Abstract
Habitual snoring may be associated with cardiovascular disease (CVD); however limited evidence exists among women. We investigated whether frequent snoring is a predictor of coronary heart disease (CHD) and stroke among 42,244 postmenopausal women participating in the Women’s Health Initiative Observational Study. Participants provided self-reported information regarding snoring habits at baseline (1993-1998) and were followed for outcomes through August 2009. Physician adjudicators confirmed CHD, defined as MI, CHD death, revascularization procedures, or hospitalized angina, and confirmed ischemic stroke. Cox proportional hazards models were used to evaluate whether snoring frequency is a significant predictor of adjudicated outcomes. We observed 2,401 incident cases of CHD over 437,899 person-years of follow up. After adjusting for age and race, frequent snoring was associated with incident CHD (HR=1.54, 95% CI 1.39-1.70) and stroke (HR=1.41, 95% CI 1.19-1.66), and all CVD (HR=1.46, 95% CI 1.34-1.60). In fully adjusted models that included CVD risk factors such as obesity, hypertension, and diabetes, frequent snoring was associated with a more modest increase in incident CHD (HR=1.14 95% CI 1.01-1.28), stroke (HR=1.19, 95% CI 1.02-1.40) and CVD (HR=1.12, 95% CI 1.01-1.24). In conclusion, snoring is associated with a modest increased risk of incident CHD, stroke and CVD after adjustment for CVD risk factors. Additional studies are needed to elucidate the mechanisms in which snoring may be associated with CVD risk factors and outcomes.
Keywords: Snoring, Cardiovascular Disease, Coronary Heart Disease, Menopause
Snoring is a correlate and early symptom of Obstructive Sleep Apnea (OSA).1 Snoring may also be associated with hypertension,2 cardiovascular disease (CVD),3,4 type II diabetes5 and metabolic syndrome.6 The current evidence related to snoring focuses primarily on studies in men, relies upon cross-sectional studies and underrepresents women.4,7,8 Habitual snoring occurs among approximately 33% of the general population, and although sleep apnea is more common among men, the prevalence of sleep disturbance and snoring increases among women as they approach and pass through menopause.9-11 Emerging evidence suggests this increase in sleep problems may be due to aging, weight gain, or menopause-induced hormonal changes, such as decreases in progesterone and estradiol, which may modulate sleep regulation and breathing.11-14 Only 1 previous study has prospectively evaluated associations of snoring with CVD among older women,3 and although that study also included pre- and perimenopausal women their findings suggested snoring was associated with a significant increase in CVD. The primary aim of the present study is to determine if self-reported snoring frequency among postmenopausal women is significantly associated with an increased risk for incident coronary heart disease (CHD), stroke and all CVD.
METHODS
The Women’s Health Initiative (WHI) is a multi-center national study of 161,808 postmenopausal women who were enrolled in either the clinical trials (WHI-CT) or observational study (WHI-OS). Methods for recruitment, inclusion and exclusion criteria, protocols and study design have been described elsewhere.15-17 This study was conducted using longitudinal data from the WHI-OS cohort, which included women who were unable or unwilling to participate in the WHI-CTs. The WHI-OS cohort is a multiethnic population comprised of 93,676 women aged 50-79 years at baseline from 40 sites around the United States. Women with prior CVD were excluded from the analysis (N=6,813), as well as those with ‘don’t know’ (N=43,839) or ‘missing’ (N=780) responses to the snoring question. Recruitment (1993-1998) was conducted through mailings to eligible women. All participants were followed for outcomes through August 2009. All participants provided informed consent, which was approved by the Institutional Review Board for each of the participating study sites.
Snoring was evaluated based on self-report at the baseline study visit. Participants were asked, ‘Over the past 4 weeks, did you snore?’ Snoring was measured as: ‘1) No, not in the past 4 weeks 2) Yes, less than once a week 3) Yes, 1 or 2 times a week 4) Yes, 3 or 4 times a week 5) Yes, 5 or more times a week 6) Don’t know.’ Individuals were classified as non-snorers, occasional snorers (<1-4 times per week), and frequent snorers (≥5 times per week). This variable was analyzed as a categorical variable with ‘non-snoring’ as the reference group.
All outcomes were adjudicated by trained physicians using medical records and death certificates. The diagnosis and adjudication of the outcomes for this study have been previously described and outlined in established protocols.18 Our primary endpoint was incident CHD, defined as incident myocardial infarction (MI), CHD death, coronary revascularization, including coronary artery bypass graft (CABG) or percutaneous transluminal coronary angioplasty (PTCA), and hospitalized angina. Participants were followed for first occurrence of the CHD outcome, and those individuals who did not develop CHD were censored at the date of death or last contact. We also assessed incident ischemic stroke and total CVD (CHD and ischemic stroke).
Baseline demographic characteristics were assessed by self-reported questionnaire at the initial visit. Race/ethnicity was categorized as ‘White/Caucasian’, ‘Black/African American’, ‘Hispanic/Latina’, ‘Asian’ and ‘Other’. Annual household income was categorized into 3 groups, <$20,000, $20,000-<$50,000, and ≥$50,000. Education was classified as a 4-level variable, ‘less than high school’, ‘high school graduate’, ‘some college’, and ‘college graduate’. Marital status was categorized as a binary variable, married or marriage-like relationship and widowed, divorced, separated or single.
Behavioral risk factors measured in the baseline questionnaire included smoking, alcohol consumption and physical activity. Smoking was categorized as current smoker, former smoker or non-smoker. Alcohol consumption was measured as servings per week and physical activity was measured as MET-hours per week, estimated from 9 questions related to expenditure of energy from recreational activity (including walking, mild, occasional and strenuous activity).
Physical measurements, including height (m), weight (kg), waist circumference (cm), and systolic and diastolic blood pressure (mm Hg) were assessed at the baseline visit by trained and certified staff. Hypertension was defined as systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg, and/or use of antihypertensive medication. Diabetes was defined by self-report of physician-diagnosed diabetes or use of diabetes medications. Hyperlipidemia was defined as use of lipid-lowering medications or having been told of high cholesterol by a physician. Depression was measured at baseline using the Burnam screening algorithm, a measure that includes 6 items from the 20-item CES-D and 2 items from the National Institute of Mental Health’s Diagnostic Interview Schedule.19 A cutpoint of 0.06 was used to identify current depressive symptomatology.
We restricted analyses to those individuals without missing data for the primary exposure and outcome. The distribution of demographic characteristics, health behaviors and cardiovascular risk factors was examined across snoring category and the corresponding associations were statistically tested using analysis of variance (ANOVA) and Chi-square tests.
Person-years of follow-up were based on time from enrollment to cardiovascular event, loss to follow-up, death or end of the study (August 2009). Crude event rates were calculated and compared across snoring categories. The Log-Rank test was used to determine if there were significant differences in survival by snoring group. Hazard Ratios (HR) were computed using Cox proportional hazards models for each outcome. Models were assessed for the proportional hazards assumption.
Covariates that were considered for the models from baseline data included age, race/ethnicity, education, income, marital status, BMI, waist-to-hip ratio (WHR), sleep duration, hypertension status, diabetes status, smoking status, alcohol consumption, physical activity, depression, hyperlipidemia and hormone medication use. The final model included only those covariates that were statistically appropriate (p<0.10) and important based on theoretical considerations. Two-way interactions for snoring and BMI were assessed and stratified analyses were conducted.
Since over 50% of participants reported ‘don’t know’ for the primary exposure, we excluded these participants for the primary analysis or ‘complete case analysis’. Recognizing that this exclusion may result in selection bias,20 we conducted a sensitivity analysis utilizing the inverse probability weighting (IPW) method21 in which a model for the probability of ‘non-missingness’ was fit, and the inverse of these probabilities were used as weights in the complete case analysis. We used SAS version 9.3 (SAS Institute, Inc., Cary, NC) to perform all statistical analyses.
RESULTS
We observed 2,401 incident cases of CHD over 437,890 person-years of follow up. At baseline, 47% of women reported no snoring, 33% reported occasional snoring and 20% reported frequent snoring (Table 1). With regards to race/ethnicity, 84.5% of participants were Caucasian, 7.5% were African American, 3.6% were Hispanic/Latino, 2.8% were Asian and 1.5% were ‘other’. Compared to the overall cohort, women who reported frequent snoring had a greater BMI, a greater proportion of current smokers, a higher systolic and diastolic blood pressure, and a higher prevalence of diabetes and depression (Table 1).
Table 1.
Baseline characteristics in the Women’s Health Initiative study population distributed by snoring status
| Variable | Overall (N=42,244) | Frequency of snoring (times/wk)
|
p-value | ||
|---|---|---|---|---|---|
| 0 (N=19,886) | <1 - 4 (N=13,734) | ≥ 5 (N=8624) | |||
| Age (years) | 62.6 ( 7.2) | 62.8 (7.5) | 62.3 (7.0) | 62.5 (7.0) | <0.001 |
| Race | |||||
| Black/African American | 7.5% | 6.3% | 7.2% | 11.0% | <0.001 |
| Caucasian | 84.5% | 87.0% | 84.7% | 78.6% | |
| Hispanic/Latino | 3.6% | 2.5% | 4.1% | 5.6% | |
| Asian | 2.8% | 2.8% | 2.5% | 3.1% | |
| Other | 1.5% | 1.5% | 1.4% | 1.8% | |
| Education | |||||
| Less than High School Grad | 4.4% | 3.2% | 4.5% | 6.8% | <0.001 |
| High School Graduate | 15.1% | 13.3% | 15.9% | 18.2% | |
| Some College | 35.6% | 33.9% | 36.6% | 37.8% | |
| College Graduate | 44.9% | 49.6% | 43.0% | 37.2% | |
| Income (% annual household) | |||||
| <$20,000 | 12.4% | 11.1% | 10.5% | 18.1% | <0.001 |
| $20,000 - $49,999 | 40.6% | 38.6% | 40.8% | 44.6% | |
| ≥$50,000 | 47.1% | 50.3% | 48.6% | 37.3% | |
| Marital status | |||||
| Married or partnered | 71.1% | 69.5% | 78.3% | 63.5% | <0.001 |
| Single/divorced/widowed | 28.9% | 30.5% | 21.7% | 36.5% | |
| Body Mass Index (mean kg/m2) | 27.2 (5.9) | 25.5 (12.1) | 27.7 (5.8) | 30.3 (6.8) | <0.001 |
| Systolic blood pressure (mm Hg) | 125.9 (17.7) | 124.1 (17.7) | 126.6 (17.4) | 128.9 (17.4) | <0.001 |
| Diastolic blood pressure (mm Hg) | 74.9 (9.3) | 74.0 (9.2) | 75.4 (9.2) | 76.3 (9.6) | <0.001 |
| Hyperlipidemia† | 9.4% | 8.0% | 10.4% | 11.3% | <0.001 |
| Diabetes Mellitus | 3.1% | 2.1% | 3.1% | 5.4% | <0.001 |
| Smoker | |||||
| Never | 52.2% | 54.7% | 51.1% | 48.3% | <0.001 |
| Past | 42.2% | 41.0% | 43.1% | 43.5% | |
| Current | 5.6% | 42.7% | 5.8% | 8.2% | |
| Alcohol (servings/week) | 2.6 (5.3) | 2.6 (5.1) | 2.9 (5.4) | 2.4 (5.4) | 0.036 |
| Depression (CES-D/DIS) | 10.5% | 8.7% | 10.3% | 15.0% | <0.001 |
| Physical activity (met-hours/wk) | 14.4 (14.8) | 16.3 (15.7) | 13.7 (13.8) | 10.46 (13.0) | <0.001 |
| WHIIRS* | 6.6 (4.5) | 6.2 (4.4) | 6.6 (4.3) | 7.4 (4.7) | <0.001 |
WHI Insomnia Rating Scale - greater number indicates higher level of sleep disturbance
Defined as individuals taking lipid-lowering medications and who had been told by a physician that they had high cholesterol
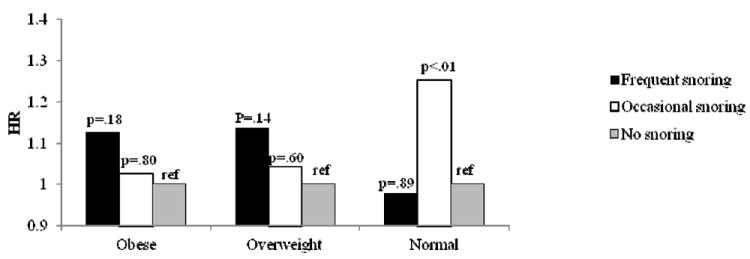
Product-limit survival estimates were assessed and the log-rank test indicated significant differences in survival rate by snoring group (p<0.001). Cox proportional hazards models adjusted for age and race indicated a 54% increased risk in CHD among frequent snorers and a 27% increased risk among occasional snorers, and similar hazard ratios were observed for stroke and CVD (Table 2). In fully adjusted models, adjusted for age, race, education, BMI, WHR, smoking, alcohol consumption, physical activity, depression, hypertension, diabetes, and hyperlipidemia, the positive associations for frequent snoring and cardiovascular outcomes were attenuated but still significant with an approximate 14% increased risk for CHD, 19% increased risk for stroke and 12% increased risk for CVD (Table 2). BMI, hypertension and diabetes were key drivers of this attenuation, and models including these covariates and sociodemographics are presented in Table 3. Because BMI is a strong confounder of the association between snoring and coronary disease, we tested for an interaction by obesity status, which was significant (p=0.024). In the stratified analysis, frequent snoring was a significant predictor of CHD among overweight and obese individuals in the age- and race-adjusted models (HR=1.29, 95% CI 1.07-1.54 for overweight; HR=1.33, 95% CI 1.10-1.61 for obese), but not normal weight individuals (HR=1.19, 95% CI 0.93-1.52). These associations were attenuated and no longer statistically significant in the fully adjusted models (Figure 1).
Table 2.
Cox proportional hazards models - snoring and incident cardiovascular disease among Women’s Health Initiative study participants (N=42,244)§
| Snoring frequency | Model adjusted for age, race | Model fully adjusted† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Person-years | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
|
|
|||||||||
| CHD* | Frequent | 609 | 86,227 | 1.54 | 1.39, 1.70 | <0.001 | 1.14 | 1.01, 1.28 | 0.038 |
| Occasional | 814 | 143,209 | 1.27 | 1.16, 1.39 | <0.001 | 1.12 | 1.01, 1.24 | 0.032 | |
| None (referent) | 978 | 208,454 | 1.00 | REF | REF | 1.00 | REF | REF | |
|
| |||||||||
| CVD‡ | Frequent | 790 | 84,671 | 1.46 | 1.34, 1.60 | <0.001 | 1.12 | 1.01, 1.24 | 0.031 |
| Occasional | 1103 | 141,122 | 1.25 | 1.16, 1.36 | <0.001 | 1.11 | 1.01, 1.21 | 0.026 | |
| None (referent) | 1354 | 205,547 | 1.00 | REF | REF | 1.00 | REF | REF | |
|
| |||||||||
| Ischemic Stroke | Frequent | 232 | 95,307 | 1.41 | 1.19, 1.66 | <0.001 | 1.19 | 1.02, 1.40 | 0.030 |
| Occasional | 349 | 153,389 | 1.29 | 1.11, 1.50 | <0.001 | 1.15 | 0.96, 1.38 | 0.140 | |
| None (referent) | 412 | 220,697 | 1.00 | REF | REF | 1.00 | REF | REF | |
Total CHD outcome includes MI, CHD death, PTCA, CABG, or hospitalized angina
CVD outcome includes MI, CHD death, PTCA, CABG, hospitalized angina, or ischemic stroke
Model adjusted for age, race, education, income, smoking, physical activity, alcohol intake, depression, diabetes, high blood pressure, BMI, WHR, hyperlipidemia
All analyses conducted among disease-free cohort and total N’s reflect this difference by outcome
Table 3.
Cox proportional hazards models - snoring and incident coronary heart disease*
| Model | Snoring Frequency | HR | 95% CI | p-value |
|---|---|---|---|---|
| 1 | Frequent | 1.46 | 1.15, 1.40 | <0.001 |
| Occasional | 1.27 | 1.32, 1.63 | <0.001 | |
| None (ref) | 1.00 | REF | REF | |
| 2 | Frequent | 1.28 | 1.14, 1.43 | <0.001 |
| Occasional | 1.19 | 1.08, 1.31 | <0.001 | |
| None (ref) | 1.00 | REF | REF | |
| 3 | Frequent | 1.33 | 1.19, 1.48 | <0.001 |
| Occasional | 1.19 | 1.08, 1.31 | <0.001 | |
| None (ref) | 1.00 | REF | REF | |
| 4 | Frequent | 1.41 | 1.27, 1.57 | <0.001 |
| Occasional | 1.25 | 1.13, 1.37 | <0.001 | |
| None (ref) | 1.00 | REF | REF | |
| 5 | Frequent | 1.21 | 1.08, 1.36 | <0.001 |
| Occasional | 1.15 | 1.04, 1.27 | 0.008 | |
| None (ref) | 1.00 | REF | REF |
Model 1: adjusted for sociodemographics (age, race, education, income)
Model 2: adjusted for sociodemographics + BMI
Model 3: adjusted for sociodemographics + hypertension
Model 4: adjusted for sociodemographics + diabetes
Model 5: adjusted for age, race, education, income, BMI, hypertension, diabetes
CHD outcome includes MI, CHD death, PTCA, CABG, or hospitalized angina
Figure 1.
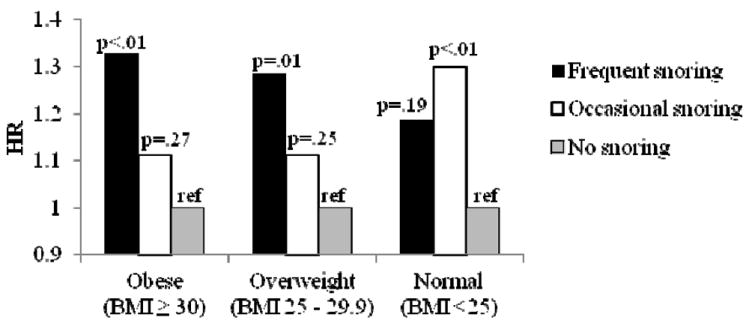
a. Cox Proportional Hazards Models Snoring and Coronary Heart Disease Stratified by Obesity Status
BMI – body mass index
HR – hazard ratio
REF – reference group
*Adjusted for age and race
b. Cox Proportional Hazards Models Snoring and Incident Coronary Heart Disease Stratified by Obesity Status
BMI – body mass index
HR – hazard ratio
REF – reference group
WHR – waist to hip ratio
**Adjusted for age, race, education, income, smoking, physical activity, alcohol intake, depression, diabetes, hyperlipidemia, BMI, WHR, hypertension
A sensitivity analysis was conducted using IPW. The weighted method generated results that also were similar to that of the complete case for the age- and race-adjusted models and indicated a 59% increased risk of CHD, 37% increased risk of stroke and 49% increased risk of CVD among frequent snorers (Table 4).
Table 4.
Sensitivity analysis - Cox proportional hazards models of snoring and incident cardiovascular disease among Women’s Health Initiative participants using inverse probability weighting (N=42,244)§
| Snoring frequency | Model adjusted for age, race | Model fully adjusted† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Person-yrs | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
|
|
|||||||||
| CHD* | Frequent | 609 | 86,227 | 1.59 | 1.42, 1.78 | <0.001 | 1.16 | 1.03, 1.30 | 0.018 |
| Occasional | 814 | 143,209 | 1.28 | 1.16, 1.42 | <0.001 | 1.12 | 1.00, 1.24 | 0.043 | |
| None (referent) | 978 | 208,454 | 1.00 | REF | REF | 1.00 | REF | REF | |
|
| |||||||||
| CVD‡ | Frequent | 790 | 84,671 | 1.49 | 1.35, 1.65 | <0.001 | 1.13 | 1.02, 1.25 | 0.018 |
| Occasional | 1103 | 141,122 | 1.26 | 1.15, 1.38 | <0.001 | 1.12 | 1.02, 1.23 | 0.026 | |
| None (referent) | 1354 | 205,547 | 1.00 | REF | REF | 1.00 | REF | REF | |
|
| |||||||||
| Stroke | Frequent | 232 | 95,307 | 1.37 | 1.15, 1.64 | 0.001 | 1.21 | 1.03, 1.43 | 0.020 |
| Occasional | 349 | 153,389 | 1.30 | 1.11, 1.52 | 0.002 | 1.14 | 0.94, 1.37 | 0.178 | |
| None (referent) | 412 | 220,697 | 1.00 | REF | REF | 1.00 | REF | REF | |
CHD outcome includes MI, CHD death, PTCA, CABG, or hospitalized angina
CVD outcome includes MI, CHD death, PTCA, CABG, hospitalized angina, or ischemic stroke
Model adjusted for age, race, education, income, smoking, physical activity, alcohol intake, depression, diabetes, high blood pressure, BMI, WHR, hyperlipidemia
All analyses conducted among disease-free cohort and total N’s reflect this difference by outcome
DISCUSSION
This prospective study among postmenopausal women demonstrates that frequency of snoring is associated with modestly increased risk of incident CHD, ischemic stroke and all CVD after adjusting for age and race. This significant association persisted but was attenuated in fully adjusted models.
These findings build on previous studies among men.4,7,8,22 Estimates from Marin et al. suggested a much weaker and non-significant association between snoring and CVD (OR=1.03, 95% CI 0.31-1.84), however the effect estimates among men with untreated mild-moderate OSA were similar to that of the postmenopausal women in our study (OR=1.15, 95% CI 0.34-2.69). Since the present study could not differentiate between simple snoring and OSA, it is likely that the snoring measure in our study included those with OSA and thus generated similar estimates. The differences between studies could also be due to power issues, as Marin and colleagues utilized a smaller sample (N=1,651) and had fewer events. Other previous studies among men reported a stronger association including a 71% increased odds of CHD, >2-fold increased risk of CVD, and >2-fold increased risk of stroke in adjusted models.4,7 These discrepancies however could be attributed to methodological differences in study design, including lack of adjustment for sociodemographics or CVD risk markers.4,7,8,22
We also evaluated the issue of potential selection bias induced by excluding the ‘don’t know’ individuals in a sensitivity analysis that used the IPW method.21 This approach is a powerful tool that addresses selection bias by creating a pseudo-population with characteristics that are more representative of the censored individuals. Similar HRs with a slightly stronger estimate of effect was observed in the IPW analysis, which further supported our findings from the complete case analysis.
It has been previously demonstrated that habitual snoring is associated with greater BMI.6,23 Our findings indicate that snoring is an independent predictor of increased CVD risk even after adjustment for obesity and other risk factors, and an interaction was present between snoring and BMI (p=0.02). When stratifying by obesity status, findings indicated a dose-response relationship between frequency of snoring and CHD risk, however this was present only among obese and overweight individuals and not among those with a BMI<25. These findings are not completely consistent with Hu and colleagues, where a positive association between snoring and CVD was observed across all BMI strata.3 Given the bidirectional relationship of obesity and snoring, we cannot rule out that obesity may be the driving force of the observed association. Compared to previous reports among women,3 our findings draw from a more diverse sample with regards to race/ethnicity and sociodemographics, and thus these results are more generalizable to the entire U.S. population. Replication of these findings is important, especially given the cardiovascular consequences of obesity and the complex relationship with snoring. Additional investigations need to evaluate the association between snoring and incident CVD and assess the relationship among obese and non-obese subjects.
Snoring may increase risk of CVD through atherosclerosis. Proposed biological mechanisms involve increased localized carotid endothelial dysfunction due to upper airway resistance and subsequent vibrations in the pharyngeal wall, a hypothesis that has been supported by animal models among rabbits.24-26 Heavy snoring is significantly associated with increased carotid intima-media thickness (IMT) and plaque (OR=1.71, 95% CI 1.22-2.39 for IMT; OR=3.63, 95% CI 2.57-5.12 for carotid bifurcation plaque),27 and there exists a dose response (p<0.04) between snoring frequency and atherosclerosis.24 Lee and colleagues also suggested that frequent snoring is associated with increased carotid atherosclerosis (OR=10.5, 95% CI, 2.1-51.8), but not with femoral atherosclerosis.24 Findings from angiography have also supported that snoring in combination with feelings of tiredness was associated with increased atherosclerosis compared to non-snorers (0.18 mm vs. 0.07 mm change, p=0.0006).25 Further research is needed to determine if snoring alone increases CVD risk through these mechanisms, or if it simply represents a marker for the presence of sleep apnea, an established as a risk factor for CVD.
Because the simple measure of self-reported snoring may represent early stages of the OSA continuum, it may serve as an early marker of sleep apnea and serve as a useful public health screening tool. Polysomnography (the gold standard for diagnosing OSA) is costly and time intensive, and so it is increasingly important to understand how self-reported snoring is associated with CVD outcomes. However, although snoring is considered a symptom of OSA, many individuals that snore do not have sleep apnea. Future research should strive to identify the relative impact of sleep symptoms versus true disorders on adverse disease outcomes.
Self-reported snoring is not a perfect measure of snoring frequency and the reliability of snoring data were not validated in this population. We conducted a sensitivity analysis among only those women that were partnered (71% of women) and found similar estimates of effect for age and race adjusted models. Although a similar trend was observed in fully adjusted models, the association was not significant among this subgroup. However, self-reported snoring has been previously validated against overnight sleep monitoring, and findings suggest self-report to be a reliable measure.28 Also, self-reported snoring over 1 month represents an integrated assessment, which cannot always be achieved by a single overnight study in a controlled setting. Because this association was assessed prospectively, it is likely that the misclassification is non-differential with respect to the outcome, resulting in an underestimation of the effect estimate, which would bias the results towards the null. Future studies should consider alternative ways to measure this exposure, such as partner-reported snoring habits or alternative measures of snoring during sleep. Because over 50% of these individuals reported ‘don’t know’ in response to the snoring question, we cannot make inferences for these individuals. It should be noted that the IPW statistical methodology does not come without some limitations, and that if the level of exposure is associated with whether snoring was reported, then the level of weights may be subject to bias.29 The IPW sensitivity analysis did however generate similar estimates of effect, which supported our primary analysis. Lastly, because we did not assess sleep apnea using polysomnography, we are unable to determine whether simple snoring without sleep apnea may increase the risk of CVD outcomes. While sleep apnea may be driving the observed association, we noted that occasional snoring was also significantly associated with CVD, suggesting that individuals who snore but potentially do not have OSA were still at increased risk for disease.
Acknowledgments
We are grateful for the contributions of the WHI study investigators and participants. Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Sources of Financial Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Finn L, Hla KM, Morgan B, Palta M. Snoring as part of a dose-response relationship between sleep-disordered breathing and blood pressure. Sleep. 1996;19(10 Suppl):S202–205. doi: 10.1093/sleep/19.suppl_10.s202. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Willett WC, Manson JE, Colditz GA, Rimm EB, Speizer FE, Hennekens CH, Stamfer MJ. Snoring and risk of cardiovascular disease in women. J Am Coll Cardiol. 2000;35:16–19. doi: 10.1016/s0735-1097(99)00540-9. [DOI] [PubMed] [Google Scholar]
- 4.Koskenvuo M, Kaprio J, Telakivi T, Partinen M, Heikkila K, Sarna S. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J (Clin Res Ed) 1987;294:16–19. doi: 10.1136/bmj.294.6563.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Delaimy WK, Manson JE, Willett WC, Stamfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002;155:387–393. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 6.Leineweber C, Kecklund G, Akerstedt T, Janszky I, Orth-Gomer K. Snoring and the metabolic syndrome in women. Sleep Med. 2003;4:531–536. doi: 10.1016/s1389-9457(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 7.Palomaki H. Snoring and the risk of ischemic brain infarction. Stroke. 1991;22(8):1021–1025. doi: 10.1161/01.str.22.8.1021. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Phillips BA, Collop NA, Drake C, Consens F, Vgontzas AN, Weaver TE. Sleep disorders and medical conditions in women. J Womens Health (Larchmt); Proceedings of the Women & Sleep Workshop; Washington, DC. March 5-6, 2007; National Sleep Foundation; 2008. pp. 1191–1199. [DOI] [PubMed] [Google Scholar]
- 10.Kapsimalis F, Kryger M. Sleep breathing disorders in the U.S. female population. J Womens Health (Larchmt) 2009;18:1211–1219. doi: 10.1089/jwh.2008.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichling PS, Sahni J. Menopause related sleep disorders. J Clin Sleep Med. 2005;1:291–300. [PubMed] [Google Scholar]
- 12.Netzer NC, Eliasson AH, Strohl KP. Women with sleep apnea have lower levels of sex hormones. Sleep Breath. 2003;7:25–29. doi: 10.1007/s11325-003-0025-8. [DOI] [PubMed] [Google Scholar]
- 13.D’Ambrosio C, Stachenfeld NS, Pisani M, Mohsenin V. Sleep, breathing, and menopause: the effect of fluctuating estrogen and progesterone on sleep and breathing in women. Gend Med. 2005;2:238–245. doi: 10.1016/s1550-8579(05)80053-1. [DOI] [PubMed] [Google Scholar]
- 14.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J of Resp and Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 15.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 suppl):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 16.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 suppl):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 17.Rossouw JE, Hurd S. The Women’s Health Initiative: recruitment complete – looking back and looking forward. J Womens Health. 1999;8:3–5. doi: 10.1089/jwh.1999.8.3. [DOI] [PubMed] [Google Scholar]
- 18.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Crique M, Daugherty S. WHI Morbidity and Mortality Committee. Ann Epidemiol. 2003;13(9 suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 19.Tuunainen A, Laner RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103:261–270. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 20.Hernan M, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 21.Hernan M, Lanoy E, Costagliola D, Robins J. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clinical Pharmacol Toxicol. 2005;98:237–242. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- 22.Partinen M, Palomaki H. Snoring and cerebral infarction. Lancet. 1985;2(8468):1326–1326. doi: 10.1016/s0140-6736(85)92625-x. [DOI] [PubMed] [Google Scholar]
- 23.Svensson M, Lindberg E, Naessen T, Janson C. Risk factors associated with snoring in women with special emphasis on body mass index: a population-based study. Chest. 2006;129:933–941. doi: 10.1378/chest.129.4.933. [DOI] [PubMed] [Google Scholar]
- 24.Lee SA, Amis TC, Byth K, Larcos G, Kairaitis K, Robinson TD, Wheatley JR. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207–1213. [PMC free article] [PubMed] [Google Scholar]
- 25.Leineweber C, Kecklund G, Janszky I, Akerstedt T, Orth-Gomer K. Snoring and progression of coronary artery disease: The Stockholm Female Coronary Angiography Study. Sleep. 2004;27:1344–1349. doi: 10.1093/sleep/27.7.1344. [DOI] [PubMed] [Google Scholar]
- 26.Cho JG, Witting PK, Verma M, Wu BJ, Shanu A, Kairaitis K, Amis TC, Wheatley JR. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep. 2011;34:751–757. doi: 10.5665/SLEEP.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Liu J, Wang W, Yong Q, Zhou G, Wang M, Sun J, Zhao D. Association of self-reported snoring with carotid artery intima-media thickness and plaque. J Sleep Res. 2011;14:1365–2869. doi: 10.1111/j.1365-2869.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 28.Telakivi T, Partinen M, Koskenvuo M, Salmi T, Kaprio J. Periodic breathing and hypoxia in snorers and controls: validation of snoring history and association with blood pressure and obesity. Acta Neurol Scand. 1987;76:69–75. doi: 10.1111/j.1600-0404.1987.tb03547.x. [DOI] [PubMed] [Google Scholar]
- 29.Hernan MA, Alonso A, Logan R, Grodstein F, Michels K, Willett W, Manson J, Robins J. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]


