Abstract
The calcium/calmodulin-dependent protein phosphatase calcineurin is required for the induction of transcriptional events that initiate and promote myogenic differentiation. An important effector for calcineurin in striated muscle is the transcription factor myocyte enhancer factor 2 (MEF2). The targeting of the enzyme and substrate to specific intracellular compartments by scaffold proteins often confers specificity in phosphatase activity. We now show that the scaffolding protein mAKAP organizes a calcineurin/MEF2 signaling complex in myocytes, regulating gene transcription. A calcineurin/mAKAP/MEF2 complex can be isolated from C2C12 cells and cardiac myocytes, and the calcineurin/MEF2 association is dependent on mAKAP expression. We have identified a peptide comprising the calcineurin binding domain in mAKAP that can disrupt the binding of the phosphatase to the scaffold in vivo. Dominant interference of calcineurin/mAKAP binding blunts the increase in MEF2 transcriptional activity seen during myoblast differentiation, as well as the expression of endogenous MEF2-target genes. Furthermore, disruption of calcineurin binding to mAKAP in cardiac myocytes inhibits adrenergic-induced cellular hypertrophy. Together these data illustrate the importance of calcineurin anchoring by the mAKAP scaffold for MEF2 regulation.
Keywords: Scaffold, AKAP, protein phosphatase, Calcineurin, MEF2
Introduction
Myogenic differentiation is the process in skeletal muscle development and regeneration by which an unstructured population of mononuclear myoblasts become striated, multinucleated myotubes [1]. This process involves complex morphological and transcriptional dynamics that ultimately results in functional myofibers capable of force generation and mechanical work. Calcineurin (CaN) is a calcium/calmodulin-dependent, serine/threonine phosphatase that has been widely studied in the context of calcium-sensitive signaling pathways, including those involved in myogenesis [2]. Previous work found that genetic loss of CaN in the skeletal muscle of mice resulted in a decrease in total fiber number and a reduction in growth, consistent with an impairment in myogenesis [3]. CaN activation induced the transcription of genes involved in oxidative fiber-selection, while pharmacological inhibition of the phosphatase in animals resulted in increased fast fiber gene expression [4]. Lastly, overexpression of constitutively active CaN was sufficient to drive muscle cell differentiation in the L6 cell line, including the increased expression of differentiation-specific genes.
CaN-dependent regulation of gene transcription in skeletal muscle was originally attributed solely to the activation of the transcription factor nuclear factor of activated T-cells (NFAT) [1]. However, oxidative fiber-specific promoters were found to be responsive to CaN stimulation even after ablation of the NFAT binding motifs within the promoter region [5]. Furthermore, inhibition of NFAT in L6 muscle cells did not prevent muscle differentiation, suggesting that other transcription factors are responsive to calcineurin stimulation and are involved in muscle differentiation [6]. Subsequently, myocyte enhancer factor 2 (MEF2), a MADS box transcription factor with four known isoforms (A-D), was shown to be required for CaN signaling in skeletal muscle development [6–10]. Expression of a constitutively active CaN mutant protein in the immortalized skeletal myoblast line C2C12 increased MEF2-induced gene transcription and DNA binding, correlating with the increased differentiation of the myoblasts into myotubes [6, 11]. In addition, skeletal muscle development in MEF2-null Drosophila embryos ceased after myoblast specification before terminal myocyte differentiation [12, 13]. Taken together, these data support the hypothesis that MEF2 transduces CaN-dependent signaling responsible for the terminal differentiation of skeletal muscle progenitor cells.
A common theme among protein phosphatases is the use of targeting subunits to localize the phosphatase in close proximity to either its substrates or upstream activators, thereby focusing the actions of the phosphatase [14]. For CaN, these anchoring proteins include AKAP5, TRESK, KSR2, RCAN and Cain/Cabin1 [14]. Among them, Cain/cabin1 is a CaN binding protein that not only inhibits phosphatase activity [15], but also binds MEF2, resulting in the suppression of MEF2-dependent transcriptional activity [16, 17]. Increased intracellular calcium results in the release of MEF2 from Cabin1 in T cells, permitting MEF2-dependent gene expression.
We have previously identified another scaffolding protein that binds and regulates MEF2 transcriptional activity [18]. The mAKAP scaffold is a ~250 kDa protein that is expressed in excitable cells such as neurons and skeletal and cardiac myocytes and that binds MEF2 family members, including MEF2A and MEF2D [18, 19]. mAKAP is localized to the nuclear envelope via direct binding to nesprin-1α, a nuclear membrane KASH domain protein [20]. In cardiac myocytes, mAKAP organizes signalosomes involved in cAMP, mitogen-activated protein kinase, calcium-dependent, and hypoxic signaling important for myocyte hypertrophy [21–26]. Recently, we found that the MADS domain of MEF2D binds directly to a N-terminal domain of mAKAP in skeletal muscle [18]. Interference of the MEF2/mAKAP interaction blunted MEF2 transcriptional activity and the expression of endogenous MEF2 target genes [18]. Importantly, disruption of MEF2/mAKAP complexes attenuated the differentiation of C2C12 myoblasts into myotubes, as evidenced by decreased cell fusion and expression of differentiation markers [18].
Intriguingly, we have also discovered that mAKAP serves as a scaffold for CaN in cardiac myocytes [27, 28]. Given that CaN and MEF2 both bind mAKAP, we now propose the hypothesis that the organization of CaN/MEF2 complexes by the mAKAP scaffold is required for MEF2 transcriptional activity in striated muscle. We show that mAKAP and CaN interact in C2C12 cells and cardiac myocytes, and that this interaction can be inhibited by a dominant negative binding site peptide based on the CaN binding domain on mAKAP. Using this peptide we reveal that calcineurin/mAKAP binding is required for MEF2 function in striated muscle. Our data support a new mechanism in which differentiation-induced CaN signaling to MEF2 in striated muscle is enhanced through the assembly of a protein complex nucleated by the mAKAP scaffold.
Materials and Methods
Expression constructs and Antibodies
pmCherry-CaNBD was constructed by inserting a cDNA fragment encoding amino acids 1285–1345 of mAKAP and a C-terminal myc tag into the Bgl II and Sal I sites in pmCherry-C1 (Clontech). Additional constructs were previously described [27]. Antibodies used in this project were as follows: mouse monoclonal anti-CaN A-subunit (Sigma-Aldrich), rabbit anti-CaNAβ (Millipore), goat polyclonal anti-dsRed for mCherry (Santa Cruz Biotechnology), mouse monoclonal anti-myc 9E10 (Santa Cruz Biotechnology), rabbit polyclonal anti-mAKAP (Covance), rabbit polyclonal anti-MEF2 (Santa Cruz Biotechnology), mouse monoclonal anti-MEF2 (Santa Cruz Biotechnology), mouse anti-myogenin (Santa Cruz Biotechnology), anti-GAPDH (Santa Cruz Biotechnology), mouse EA-53 anti-α-actinin (Sigma-Aldrich), and rabbit anti-rat ANF (US Biological). The MF-20 antibody developed by Donald A. Fischman, M.D. was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Cell Culture and Transfection
C2C12 cells were maintained as previously described [18]. Cells were passaged at low density in Growth Medium [DMEM (Invitrogen)] supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen). Cells were carefully monitored to prevent spontaneous differentiation as a result of overgrowth. To induce differentiation, cells at approximately 80% confluence were washed with PBS to remove all growth factors and media was replaced with Differentiation Medium (DMEM supplemented with 2% horse serum and 1% penicillin/streptomycin).
Two days prior to transfection, cells were plated at 40% confluence in Growth Medium. Cells were washed twice with PBS, trypsinized, counted, and pelleted. Approximately 2x106 cells per transfection were resuspended in supplemented Solution V from the Lonza Cell Electroporation kit (Lonza, Walkersville, MD). The cells were transferred to a cuvette containing 2 µg DNA in TE buffer, electroporated using Program T-017 of the Lonza electroporation unit, and transferred immediately to pre-warmed growth medium and allowed to recover for 8 hours. Cells were plated on 24 well plates for luciferase analysis, 60 mm dishes for biochemical experiments, and glass coverslips for microscopy.
Neonatal cardiac myocytes (ventricular only, over 90% free of fibroblasts) were prepared from 2–3-day old Sprague-Dawley rats and cultured as previously described [27]. The myocytes were transfected with Transfast (Promega) as suggested by the manufacturer (1–5% efficiency), and cultured in maintenance medium (79% DMEM, 20% Media 199, and 1% penicillin/streptomycin). Myocyte immunocytochemistry and morphometrics were performed as previously described by digital wide-field fluorescent microscopy using IPLab 4.0 software (BD Biosciences) [28]. Each n represents the results of experiments using separate primary cultures. Within each experiment, ~25 cells were measured for each condition for both morphometric and ANF expression studies. ON-TARGETplus siRNA oligonucleotides were mAKAP (GAC GAA CCU UCC UUC CGA A UU) and On-Targetplus Non-targeting siRNA #1 from Dharmacon. siRNA were transfected using Dharmafect 1 (Thermofisher) as recommended by the manufacturer using cells cultured in maintenance medium supplemented with 4% horse serum.
HEK293 cells were transfected using Lipofectamine (Life Technologies) following the manufacturer’s directions. Cells were plated 6 hours prior to transfection and analyzed one day after transfection.
Immunoprecipitation
Immunoprecipitations were carried out using 2–3 μg antibody. Cells were lysed in HSE lysis buffer (5% glycerol, 1% Triton X-100, 20 mM HEPES, pH 7.4, 50 mM NaCl, and 5 mM EDTA and protease inhibitors) and, following centrifugation, the soluble lysate was incubated with Protein G-agarose beads charged with antibody overnight at 4° C with rotation. Beads were washed three times with lysis buffer and subjected to SDS-PAGE electrophoresis followed by immunoblotting.
Luciferase Vectors and Assay
One day after splitting cells 1:6 as described above, adenoviral stocks were added to the culture medium. MEF2-Luciferase (Seven Hills Bioreagents), which contains three copies of the MEF2 consensus E-box sequence upstream of the firefly luciferase gene and control β-galactosidase adenoviruses were added at an MOI of 225 and 0.2, respectively. These cells were then returned to the incubator until transfection the following day as described above. For experiments using virus that expresses the dominant active calcineurin A 1–398 mutant (Seven Hills Bioreagents), virus was added at an MOI of 25. Eight to ten hours post-transfection, cells were examined by phase contrast microscopy to ensure adherence, and transfection efficiency was determined by visualizing mCherry fluorescence.
For the reporter assay, cells were washed once with PBS and incubated for 10 minutes at room temperature with 80 µl of 1X Reporter Lysis Buffer from the Luciferase Assay System (Promega). Twenty and 50 µl cell lysate were used for luciferase and β-galactosidase measurement, respectively, and the remaining lysate was saved for Western blot analysis. Samples were assayed according to the manufacturer’s instructions in an injecting luminometer (Biotek Synergy 2). Normalized MEF2 activity was obtained by dividing luciferase by β-galactosidase assay values. Statistical significance was determined using a two-tailed Student’s t-test, p values are as reported. Data is expressed as mean ± s.e.m.
Statistics
All data are expressed as the mean ± s.e.m. for n experiments. p-values were calculated using two-tailed Student’s t-tests, paired or un-paired as appropriate, and are not corrected for multiple comparisons. Repeated symbols represent p-values of different orders of magnitude: * p<0.05, ** p<0.005, and *** p < 0.0005.
Results
MEF2 transcriptional activity is mediated by calcineurin
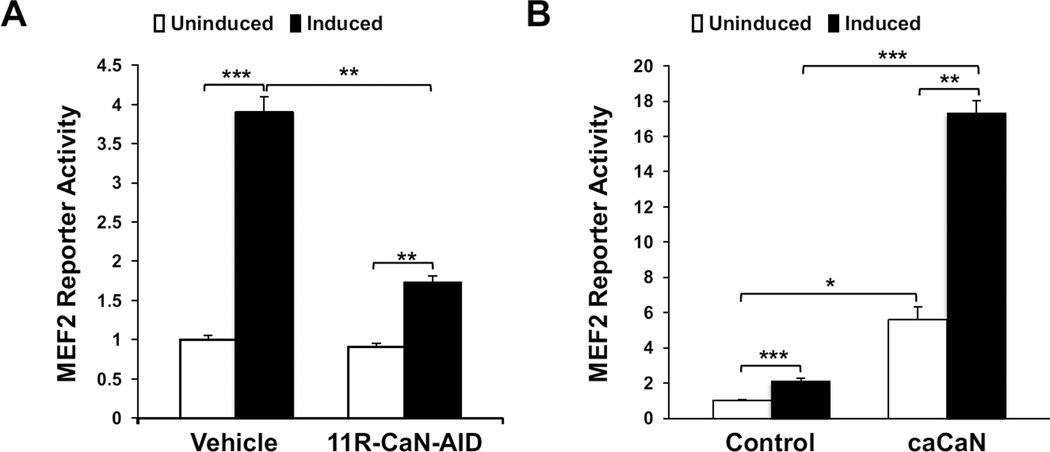
To confirm the role of CaN in the stimulation of MEF2 activity during the differentiation of C2C12 myoblasts, MEF2 activity was examined in the presence and absence of a cell permeable inhibitory peptide based on the CaN autoinhibitory domain (11R-Can-AID, 50 µM). Vehicle-treated cells cultured in differentiation medium exhibited increased MEF2 reporter activity in response to differentiation media, while inhibition of CaN significantly reduced the differentiation-induced increase in transcriptional activity (Figure 1A). Conversely, expression of a constitutively active CaN mutant protein increased MEF2 activity in C2C12 cells when cultured in both growth and differentiation media (Figure 1B). These results demonstrate that CaN is important for the activation of MEF2 transcriptional activity during myoblast differentiation.
Figure 1. CaN regulates MEF2 gene transcription in C2C12 cells.
A) C2C12 cells cultured in growth medium and infected with an adenoviral MEF2 luciferase reporter and β-galactosidase control were treated with 50 μM cell-permeable CaN autoinhibitory peptide (11R-CaN-AID, Calbiochem) for 2 hours. The resulting transcriptional activity was assayed at 0 (uninduced) and 12 hours (induced) after the cells were switched to differentiation medium. B) C2C12 cells infected with adenovirus for MEF2 luciferase and β-galactosidase reporters and for a constitutively active CaNA® 1–398 mutant protein were cultured as in (A). For both A and B, MEF2 activity was normalized using β-galactosidase assay. n = 3.
Calcineurin associates with mAKAP in C2C12 cells during differentiation
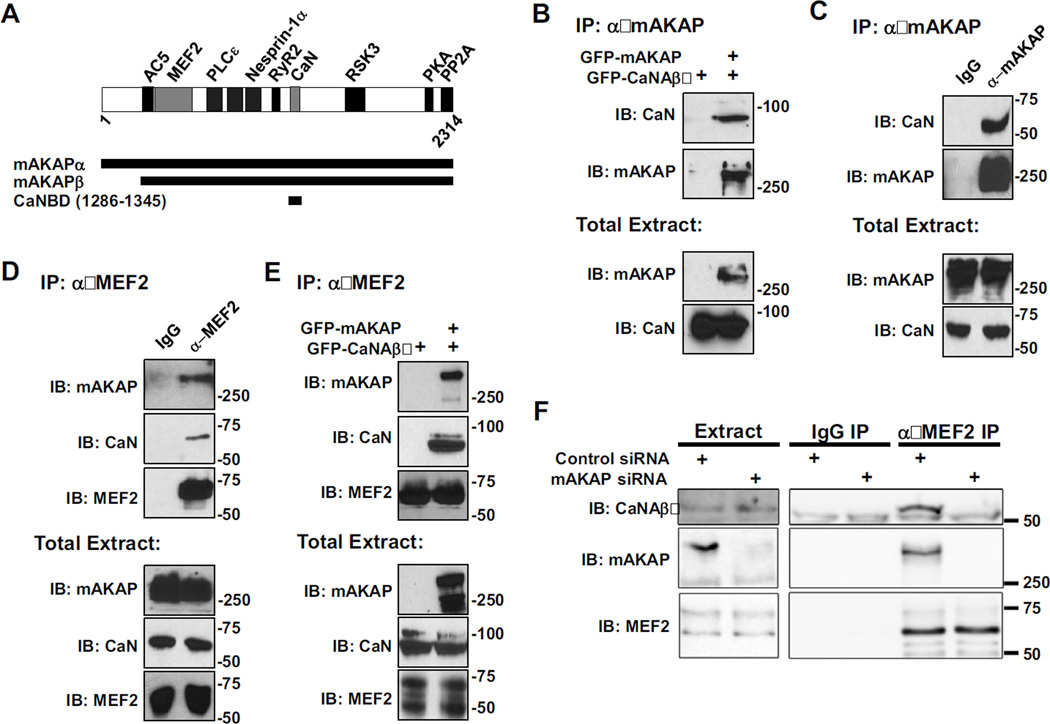
We have previously shown that both CaN and MEF2 bind directly to discret domains on mAKAP (Figure 2A) [18, 27]. As shown in Figure 2B, CaN catalytic subunit Aβ (CaNAβ) is immunoprecipitated with mAKAP antibody when both proteins are expressed in HEK293 cells as GFP-fusion proteins. Likewise, endogenous CaNA/mAKAP complexes can be isolated from C2C12 cells (Figure 2C). These data show that CaN and mAKAP will bind not only in cardiac myocytes, but also in other cells that express mAKAP.
Figure 2. mAKAP is the scaffold for a signaling complex consisting of CaN and MEF2.
A) mAKAP domain structure. The stippled domains are the spectrin-like repeat domains [18–28]. Direct binding partners whose sites have been finely mapped in mAKAPβ are shown. mAKAPβ (the 227 kDa, alternatively-spliced isoform predominant in striated muscle) starts at residue 245 of mAKAPα (the 254 kDa, alternatively-spliced isoform predominant in neurons) [34]. All fragments are numbered per mAKAPα. B) Protein complexes were immunoprecipitated using a mAKAP antibody from HEK293 cells expressing GFP-tagged mAKAPβ (255 kDa) and CaNAβ (87 kDa). Protein in the immunoprecipitates (top panel) and in total extracts (bottom panels) were detected using CaN catalytic subunit and mAKAP-specific antibodies. C and D) Protein complexes were immunoprecipitated from C2C12 cell lysate using a mAKAP (C), MEF2 (D), or control IgG antibody and associated proteins were detected as in A. Note that like other AKAPs, mAKAP tends to migrate slower in SDS-PAGE than predicted by its molecular weight. E) MEF2 antibody was used to immunoprecipitate protein complexes from HEK293 cells expressing GFP-CaNAβ and GFP-mAKAPβ. CaN and MEF2 were associated in HEK293 cells only when co-expressed with mAKAP. F) MEF2 antibody was used to immunoprecipitate endogenous protein complexes from neonatal rat cardiac myocytes transfected with control or mAKAP-specific siRNA oligonucleotides. Endogenous CaNAβ (59 kDa) and mAKAPβ were present in MEF2 immunoprecipitates only when mAKAPβ was expressed. All blots shown are representative of experiments repeated at least three times.
We have published that binding of CaN is enhanced by activation of the phosphatase, and unlike other CaN scaffolds, binding to mAKAP did not inhibit CaN activity [27], suggesting that mAKAP functions to direct active phosphatase towards relevant substrates associated with the scaffold. We now show that CaNA and mAKAP are immunoprecipitated with MEF2 antibodies from C2C12 cells, suggesting that a complex of the phosphatase, its substrate, and the scaffold protein exists in these cells (Figure 2D). To determine if mAKAP could be responsible for the association of CaN and MEF2, HEK293 cells were transfected with GFP-CaNAβ in the presence and absence of GFP-mAKAP. Endogenous MEF2 was immunoprecipitated from these cells, and association of CaNAβ and mAKAP was determined by immunoblot. As shown in Figure 2E, CaNAβ was only detected in MEF2 immunoprecipitates when co-expressed with mAKAP. Accordingly, to show that endogenous ternary complexes exist in striated myocytes, MEF2 antibody was used to immunoprecipitate complexes from neonatal rat cardiac myocytes transfected with control or mAKAP siRNA (Figure 2F). Consistent with the data obtained using recombinant proteins, endogenous mAKAP and CaNAβ were co-immunoprecipitated with MEF2 antibody only in cells expressing mAKAP. These data, taken together, demonstrate that one function of mAKAP is to anchor CaN and MEF2 within the same signaling complexes.
Disruption of the Calcineurin/mAKAP Interaction
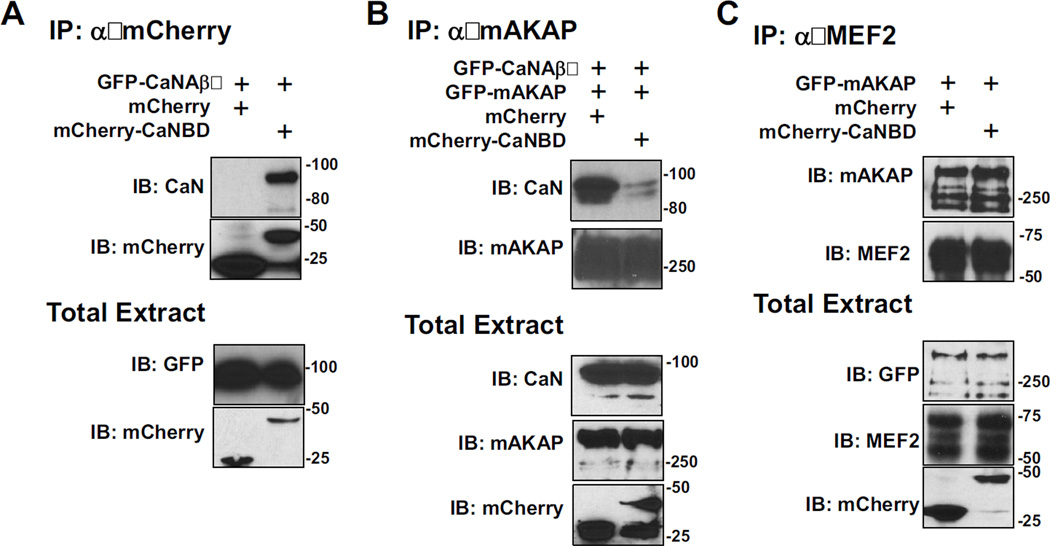
We next sought to develop reagents that could disrupt CaN/MEF2/mAKAP signaling complexes in vivo. We have previously mapped the CaN binding site (CaNBD, Figure 2A) on mAKAP to amino acid residues 1286–1345, such that a purified, bacterially-expressed His6-tagged mAKAP 1286–1345 fragment bound GST-tagged CaNAβ in pull-down assays [27]. To facilitate our current studies, we fused the mAKAP CaNBD to mCherry fluorescent protein. When expressed in HEK293 cells, GFP-CaN co-precipitated with mCherry-CaNBD, but not mCherry alone (Figure 3A), confirming this domain of mAKAP can bind to CaN in cells independent of the full-length protein.
Figure 3. In vivo disruption of CaN-mAKAP binding.
As indicated, HEK293 cells were transfected with plasmids to express GFP-tagged CaNAβ (87 kDa) and mAKAPβ (255 kDa) and either mCherry control (29 kDa) or mCherry-CaNBD (mAKAP 1286–1345, 37 kDa). A) Protein complexes were immunoprecipitated using a mCherry-specific antibody. Proteins in the immunoprecipitates (top panels) and in total cellular extracts (bottom panels) were detected with mCherry and GFP antibodies. B) Protein complexes were immunoprecipitated using a mAKAP-specific antibody. C) Protein complexes were immunoprecipitated using a MEF2-specific antibody. mCherry-CaNBD that binds CaNAβ competed the binding of mAKAP to CaNAβ, but not MEF2. All blots shown are representative of experiments repeated at least three times.
Importantly, expression of mCherry-CaNBD competed full-length mAKAP/CaN binding in cells. HEK293 cells were transfected with GFP-mAKAP, GFP-CaNAβ and either mCherry control, or mCherry-CaNBD. Protein complexes were immunoprecipitated using a mAKAP specific antibody, and association of CaN was determined by western blot analysis. As shown in Figure 3B, the mAKAP/CaN association was significantly reduced when mCherry-CaNBD was co-expressed. This peptide did not affect MEF2 binding to the AKAP, as expected since MEF2 binds a separate, N-terminal mAKAP domain (Figures 2A and 3C). Hence, over-expression of mCherry-CaNBD can specifically displace CaN from mAKAP complexes in vivo.
Functional significance of the mAKAP/PP2B interaction on MEF2 activity
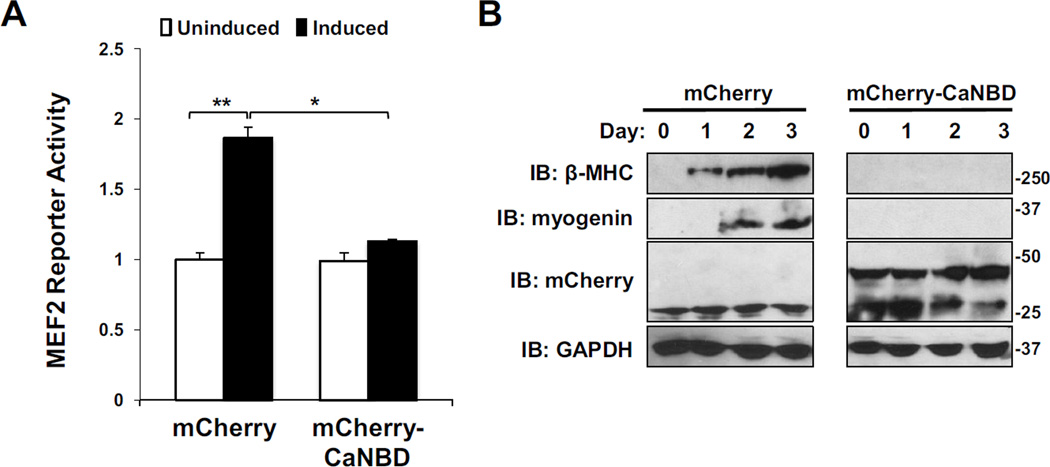
To address the involvement of mAKAP in the regulation of MEF2 by CaN, a dominant interference approach was employed. C2C12 cells were infected with adenovirus encoding the firefly luciferase gene under the control of the MEF2 consensus sequence to monitor MEF2 transcriptional activity. These cells were also transiently transfected with plasmids expressing either mCherry or mCherry-CaNBD. In control experiments, MEF2 activity was significantly increased within 12 hours of the induction of differentiation (Figure 4A). In contrast, cells expressing the mCherry-CaNBD fragment showed no increase in luciferase activity following the induction of differentiation. These results suggest that CaN tethering to mAKAP is a necessary step in the activation of MEF2 during the induction of myogenesis.
Figure 4. mAKAP-bound CaN regulates MEF2 transcriptional activity.
A) C2C12 cells cultured in growth medium were infected with MEF2 luciferase and β-galactosidase control adenoviruses and transfected with expression plasmids for either mCherry alone or mCherry-CaNBD. Reporters were assayed at 0 (uninduced) and 12 hours (induced) after the cells were placed in differentiation medium. MEF2 activity was normalized using β-galactosidase assay. n = 3. B) C2C12 cells infected with adenovirus that express either mCherry or mCherry-CaNB were cultured in differentiation media for up to 3 days. β-MHC, myogenin, mCherry and GAPDH in total cellular extracts were assayed by western blotting. β-MHC and myogenin were consistently undetectable in the cells expressing the competing peptide. n > 3.
These changes in MEF2 luciferase activity correlated with changes in MEF2-dependent gene transcription. C2C12 cells were infected with adenovirus that expresses either mCherry or mCherry-CaNBD. Immunoblot analysis of C2C12 lysates taken serially after the induction of differentiation revealed that displacement of CaN from the mAKAP scaffold blocked expression of β-myosin heavy chain (β-MHC) and myogenin (Figure 4B).
Calcineurin Binding to mAKAP is required for induction of cardiac hypertrophy
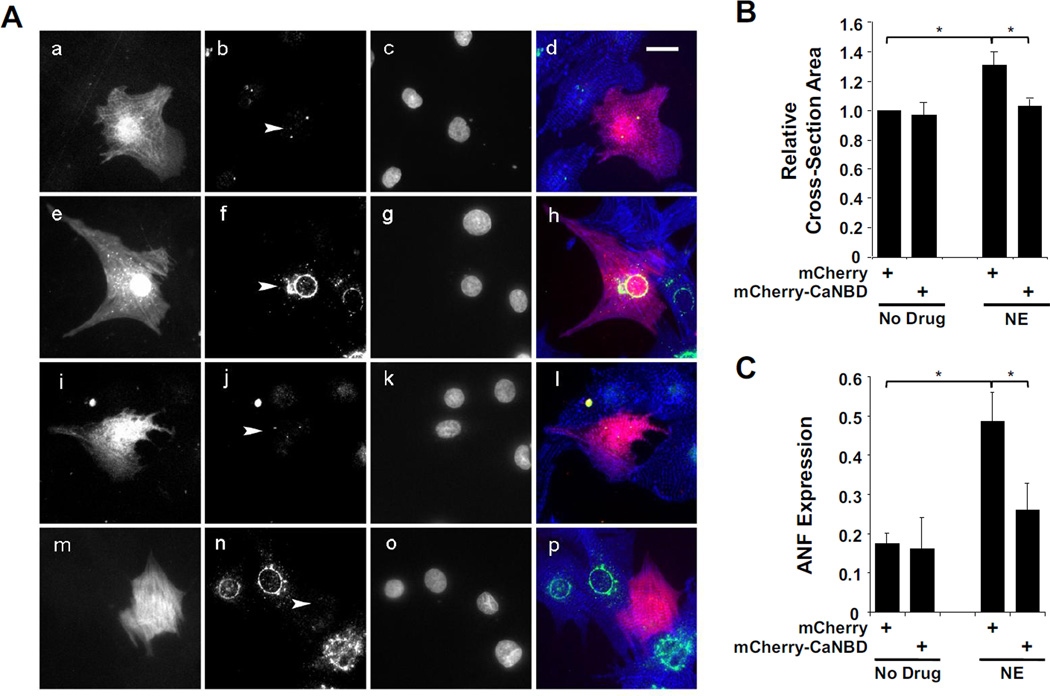
In addition to skeletal muscle differentiation, MEF2 is also important for the transactivation of hypertrophic gene transcription in the stressed heart, another striated muscle [29, 30]. To investigate if dissociation of mAKAP/CaN complexes by peptide competition would also inhibit cardiac myocyte hypertrophy, primary neonatal rat cardiac myocytes were transfected with expression plasmids for either control mCherry or mCherry-CaNBD and then stimulated for two days with the adrenergic agonist norepinephrine (NE,10 µM; Fig. 5A). Morphologic myocyte hypertrophy was assayed by measuring the cellular cross-section area on mCherry images (Figure 5A). NE-treatment increased the cross-section area of mCherry-expressing myocytes 31 ± 9 %. Remarkably, treated and non-treated myocytes expressing mCherry-CaNBD were similar in size (0.97 ± 0.08 and 1.02 ± 0.06 fold, respectively) to untreated, control myocytes expressing mCherry alone. Atrial natriuretic factor (ANF) is a marker for hypertrophy encoded by the MEF2 transactivated Nppa gene [31]. ANF expression was detected in 49 ± 7 % and 26 ± 7 %, respectively, of mCherry and mCherry-CaNBD expressing myocytes following adrenergic stimulation (Figure 5C). ITogether, these data support the hypothesis that the recruited binding of CaN to the mAKAP scaffold is required for MEF2-mediated events in striated muscles.
Figure 5. Displacement of CaN from mAKAP in rat neonatal cardiac myocytes inhibits norepinephrine-stimulated hypertrophy.
A) Primary rat neonatal ventricular myocytes were transfected with expression vectors for either mCherry fluorescent protein (panels a-h) or a mCherry-CaNBD fusion protein (panels i-p). The myocytes were cultured for two days in serum-free medium with (panels e-h and m-p) or without (panels a-d and i-l) 10 µM NE. Cells were stained with antibodies for ANF and α-actinin (green and blue in composite panels d, h, l, and p, respectively) and Hoechst DNA stain (panels c, g, k, and o). Grayscale images are included of the mCherry channel (panels a, e, i and m) and ANF staining (panels b, f, j, and n; arrowheads point to the nuclei of the transfected myocytes). Scale bar in panel d indicates 20 µm. B) Fold cross-section area is indicated for each condition shown in A. The data are normalized to the values for untreated, mCherry-expressing cells (990 ± 91 µm2). p(ANOVA) = 0.01; n = 5. C) The fraction of cells displaying peri-nuclear prepro-ANF staining with the ANF antibody is indicated for each condition shown in A. p(ANOVA) = 0.02; n = 4. For both B and C, ~25 cells were measured for each condition for each replicate experiment performed with separate primary cultures.
Discussion
The spatial regulation of protein phosphatase signaling is an area of recent intense interest. As these enzymes display significant substrate promiscuity in vitro, a pressing question is how protein phosphatase activity is directed to specific targets in vivo 32]. This is particularly important for transcription factors, where their transcriptional activity is extensively regulated by phosphorylation events. Here, we demonstrate how the scaffolding protein mAKAP directs the protein phosphatase CaN towards the transcription factor MEF2 in order to regulate MEF2-dependent gene transcription. We found that mAKAP nucleates a protein complex consisting of the scafold, CaN and MEF2 in both C2C12 and cardiac myocytes.
Overexpression of a peptide that mimics the CaN binding domain on mAKAP disrupted the association of CaN with mAKAP, but not the mAKAP/MEF2 interaction., This disrupting peptide attenuates the differentiation-induced increase in MEF2 luciferase activity in C2C12 cells, as well as the MEF2-regulated changes in skeletal muscle gene expression. This effect is not restricted only to skeletal muscle, as complex disruption prevented the increase in cell size and ANF gene expression induced by treatment of rat neonatal cardiac myocytes with norephinephrine, a phenomenon that requires MEF2 gene transcription. Thus, this work demonstrates the importance of the mAKAP scaffold in coordinating a CaN/MEF2 signaling pathway that controls MEF2-dependent events in striated muscle.
The molecular mechanism by which mAKAP-bound CaN regulates MEF2 gene transcription in striated muscle cells is still unclear, but likely involves dephosphorylation of the transcription factor. Due to binding by nesprin-1α, mAKAPβ is primarily located at the nuclear envelop in myocytes [20], where mAKAPβ recruits a discrete pool of CaN [27]. Like others [7], we have found that MEF2 is primarily nuclear in myocytes (data not shown). We suggest that the pool of CaN associated with mAKAPβ regulates MEF2 function by promoting the de-phosphorylated state of MEF2, stimulating the increased gene transcription seen during myogenic differentiation and or cardiac hypertrophy [7]. In hippocampal neurons, dephosphorylation of MEF2A by CaN is seen during calcium-induced differentiation, which enhances MEF2 reporter activity and is critical for neuronal dendritic outgrowth [33]. We have not yet, however, been able to detect CaN-dependent dephosphorylation of MEF2 in myocytes with the currently available phospho-specific antibodies, albeit, other CaN-dependent effects on MEF2 co-factors cannot be ruled out. Interestingly, mAKAP-bound CaN also regulates the transcription factor NFATc3 in cardiac muscle [27]. When a mutant mAKAP lacking the CaN binding domain was expressed in rat neonatal cardiomyocytes along with NFATc3, the dephosphorylation of the transcription factor was attenuated. This finding lends credence to the hypothesis that mAKAP-bound CaN catalyzes the dephosphorylation of MEF2 in skeletal muscle.
In summary, we have identified a multimolecular signaling complex in striated muscle consisting of CaN, MEF2 and the scaffold protein mAKAP. This work provides a greater understanding of the regulation of MEF2 gene transcription in striated muscle and reveals a novel mechanism that confers specificity to the CaN-MEF2 gene regulatory pathway.
Highlights.
Calcineurin regulates MEF2 activity in striated muscle.
mAKAP is a calcineurin scaffold in striated muscle.
The mAKAP/calcineurin interaction can be disrupted using a competitive binding peptide
Disruption of the interaction prevents differentiation-induced MEF2 gene transcription in C2C12 cells
Disruption of the interaction also prevents induction of cardiac hypertrophy in neonatal cardiac myocytes
Acknowledgements
This work contains data from the doctoral thesis of Maximilian Vargas (UCHC, Farmington, CT, USA). This work was supported by NIH grants HL82705 to KDK and HL075398 to MSK and American Heart Association awards to JL (Scientific Development Grant).
Abbreviations
- mAKAP
muscle specific A-kinase anchoring protein
- MEF2
myocyte enhancer factor 2
- CaN
calcineurin
- CaNBD
calcineurin binding domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wakelam MJ. The fusion of myoblasts. Biochem J. 1985;228:1–12. doi: 10.1042/bj2280001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol. 2000;149:657–666. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem. 2004;279:26192–26200. doi: 10.1074/jbc.M313800200. [DOI] [PubMed] [Google Scholar]
- 4.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvo S, Venepally P, Cheng J, Buonanno A. Fiber-type-specific transcription of the troponin I slow gene is regulated by multiple elements. Mol Cell Biol. 1999;19:515–525. doi: 10.1128/mcb.19.1.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friday BB, Mitchell PO, Kegley KM, Pavlath GK. Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation. 2003;71:217–227. doi: 10.1046/j.1432-0436.2003.710303.x. [DOI] [PubMed] [Google Scholar]
- 7.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 8.Naya FJ, Olson EN. MEF2: a transcriptional target for signlaing pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol. 1999;1999:6. doi: 10.1016/s0955-0674(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 9.Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, R B-D, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 2001;20:6414–6423. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naya FJ, Wu C, Richardson JA, Overbeek P, Olson EN. Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependenttransgene. Development. 1999;126:2045–2052. doi: 10.1242/dev.126.10.2045. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000;19:1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lilly B, Zhao B, Ranganayakulu G, Paterson BM, Schulz RA, Olson EN. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267:688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- 13.Bour BA, O'Brien MA, Lockwood WL, Goldstein ES, Bodmer R, Taghert PH, Abmayr SM, Nguyen HT. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21:91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai MM, Burnett PE, Wolosker H, Blackshaw S, Snyder SH. Cain, a novel physiologic protein inhibitor of calcineurin. J Biol Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- 16.Esau C, Boes M, Youn H-D, Tarrerson L, Liu JO, Chen J. Deletion of Calcineurin and Myocyte Enhancer Factor 2 (MEF2) Binding Domain of Cabinl Results in Enhanced Cytokine Gene Expression in T Cells. J Exp Med. 2001;194:1449–1459. doi: 10.1084/jem.194.10.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youn HD, Sun L, Prywes R, Liu JO. Apoptosis of T cells mediated by Ca2-induced release of the transcription factor MEF2. Science. 1999;286:790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- 18.Vargas MA, Tirnauer JS, Glidden N, Kapiloff MS, Dodge-Kafka KL. Myocyte enhancer factor 2 (MEF2) tethering to muscle selective A-kinase anchoring protein (mAKAP) is necessary for myogenic differentiation. Cell Signal. 2012;24:1496–1503. doi: 10.1016/j.cellsig.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci. 1999;112(Pt 16):2725–2736. doi: 10.1242/jcs.112.16.2725. [DOI] [PubMed] [Google Scholar]
- 20.Pare GC, Easlick JL, Mislow JM, McNally EM, Kapiloff MS. Nesprin-lalpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res. 2005;303:388–399. doi: 10.1016/j.yexcr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Kritzer MD, Carlisle Michel JJ, Le A, Thakur H, Gayanilo M, Passariello CL, Negro A, Daniel JB, Oskouei B, Sanders M, Hare JM, Hanauer A, Dodge-Kafka KL, Kapiloff MS. Anchored p90 Ribosomal S6 Kinase 3 is Required for Cardiac Myocyte Hypertrophy. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.112.276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Malik S, Kelley GG, Kapiloff MS, Smrcka AV. Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPbeta) and integrates multiple hypertrophic stimuli in cardiac myocytes. J Biol Chem. 2011;286:23012–23021. doi: 10.1074/jbc.M111.231993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem. 2009;284:23540–23546. doi: 10.1074/jbc.M109.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong W, Goehring AS, Kapiloff MS, Langeberg LK, Scott JD. mAKAP compartmentalizes oxygen-dependent control of HIF-lalpha. Sci Signal. 2008;1:ral8. doi: 10.1126/scisignal.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Negro A, Lopez J, Bauman A, Henson E, Dodge-Kafka K, Kapiloff MS. The mAKAPp scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol. 2010;48:381–394. doi: 10.1016/j.yjmcc.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci. 2005;118:5637–5646. doi: 10.1242/jcs.02675. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Phan D, van Rooij E, Wang D, McAnally J, Qi X, Richardson J, Hill J, Bassel-Duby R, Olson E. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest. 2008;2008:124–132. doi: 10.1172/JCI33255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 31.Morin S, Charron F, Robitaille L, Nemer M. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO. 2000;J19:2046–2055. doi: 10.1093/emboj/19.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- 34.Michel JJ, Townley IK, Dodge-Kafka KL, Zhang F, Kapiloff MS, Scott JD. Spatial restriction of PDKl activation cascades by anchoring to mAKAPalpha. Mol. Cell. 2005;20:661–672. doi: 10.1016/j.molcel.2005.10.013. [DOI] [PubMed] [Google Scholar]