Abstract
BACKGROUND
Vaccines have treatment potential for methamphetamine (MA) addiction. We tested whether a conjugate vaccine against MA (succinyl-methamphetamine–keyhole limpet hemocyanin carrier protein; SMA-KLH) would generate MA antibodies and alter MA-induced behaviors.
METHODS
Mice were injected with SMA-KLH and received booster administrations 3-and 20-weeks later. Serum antibody titers reached peak levels by 4–6 weeks, remained at a modest level through 18-weeks, peaked again at 22-wks after the second boost, and were still elevated at 35-weeks. At 7 weeks, groups of vaccinated and non-vaccinated mice were administered one of three MA doses (1, 2, or 3 mg/kg) to assess locomotor activity.
RESULTS
Non-vaccinated mice showed dose-dependent effects of MA with hypolocomotion at the lowest dose and elevated activity levels at the highest dose. Both dose effects were reduced in SMA-KLH groups, particularly low dose-induced hypolocomotion at later times post MA administration. Separate groups of vaccinated and non-vaccinated mice were trained in MA place conditioning at 30-weeks with either 0 (vehicle) or 0.5 mg/kg MA. Although times spent in the MA-paired side did not differ between groups on Test vs. Baseline sessions, SMA-KLH mice conditioned with MA showed reduced conditioned approach behaviors and decreased conditioned activity levels compared to control groups.
CONCLUSION
These data suggest SMA-KLH attenuates the ability of MA to support place conditioning and reduces or delays its locomotor effects. Overall, results support SMA-KLH as a candidate MA vaccine.
Keywords: conjugate vaccine, keyhole limpet hemocyanin, monophosphoryl lipid A, serum antibody titers, locomotor activity, conditioned place preference
1. INTRODUCTION
Methamphetamine (MA) abuse has grown at alarming rates in the United States over the past two decades and is spreading across Southeast and East Asia (Gonzales et al., 2010; McKetin et al., 2008). Currently, there are no FDA approved medications for treating MA addiction. The highly addictive effects of MA likely relates to its known central and peripheral sympathomimetic effects (Darke et al., 2008; Fowler et al., 2007; Volkow et al., 2010) and to its ability to release multiple neurotransmitters, including dopamine (DA), norepinephrine (NE), serotonin (5-HT), histamine, and gamma-aminobutyric acid (GABA), from synaptic vesicles (Sulzer et al., 2005). Due to this multiplicity of effects, MA is much less likely than other drugs of abuse to be treated effectively with specific pharmacological antagonists or substitute agonists.
An increasingly appealing approach to drug addiction treatment is to use conjugate drug vaccines to induce specific antibody blockade of abused drugs. Conjugate vaccines developed against cocaine and nicotine have progressed to clinical trials (Hatsukami et al., 2005; Martell et al., 2009). Data from these trials suggest that many patients may not produce a sufficient antibody response, but of those who do, drug use is reduced and abstinence rates can be quite good (Hatsukami et al., 2011; Martell et al., 2009; Maurer and Bachmann, 2007). An anti-MA vaccine could also be a viable treatment approach for this addiction. Several laboratories have been working on evaluating the best composition for an MA vaccine. For example, Janda’s group has recently evaluated three MA-KLH conjugate vaccines that generated substantial antibody titers with good affinity (Moreno et al., 2011). There are several choices in vaccine construction, including hapten design, selection of the carrier protein, the chemical positioning of a linker between the target antigen and the carrier protein, and selection of the adjuvant (Byrnes-Blake et al., 2001; Moreno et al., 2011; Peterson et al., 2007).
In theory, an anti-MA vaccine would generate antibodies that bind to MA so that when MA is subsequently introduced into the bloodstream, those antibodies would bind to it and form antibody-MA complexes within the circulatory system. Such complexes should be too large to readily cross the blood–brain barrier and therefore would reduce the rate or amount of MA entry into the brain. Antibody-bound drug would then be slowly released from antibody binding in the equilibrium state as residual-free drug to be metabolized and eliminated. Reduction in either rate or amount of MA entering the brain should attenuate its behavioral effects, including its ability to be rewarding. Here, we report on the effects of one such construct, succinyl-methamphetamine-keyhole limpet hemocyanin (SMA-KLH), in mice.
We assessed the ability of SMA-KLH to generate antibodies in mice by measuring serum titers across a 35-week period. In addition, we tested the functional effects of SMA-KLH by examining whether vaccinated mice would show attenuated behaviors induced by MA administration. Two behavioral assays were chosen based on known actions of MA and other psychostimulants in mice and rats. These were locomotor activity and conditioned place preference (CPP). MA and other psychostimulants, such as cocaine, increase locomotor activity at moderate doses and can induce stereotypic responses, such as sniffing, head bobbing, and other in-place activities, at higher doses (Antoniou et al., 1998; Brien et al., 1978; Ellinwood and Balster, 1974; Kuczenski and Segal, 1989). However, at very low doses, cocaine and amphetamine can cause hypolocomotion in rats and mice (George, 1989, 1990) and we recently confirmed this observation with MA in mice (Kitahama and Valatx, 1979; Singh et al., submitted). CPP is a procedure that has been used to assess the rewarding properties of many drugs (Bardo and Bevins, 2000; Carr et al., 1989; Schechter and Calcagnetti, 1993; Tzschentke, 1998). In CPP, drug administrations are paired with a distinct context while vehicle is paired with a different context. After several conditioning trials, the animal is allowed access to both contexts and the degree to which it approaches and spends more time in the drug-paired context is thought to measure drug reward.
2. METHODS
2.1Animals and housing
Female BALB/c mice were bred in the Houston VAMC vivarium from mice originally purchased from Charles River Laboratories (Wilmington, MA). Mice were 8 wks of age and weighed approximately 20 g at the start of the study. They were group-housed (5 per cage) under temperature-and humidity-controlled conditions with a 12:12 h light/dark cycle (lights on from 0600). Food and water were available ad libitum. Procedures were approved by the Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (1996).
2.2 Drug
(+) Methamphetamine hydrochloride (MA; Research Triangle Institute, Research Triangle, NC) was dissolved in PBS and prepared as salt base. MA was administered at a dose of 1, 2 or 3 mg/kg (IP) in a volume of 5 ml/kg for the locomotor study. A dose of 0.5 mg/kg (SC) in a volume of 4 ml/kg was used for the conditioned place preference (CPP) study. These doses were chosen based on previous studies (Brien et al., 1978; Itzhak and Ali, 2002; Shabani et al., 2011b; Wheeler et al., 2009).
2.3 Generation of a carrier protein/methamphetamine conjugate
The hapten, succinyl methamphetamine (SMA), was prepared by refluxing a solution of methamphetamine HCl, succinic anhydride, and triethyl amine in CH2Cl2 for several hours. The solution was washed with 10% HCl and saturated NaCl, dried over anhydrous Na2SO4 and the solvent removed on a rotary evaporator. Thin layer chromatography (Analtech Uniplate) using 5% methanol in CH2Cl2 showed no starting material. Conjugation to keyhole limpet hemocyanin (KLH) (Thermo Fisher Scientific; Houston, TX) or fish gelatin (FG) involved first making a solution of 10 mg KLH or FG in 1-mL phosphate-buffered saline (PBS; pH 7.4). Then, a solution of sulfoNHS and EDC in 0.5 mL PBS was prepared and a solution of SMA in 10 μL DMSO was added. After several hours of stirring at room temperature, this solution was added to the KLH or FG solution. The pH was adjusted to ~7.5 with NaOH and stirring continued overnight at room temperature. The conjugate protein solution was passed through an NAP-25 column (GE HealthCare; Fairfield, CT) equilibrated with PBS and 2.5 mL was collected, giving a final concentration of KLH or FG conjugate of 4 mg/mL. Hapten structure was confirmed by NMR and conjugation conditions to carrier proteins were selected by maximum ELISA responses with anti-methamphetamine antisera.
2.4 Immunization, blood collection, and behavioral testing schedules
Mice were administered (SC) a vaccine consisting of 200-μl PBS containing 100-μg of SMA-KLH and 50-μg of monophosphoryl lipid A (MPL; Sigma; St. Louis, MO). They received a booster injection with the same vaccine formula twice, once at 3-weeks and again at 20-weeks after the initial injection. Blood was collected at weeks 2, 4, 6, 8, 12, 22, 24, 26, 35 and 40 after initial immunization and allowed to clot at room temperature for 2-hrs. Samples were centrifuged (4000-rpm for 15-m) and sera collected and stored at −80 °C until ELISAs were performed (see below). The locomotor study was conducted at week 7 and the conditioned place preference (CPP) study from weeks 31–33 as shown in the timeline in Fig. 1.
Figure 1.
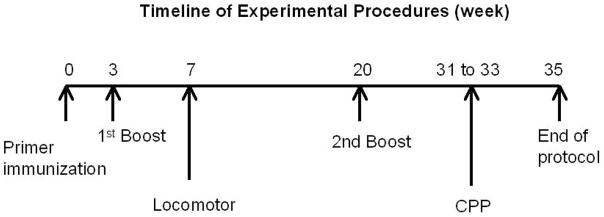
The timeline of the vaccine administrations and behavioral tests is presented.
2.5 Determination of serum anti-MA titers by ELISA
To measure specific anti-MA antibody, ELISA plates (Immulon 2HB, Daigger, Vernon Hills, IL) were coated overnight in carbonate buffer (0.05M; pH 9.6) using SMA conjugated to FG, a heterologous carrier protein. Background antibody binding to the carrier alone (which was very low in most samples) was subtracted from every sample to ensure that the results reflected antibodies specific for the hapten, MA.
Pooled (n=30) or individual serum samples were added to plates in three fold serial dilutions starting at 1:5000 or 1:15000 in PBS-tween (0.1%) and incubated for 2-h. After washing with PBS-Tween, goat anti-mouse IgG conjugated to horseradish peroxidase (Southern Biotech, Birmingham, AL) was then incubated in the plates for 30-min. The plates were again washed and then substrate (Tetramethylbenzidine, Sigma, St. Louis, MO) was incubated in the plates for 45-min. Reactions were stopped with 1M HCl. The optical density (O.D.) was measured on a microplate reader (LabX, Canadian; ON, Canada).
2.6 Locomotor activity procedure
The locomotor activity study was performed at week 7. Horizontal and vertical activity levels were tabulated and analyzed via the system of infrared beams located on two sets of bars (1″ and 2.25″ above the floor of the test cage) using a commercially-available, automated hardware and software system (Opto-M3; Columbus Instruments; Columbus, OH). Mice were habituated to the apparatus and procedure during two, 60-min sessions. Briefly, mice were brought into the procedure room and, after a short acclimatization period, were placed individually into one of the 15 acrylic test cages (17.5″ L × 17.5″ W × 8.0″ H). On the day following habituation sessions, the MA test was conducted. Mice were placed in the apparatus for 60-min to obtain baseline levels of activity (last 10-min of this session). After this time, separate groups of mice (n’s=5–15) were administered one of the three MA doses (1, 2, or 3 mg/kg) and immediately placed back into the chamber for 90-min. Data on horizontal and vertical activity levels were tabulated in 10-min time blocks in order to test if absolute levels of activity were altered by the vaccine as well as the time course of the effects of MA administration. The study was conducted under dim lighting conditions between 0900 and 1200 hr.
2.7 Conditioned place preference procedure
The conditioned place preference (CPP) procedure was conducted during weeks 31–33 using a commercially-available system (San Diego Instruments; San Diego, CA). The CPP apparatus consisted of two visually and tactilely distinct sides (13 1/8″ L × 13 1/2″ W × 8 1/8″ D ea). One side was black and had a coarse, wire-mesh floor and the other side had vertically-striped, black and white, walls with a smooth floor. Lighting had been adjusted in the black compartment so that times spent on each side were similar. A black, acrylic barrier could be inserted between the two compartments to confine the animal to a side during training trials. When the barrier was removed during test sessions, the mouse was placed facing a wall on the seam separating the two sides at the start of the session. A 4 × 15 photobeam array was in place on each side to tabulate the times the mouse spent on each side, as well as other measures, using the Photobeam Activity System (PAS v 2.0) software program (San Diego Instruments). Data on ambulatory activity levels, numbers of entries and exploratory nose pokes into each side, as well as times (sec) spent on each side were recorded.
On the first day, mice were placed into the apparatus with the barrier removed for 20-min to habituate them to the procedure and apparatus. The next day of the CPP procedure was the Baseline session. In this session, each mouse was placed into the apparatus with the barrier removed and allowed to move between compartments for 20-min. This study utilized an unbiased procedure in which the side assigned to be the drug-conditioned compartment was not based on initial side preference during the Baseline session. Any mouse that showed a strong compartment bias (>75% time spent in one compartment) was excluded. Two days later, conditioning trials began. On four alternating days, mice were administered their assigned MA dose (0 or 0.5 mg/kg) and confined to one compartment of the apparatus and on the other days, mice were administered vehicle injections and confined to the other compartment. The apparatus was cleaned with Clidox after each session. Each of these training sessions was 20-min in duration with one session performed per day. Mice assigned to receive 0 (vehicle) mg/kg MA received vehicle injections on all training sessions. Following the eight training sessions, a test session was run with no drug injections. This session was conducted as in the Baseline session in which the barrier was removed and the mouse had access to both compartments of the apparatus for 20-min. This study was conducted under dim lighting conditions between 0900 and 1200 hr.
2.8 Data analysis
All data are expressed as the mean ± SEM. Two measures of locomotor activity were obtained – horizontal and vertical – assessed by the number of beam breaks of the lower and upper infrared beam bars, respectively. Horizontal activity and vertical activity levels were analyzed in separate 2 × 3 × 9 analysis of variance (ANOVAs) representing the between group factors of Vaccine and MA Dose groups with repeated measures on time (nine, 10-min time blocks) post-MA administration. We also assessed the time of peak activity for both horizontal and vertical activity measures individually by mouse. These data were analyzed using 2 × 3 (Vaccine group X Dose) ANOVAs. Significant main effects or interactions were followed by post-hoc comparisons (Newman- Keuls). The P value was set at 0.05.
Four measures were obtained from the CPP study. These included an assessment of ambulatory activity in the drug-paired side, and three assessments of approach behavior: 1) numbers of entries into the drug-paired side; 2) numbers of nose pokes exploring the drug-paired side; and 3) times (sec) spent on the drug-paired side. These measures at Baseline were used as co-variates and analyzed in separate 2 × 2 ANOCOVAs per measure representing the between group factors of Vaccine and MA Dose groups. The P value was set at 0.05.
3. RESULTS
3.1 Serum anti-MA titers
Titers of MA antibodies induced by administration of SMA-KLH were assessed from pooled samples at several time points after the initial injection as seen in Fig. 2. The initial administration of the MA vaccine leads to a modest level of antibody production seen at 2 weeks. Higher titers of MA antibodies are generated after the first vaccine boost given at week 3. Titers are quite high at 4 weeks, peak at 6 weeks, and then decrease to lower levels seen through week 18. There is a stable titer of antibodies from weeks 8 to 12 and, by 18-weeks, levels had decreased to about that seen after the initial vaccine administration (week 2). The second boost, given at week 20, results in another substantial antibody peak seen at 22 weeks after which titers decrease slightly but are maintained at moderate levels for the remaining 12 weeks of the study.
Figure 2.
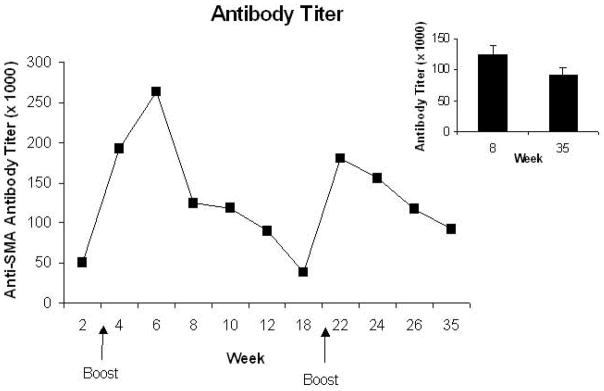
The pooled sera anti-MA antibody titers obtained at various times points across the 35-week protocol are shown. Note that a second vaccine administration was given at 3-weeks and a third administration at 20-weeks. The arrows depict these time points. The inset shows the mean (±S.E.M.) of the titers at the two times for which assays were conducted on individual mouse sera including the two mice that did not produce antibodies.
Sera samples from individual mice were examined at two times, 8 weeks and 35 weeks, after the initial vaccination (see Inset for Fig. 2). There is no difference in antibody titers between these two time points, P>0.10. The antibody titers at 8 weeks are highly correlated with the titers at 35 weeks, r=0.81; P<0.0001. That is, mice that generate high titers of antibodies at the early time are very likely to show high titers at the later time point and vice versa. However, no MA antibodies were detected in two of the 30 mice at either time point. These two mice were not employed in the behavioral studies.
3.2 Locomotor activity
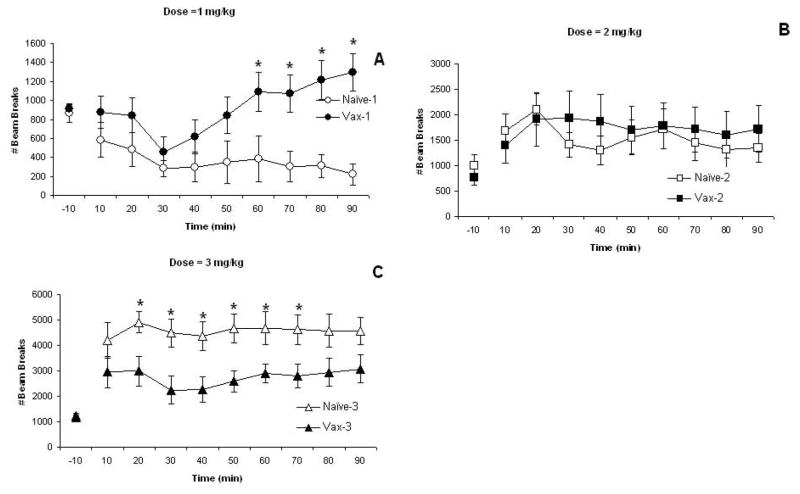
The first measure of locomotor activity is horizontal movements. These data are shown in Fig. 3 with data from each MA dose presented in separate panels (note scale differences across doses). There were no significant differences in baseline (−10 min) horizontal activity measures, P’s>0.10. MA leads to dose-dependent effects on horizontal activity in naïve, control mice and in the vaccinated mice as supported by the significant Dose effect, F(2,54)=37.00; P<0.0001. Activity levels are much greater after administration of the 3 mg/kg dose of MA compared to the other doses. Horizontal activity levels change over the session as supported by the significant Time effect, F(8,432)=2.36; P<0.05. This likely reflects that the lowest MA dose induces low levels of horizontal activity, a decrease from pre-drug administration levels, across the session (Fig. 3A). This effect is particularly noticeable in naïve mice. Vaccinated mice also show an initial hypolocomotor response but activity returns to pre-drug administration levels (or slightly higher) by 50-min. Indeed, the vaccine significantly affected horizontal activity differentially by dose as supported by the significant Vaccine X Dose interaction effect, F(2,54)=5.97; P<0.005. This interaction may also reflect that at the highest MA dose, the elevated activity levels of the vaccinated mice dips a bit at 30–40 min unlike the levels of the naïve mice that remains elevated across the entire session (Fig. 3C). Post-hoc comparisons by dose show significant vaccine group differences from 60–90 min at the lowest dose (Fig. 3A) and from 20–70 min at the highest dose (Fig. 3C), P’s<0.05, with no group differences seen at any time point for the 2 mg/kg dose (Fig. 3B), P’s>0.10.
Figure 3.
The mean (± S.E.M.) numbers of horizontal beam breaks are shown in 10-min time blocks for naïve (non-vaccinated) mice (open symbols) and for vaccinated mice (closed symbols) that were administered 1- (Panel A; circles), 2- (Panel B; squares), or 3- (Panel C; triangles) mg/kg MA. Activity levels at −10-min represent the last time block of a 60-min pre-drug injection baseline. These data show significant Dose and Vaccine Group X Dose effects as well as a significant Time effect. See text for statistics. *represents significant post-hoc Vaccine group effects.
Time to peak horizontal activity levels are given in Table 1 by vaccine and MA dose groups. Peak activity levels occur later in the Vaccine groups compared to the naïve groups, particularly at the lowest MA dose. This statement is supported by the significant main effect of Vaccine group, F(1, 54)=5.81; P<0.02, and by its significant interaction with Dose, F(2,54)=4.28; P<0.02. There was also a trend towards significance for the main effect of Dose, F(2,62)=2.93; P<0.10. Post-hoc comparisons show that the vaccine group administered 1 mg/kg MA differs significantly from all three naïve groups and from the vaccine group administered 2 mg/kg MA, P’s <0.05. Because the lowest MA dose caused hypolocomotion, particularly in the naïve mice, we also examined time to recovery – the time when activity returned to the level seen at baseline (i.e., −10 min). However, less than half of the naïve mice showed recovery compared to almost 75% of vaccinated mice. Of those mice that showed recovery from hypolocomotor-inducing effects of the 1 mg/kg MA dose, there was no difference in latency to return to baseline activity levels (16- vs 23-min; naïve vs. vaccinated).
Table 1.
Mean (± S.E.M.) time (min) of peak response for horizontal activity and vertical activity is presented by vaccine group and methamphetamine dose (mg/kg).
| MA DOSE | HORIZONTAL ACTIVITY | VERTICAL ACTIVITY | ||
|---|---|---|---|---|
| Naïve | Vaccine | Naïve | Vaccine | |
| 1 | 27.0 (6.0) | 71.3 (6.2) * | 21.1 (6.5) ** | 69.3 (7.0) |
| 2 | 29.3 (6.1) | 37.0 (8.7) | 47.1 (9.3) | 63.0 (10.8) |
| 3 | 50.0 (13.8) | 50.0 (15.1) | 88.0 (2.0) | 68.0 (10.2) |
Vaccine group tested with 1 mg/kg MA differs significantly from all three naive groups (horizontal).
Naive group tested with 1 mg/kg MA differs significantly from all three vaccine groups (vertical).
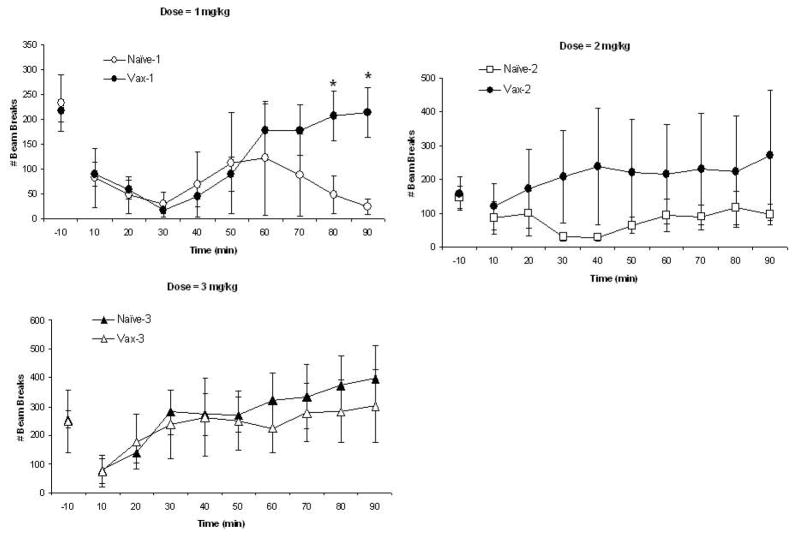
The second measure of locomotor activity is vertical movements and these data are shown in Fig. 4 with data from each MA dose presented in separate panels (note scale differences across doses). There were no significant differences in baseline (−10 min) vertical activity measures, P’s>0.10. Vertical activity levels change over the session as supported by the significant Time effect, F(8,432)=7.72; P<0.0001. These changes over session differ by dose group as supported by the significant Dose X Time interaction, F(16,432)= 2.07; P<0.01. This likely reflects that vertical activity levels increase over the session in the groups that received the highest MA dose (Fig. 4C). Vertical activity also varies over time in groups administered the lowest MA dose as seen in Fig. 4A; decreased activity is seen initially followed by increased activity at 40–50 min for the vaccine group. These statements are supported by the significant Vaccine group X Dose X Time interaction, F(16,432)= 1.78; P<0.05. Post-hoc comparisons by dose showed significant vaccine group differences at 80–90 min at the lowest dose (Fig. 4A) with no group differences seen at the 2 mg/kg (Fig. 4B) or 3 mg/kg (Fig. 4C) doses, P’s>0.10. Note that there is a good deal of variability in these measures, particularly for the vaccine group administered the 2 mg/kg MA dose and the naïve group administered the 1 mg/kg MA dose. In fact, variability is high prior to MA administration (−10 min), particularly for the 1 and 3 mg/kg doses.
Figure 4.
The mean (± S.E.M.) numbers of vertical beam breaks are shown in 10-min time blocks for the naïve (non-vaccinated) mice (open symbols) and for vaccinated mice (closed symbols) that were administered 1- (Panel A; circles), 2- (Panel B; squares), or 3- (Panel C; triangles) mg/kg MA. Activity level at −10-min represents the last time block of a 60-min pre-drug injection baseline. These data show a significant Time effects as well as significant interactions of Time with MA Dose and Vaccine groups. See text for statistics. *represents significant post-hoc Vaccine X Dose effects.
Time to peak vertical activity levels are given in Table 1 by vaccine and MA dose groups. Peak activity levels occur later as dose increases in the Vaccine groups whereas time to peak activity in the naïve groups does not differ by MA dose. This statement is supported by the significant main effect of Dose group, F(2,52)=4.62; P<0.02, and by the significant interaction of Vaccine X Dose, F(2,52)=5.28; P<0.01. There was also a trend towards significance for the main effect of Vaccine, F(2,54)=3.2; P<0.08. Post-hoc comparisons show that the naive group administered 1 mg/kg MA differs significantly from all three vaccine groups and from the naive group administered 3 mg/kg MA, P’s <0.05.
3.3 Conditioned place preference
Six mice were eliminated from the conditioned place preference (CPP) analysis due to bias towards one compartment during the Baseline test (>75% time in one compartment). The measures obtained on the drug side during the Test session (post-conditioning) from the remaining mice (n’s = 10–15/group) are analyzed using measures obtained on this side during the Baseline session (preconditioning) as co-variates in the ANOCOVAs.
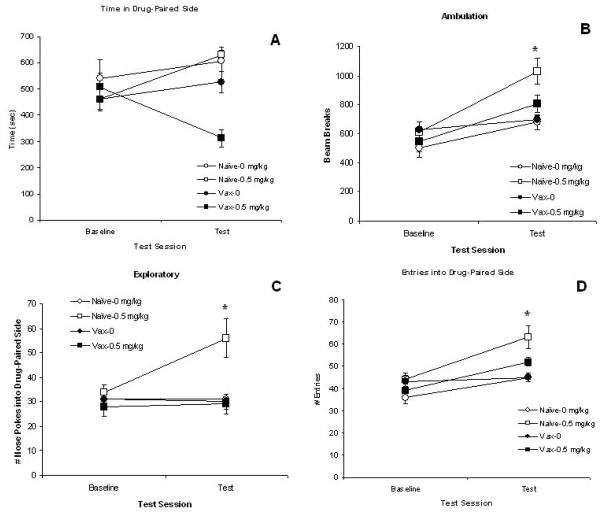
Four measures are examined and these data are shown in Fig. 5. First, we present times (sec) spent on the drug-paired side (Fig 5A). Although it appears that times spent in the MA-paired side differ in the vaccinated mice conditioned with MA, there is no significant difference between vaccine groups or between MA groups (Vehicle-vs. Drug-trained), P’s>0.10. None of the interactions is significant either, P’s>0.10. However, all of the other three measures do show significant Vaccine or MA dose group effects. Next, data on conditioned locomotor activity is shown in Fig. 5B. Conditioned activity levels are higher in mice conditioned with MA compared to mice conditioned with vehicle (0 mg/kg) as supported by the significant MA Dose group, F(1,44)=19.87; P<0.0001. These levels are also higher in naïve mice compared to vaccinated mice, particularly for the 0.5 mg/kg dose groups. This statement is supported by the significant Vaccine group effect, F(1,44)=5.20; P<0.05. Post-hoc comparisons show that the naïve group conditioned with 0.5 mg/kg MA differs from all other groups, P’s<0.01.
Figure 5.
The measures obtained on the Baseline and Test sessions of the place conditioning study are shown for the naïve (non-vaccinated) mice (open symbols) and for the vaccinated mice (closed symbols). Panel A presents the mean (± S.E.M.) time (sec) spent on the drug-paired side. Panel B presents the mean (± S.E.M.) amount of ambulatory activity in the drug-paired side. Panel C presents the mean (± S.E.M.) numbers of exploratory nose pokes into the drug-paired side. Panel D presents the mean (± S.E.M.) numbers of entries into the drug-paired side. Circles represent the groups administered vehicle (0 mg/kg MA) and squares represent the groups administered 0.5 mg/kg MA. * P<0.05 for the Vaccine Group X MA Dose Group interaction. See text for statistics.
The next measure assessed is the approach behavior of exploratory pokes into the drug-paired side and these data are presented in Fig. 5C. The naïve group conditioned with MA exhibited the greatest number of exploratory pokes into the drug-paired side on the Test session compared to the other three groups. This statement is supported by the significant main effects of Vaccine group, F(1,44)=8.16; P<0.01, and MA dose group, F(1,44)=5.57; P<0.05, as well as the significant interaction of Vaccine X MA group, F(1,44)=6.65; P<0.02. This is further confirmed by post-hoc comparisons that show that the naïve group conditioned with 0.5 mg/kg MA differs from all other groups, P<0.01. The final measure of approach behavior is the numbers of entries into the drug-paired side. These data are shown in Fig. 5D. Groups conditioned with MA enter the drug-paired side more than groups conditioned with vehicle as supported by the significant MA dose group effect, F(1,44)=14.37; P<0.0005. There is also a trend to see greater number of entries in naïve groups compared to vaccine groups, F(1,44)=3.34; P<0.08. Post-hoc comparisons show that the naïve group conditioned with 0.5 mg/kg MA differs from all other groups, P<0.01.
4. DISCUSSION
Results from this evaluation of a succinyl-methamphetamine- keyhole limpet hemocyanin (SMA-KLH) conjugate vaccine support its potential as an anti-MA vaccine. We have shown that the conjugate used in this study, composed of succinyl-methamphetamine molecules covalently linked to a carrier protein derived from the keyhole limpet hemocyanin (KLH) carrier protein and suspended in a monophosphoryl lipid A (MPL) adjuvant, generates MA antibodies in sera of mice. And, the vaccine has functional consequences as seen in the behavioral tests. Overall, these findings show support for the effectiveness of this approach to the construction of an anti-MA vaccine.
Antibodies were detected in sera after the initial vaccine administration. Although this titer is low, a second administration increased levels considerably. The third administration at week 20 was sufficient to boost antibody levels and maintain them for the additional 17 weeks of the study. However, this additional boost did not increase the peak antibody titer above that seen at 6 weeks. The mice were 28 weeks of age when they received the third vaccine administration and they may have exceeded the best age for immunization. Nonetheless, antibody titers at 8 weeks were highly correlated with titers at 35 weeks suggesting that those mice that generate high titers initially continue to generate high titers several months later after additional boost administrations. Antibodies were not produced at either time point in two of the mice. We have no explanation for this deviation from the other mice but should point out that this lack of antibody production occurred in less than 7% of the sample. Overall, these data demonstrate that this schedule of SMA-KLH vaccination generated MA antibodies at relatively high titers that could last over the 35 weeks of the study.
The functional significance of the antibody titers can be determined by testing the ability of the vaccination to alter behaviors induced by the drug. In the first functional test, we demonstrate that the SMA-KLH vaccine alters the acute effects of MA in a dose-dependent manner. At low doses, MA can cause hypolocomotion (Kitahama and Valatx, 1979), a phenomenon we have confirmed and extended previously (Singh et al., submitted) as well as in the present paper. However, the duration of this prolonged hypolocomotor effect is shortened by the SMA-KLH vaccine (Fig. 3A). In fact, horizontal activity levels increase above baseline at the latest time points in vaccinated mice at this dose. This pattern can also be seen in the time of peak response data (Table 1). Peak activity occurs later in the vaccinated vs naïve mice at the lowest MA dose. In contrast to the low dose hypolocomotor effect, horizontal activity levels are quite elevated after administration of the highest MA dose in the naïve group. This hyperlocomotor effect is decreased in the SMA-KLH vaccine group (Fig. 3C). Thus, the SMA-KLH vaccine appears to reduce both the hypolocomotor and hyperlocomotor effects on horizontal activity induced by MA. The effects of the vaccine on vertical activity are less robust that may be due, in part, to the large degree of variability in this measure. Nonetheless, the vaccine had a similar effect on vertical activity induced by the lowest MA dose as seen in horizontal activity. That is, the SMA-KLH vaccine shortened the duration of the prolonged decreased vertical activity seen in naïve mice (Fig. 4A).
The second behavioral test evaluated if the vaccine could decrease the ability of MA to support context conditioning using conditioned place preference (CPP). Indeed, three of four measures of MA conditioned were either significantly decreased or showed a trend toward a decrease in the vaccinated mice. In addition to attenuating conditioned locomotor activity, the SMA-KLH vaccine led to a complete block of the approach behavior of exploratory pokes into the MA-paired side. Although the typical measure of CPP, shift in time spent on the drug-paired side, failed to show significance, this may reflect that the MA dose used was somewhat low. We had chosen to test CPP with this low MA dose (relative to the locomotor study) based on previous studies conducted with MA CPP in mice (Shabani et al., 2011a, 2011b). In these studies, 0.5 mg/kg MA was more effective in supporting CPP than higher doses (1 and 2 mg/kg) and these results are consistent with other reports that assessed CPP with just the 0.5 mg/kg dose (Itzhak et al., 2002; Wheeler et al., 2009). However, there are large mouse strain differences in the ability of a psychostimulant to induce locomotor activity and to be self-administered (Thomsen and Caine, 2011) and the strains of mice employed differed across these CPP studies.
Other MA vaccines have been tested for functional effects in various behavioral assays. The Owens group evaluated the ability of monoclonal antibodies to affect MA-induced locomotor activity and found effects similar to those reported upon in the present study. These include shorter duration of increased activity as well as a delay in the peak response in rats (Byrnes-Blake et al., 2005; Gentry et al., 2006). The higher affinity antibody, mAb6H4, was more effective than the lower affinity antibody, mAb6H8, in reducing MA-induced locomotor activity (Byrnes-Blake et al., 2005). Yet, both antibodies were capable of attenuating MA self-administration in rats (McMillan et al., 2004) and the low affinity antibody also shifted the MA dose-response curve to the right in rats and pigeons trained to discriminate cocaine (McMillan et al., 2002). Rats vaccinated with an active type of MA vaccine actually learned to discriminate active and inactive levers more quickly than control rats although they showed decreases in MA self-administration under some conditions but not others (Duryee et al., 2009). It is difficult to make direct comparisons between our results and those of the other investigators. For example, our study employed mice and all the other studies used rats or pigeons. Further, the route of administration (IV) in the earlier locomotor activity studies differed from the route used in the present study (IP).
We believe this is the first report to demonstrate that an MA vaccine altered approach behaviors conditioned with MA using the place conditioning procedure. A vaccine against cocaine that was also constructed with a KLH conjugate reduced cocaine CPP in rats (Ettinger et al., 1997). However, this earlier study used a biased procedure and did not include vehicle-trained groups. Thus, it is difficult to interpret these findings as the vaccine may have disrupted habituation processes (Aguilar et al., 2009; Bardo and Bevins, 2000). Nonetheless, that we showed the SMA-KLH vaccine reduced or blocked MA conditioned approach behaviors using a non-biased procedure and vehicle control groups complements the results of studies using monoclonal antibodies and MA self-administration. Future studies with SMA-KLH utilizing self-administration should be conducted in order to make conclusive interpretations.
This vaccine may be improved by modifying the carrier protein or the adjuvant. Such improvements are aimed at developing a vaccine for human use. While KLH is a very effective carrier, it is a very large protein that makes quality control over its haptenation process difficult (Josefsberg and Buckland, 2012). Smaller proteins such as, tetanus toxid, diptheria toxoid, or inactivated cholera toxin, may be better suited for manufacturing a human vaccine, although they may have lower immunogenicity (Dagan et al., 2010). The adjuvant used in the current study, MPL, is FDA-approved but is not available for general use due to patent protection. The only adjuvant that is available for general use is alum and it may not be as effective as MPL (Montomoli, 2010). Thus, these factors need to be considered when designing future studies.
Acknowledgments
Role of Funding Source
This research was supported by grants DP1DA033502 and U01023898 from The National Institute on Drug Abuse (NIDA). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Drs. TA Kosten, TR Kosten, and Orson have received funding from the NIH and the Department of Veterans Affairs.
The authors thank Y. Wu and B. Mao for their excellent technical assistance.
Footnotes
Contributors
All authors made significant contributions to the conduct of the study and the manuscript that reports on the results. Drs. Xiaoyun Shen and Therese A Kosten contributed equally to the conduct of the research and preparation of this manuscript. Specific contributions are as follows: Dr. Shen developed and wrote the protocol, oversaw the conduct of the study, and managed the data sets. Dr. Therese Kosten wrote the manuscript, designed the behavioral studies, crafted the figures, and performed the statistical analyses. Ms. Angel Lopez conducted ELISA studies, helped manage the data sets, optimized the conditioned place preference procedure, and conducted the CPP study. Dr. Berma Kinsey generated the conjugate vaccine and contributed to the writing of the paper. Dr. Thomas Kosten contributed to the writing of the paper. Dr. Frank Orson supervised the research staff and contributed to the writing of the paper.
Conflict of interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar MA, Rodriguez-Arias M, Minarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59:253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Antoniou K, Kafetaopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. d-amphetamine, cocaine and caffeine: a comparative study of acute effeccts on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–196. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Brien JF, Kitney JC, Peachey JE, Rogers BJ. Methamphetamine-induced behavioural effects and brain concentrations of methamphetamine and its metabolite amphetamine in mice. Res Commun Chem Pathol Pharmacol. 1978;22:313–318. [PubMed] [Google Scholar]
- Byrnes-Blake KA, Carroll FI, Abraham P, Owens SM. Generation of anti- (+)methamphetamine antibodies is not impeded by (+)methamphetamine administration during active immunization of rats. Int Immunopharmacol. 2001;1:329–338. doi: 10.1016/s1567-5769(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Byrnes-Blake KA, Laurenzana EM, Landes RD, Gentry WB, Owens SM. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur J Pharmacol. 2005;521:86–94. doi: 10.1016/j.ejphar.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman J, Cooper S, editors. The Neuropharmacological Basis of Reward. Oxford University Press; New York: 1989. pp. 264–319. [Google Scholar]
- Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: a review. Vaccine. 2010;28:5513–5523. doi: 10.1016/j.vaccine.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–262. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, Sanderson SD. Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine. 2009;27:2981–2988. doi: 10.1016/j.vaccine.2009.02.105. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- Ettinger RH, Ettinger WF, Harless WE. Active immunization with cocaine-protein conjugate attenuates cocaine effects. Pharmacol Biochem Behav. 1997;58:215–220. doi: 10.1016/s0091-3057(97)00005-1. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Kroll C, Ferrieri R, Alexoff D, Logan J, Dewey SL, Schiffer W, Schlyer D, Carter P, King P, Shea C, Xu Y, Muench L, Benveniste H, Vaska P, Volkow ND. PET studies of d-methamphetamine in primates: comparison with l- methamphetamine and (−)-cocaine. J Nucl Med. 2007;48:1724–1732. doi: 10.2967/jnumed.107.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry WB, Laurenzana EM, WIlliams DK, West JR, Berg RJ, Terlea T, Owens SM. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol. 2006;6:968–977. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- George FR. Cocaine produces low dose locomotor depressant effects in mice. Psychopharmacology. 1989;99:147–150. doi: 10.1007/BF00442799. [DOI] [PubMed] [Google Scholar]
- George FR. Cocaine produces low dose locomotor depressant effects in NBR and F344 rats. Pharmacol Biochem Behav. 1990;37:795–798. doi: 10.1016/0091-3057(90)90565-y. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Ann Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Donzales G, Aigotti RN, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, Kessler PD, Niknian M, Kalnik MW, Rennard SI. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Thera. 2011;89:392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, deVos A, Horwith G, Pentel PR. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Thera. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. Behavioral consequences of methamphetamine-induced neurotoxicity in mice: relevance to the psychopathology of methamphetamine addiction. Ann N Y Acad Sci. 2002;965:127–135. doi: 10.1111/j.1749-6632.2002.tb04156.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Ali SF. Methamphetamine-induced dopaminergic neurotoxicity in mice: long-lasting sensitization to the locomotor stimulation and desensitization to the rewarding effects of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1177–1183. doi: 10.1016/s0278-5846(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Josefsberg JO, Buckland B. Vaccine process technology. Biotechnol Bioeng. 2012;109:1443–1460. doi: 10.1002/bit.24493. [DOI] [PubMed] [Google Scholar]
- Kitahama K, Valatx JL. Strain differences in amphetamine sensitivity in mice. Psychopharmacology. 1979;66:189–194. doi: 10.1007/BF00427629. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenzana EM, Kyrnes-Blake KA, Milesi-Halle A, Gentry WB, Williams DK, Owens SM. Use of anti-(+)-methamphetamine monoclonal antibody to significantly alter (+)-methamphetamine and (+)-amphetamine disposition in rats. Drug Metab Dispos. 2003;31:1320–1326. doi: 10.1124/dmd.31.11.1320. [DOI] [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatr. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer P, Bachmann MF. Vaccination against nicotine: an emerging therapy for tobacco dependence. Exp Opin Invest Drugs. 2007;16:1775–1783. doi: 10.1517/13543784.16.11.1775. [DOI] [PubMed] [Google Scholar]
- McKetin R, Kozel N, Doublas J, Ali R, Vicknasingam B, Lund J, Li JH. The rise of methamphetamine in Southeast and East Asia. Drug Alcohol Rev. 2008;27:220–228. doi: 10.1080/09595230801923710. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Hardwick WC, Li M, Gunnell MG, Carroll FI, Abraham P, Owens SM. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther. 2004;309:1248–1255. doi: 10.1124/jpet.103.061762. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Hardwick WC, Li M, Owens SM. Pharmacokinetic antagonism of (+) -methamphetamine discrimination by a low-affinity monoclonal anti-methamphetamine antibody. Behav Pharmacol. 2002;13:465–473. doi: 10.1097/00008877-200209000-00019. [DOI] [PubMed] [Google Scholar]
- Montomoli E. Current adjuvants and new perspectives in vaccine formulation. Exp Rev. 2010;10:1053–1061. doi: 10.1586/erv.11.48. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Mayorov AV, Janda KD. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J Am Chem Soc. 2011;4:6587–6595. doi: 10.1021/ja108807j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EC, Gunnell M, Che Y, Goforth RL, Carroll FI, Henry R, Liu H, Owens SM. Using hapten design to discover therapeutic monoclonal antibodies for treating methamphetamine abuse. J Pharmacol Exp Ther. 2007;322:30–39. doi: 10.1124/jpet.106.117150. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Trends in place preference conditioning with a cross- indexed bibliography, 1957–1991. Pharmacol Biochem Behav. 1993;17:21–41. doi: 10.1016/s0149-7634(05)80228-3. [DOI] [PubMed] [Google Scholar]
- Shabani S, McKinnon C, Cunningham C, Phillips T. Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology. 2011a;62:1134–1141. doi: 10.1016/j.neuropharm.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011b;10:625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RAK, Kosten TA, Kinsey BM, Shen X, Lopez AY, Kosten TR, Orson FM. Dose-dependent changes in the locomotor responses to methamphetamine in BALB/c mice: Low doses induce hypolocomotion. doi: 10.1016/j.pbb.2012.08.013. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol. 2011;19:321–341. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, Thanos PK, Alexoff D. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PloS One. 2010;7:5. doi: 10.1371/journal.pone.0015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]