Chicago: Riverside; 1986. [Google Scholar]
- Waters E, Doyle J, Wolfe R, Wright M, Wake M, Salmon L. Influence of parental gender and self-reported health and illness on parent-reported child health. Pediatrics. 2000;106(5):1422–1428. doi: 10.1542/peds.106.6.1422. [DOI] [PubMed] [Google Scholar]