Abstract
Purpose
To develop angiographic models of embolic stroke in the rabbit using pre-formed clot or microspheres to model clinical situations ranging from transient ischemic events to severe ischemic stroke.
Materials and Methods
New Zealand White rabbits (N=151) received angiographic access to the internal carotid artery (ICA) from a femoral approach. Variations of emboli type and quantity of emboli were tested by injection into the ICA. These included fresh clots (1.0-mm length, 3–6 h), larger aged clots (4.0-mm length, 3 days), and 2 or 3 insoluble microspheres (700–900 μm). Neurological assessment scores (NAS) were based on motor, sensory, balance, and reflex measures. Rabbits were euthanized at 4, 7, or 24 hours after embolization, and infarct volume was measured as a percent of total brain volume using 2,3,5-triphenyltetrazolium chloride (TTC).
Results
Infarct volume percent at 24 hours after stroke was lower for rabbits embolized with fresh clot (0.45% ± 0.14%), compared with aged clot (3.52% ± 1.31%) and insoluble microspheres (3.39% ± 1.04%). Overall NAS (including posterior vessel occlusions) were positively correlated to infarct volume percent measurements in the fresh clot (r=0.50), aged clot (r=0.65) and microsphere (r=0.62) models (p<0.001).
Conclusion
The three basic angiographic stroke models may be similar to human transient ischemic attacks (TIA) (fresh clot), major strokes that can be thrombolysed (aged clot), or major strokes with insoluble emboli such as atheromata (microspheres). Model selection can be tailored to specific research needs.
Keywords: Stroke, Animal models, Rabbit, Angiography, Focal cerebral ischemia
1. Introduction
Animal models of ischemic stroke have been used for many years with well-developed models in rats and mice predominating. Neuroprotective drugs were effective in these models and demonstrated numerous mechanisms (Carmichael, 2005). The need for demonstrations of efficacy in different animal models prompted us to return to the rabbit model, which was classically used to demonstrate effective stroke treatment with tPA (Zivin et al., 1985). Our and other’s innovations include 1) modifications to the surgical approach to move it away from the neck and brain, 2) angiographic access to the cerebral vasculature, and 3) several types of emboli (Kirchhof et al., 2002; Kong et al., 2004; Jahan et al., 2008; Culp et al., 2008). End point assessments include 1) infarct volume as a percent of total brain volume, 2) neurological and behavior abnormalities, and 3) serum analysis for markers of brain damage (Zivin et al., 1992; Lapchak et al., 2004; Lapchak et al., 2007; Lapchak et al., 2011; Woods et al., 2011). CT and MRI imaging, commonly used in humans for location and volume assessment, are infrequently used in animal studies, perhaps due to availability, scale issues, and high costs.
Animal experiments yield more variable results than in vitro studies due to the greater complexities of in vivo models. The largest rabbit experience, Zivin’s and Lapchak’s extensive volume of work, uses a surgical approach to the carotid artery with many small clots flushed into the circulation (Zivin et al., 1985; Zivin et al., 1992; Lapchak et al., 2004; Lapchak et al., 2007; Lapchak et al., 2011). These clots result in widely scattered infarcts throughout the entire brain. These reports are based on end points measuring the dose of small clots required to produce any detectable neurological defect or animal death. Several variations using soluble clot emboli including a large clot version have been reported by this group.
While several reports have demonstrated super-selective microcatheter angiographic techniques in small series of animals, the technical skill and expensive equipment limit application (Kirchhof et al., 2002; Jahan et al., 2008; Culp et al., 2008).
Our stroke model (Culp et al., 2007; Brown et al., 2010; Brown et al., 2011; Flores et al., 2011; Culp et al., 2011; Culp et al., 2012) uses a simplified subselective rabbit angiographic technique. This is designed to model the human embolic stroke in which occlusive material, usually clot or atheroma, is suddenly imposed on normal cerebral blood vessels. It avoids surgical distortions in regional arteries supplying the brain and provides a pure embolic defect. A 3 Fr catheter from a femoral artery approach accesses an internal carotid artery (ICA) and shows detailed angiographic anatomy (Fig 1a–c). Subsequently, an embolus is delivered to the ICA followed by angiography to assess embolus placement and vascular occlusion (Fig 1d). These emboli are flow-directed to the middle cerebral artery (MCA) or anterior cerebral artery (ACA) in most cases. Three principal variations based on the nature and volume of the embolus are presented here and can provide the basic building blocks for future techniques to meet specific research needs. We report here 151 rabbits that received angiography and embolic ischemia without therapy.
Figure 1. Selective rabbit intracranial angiography.
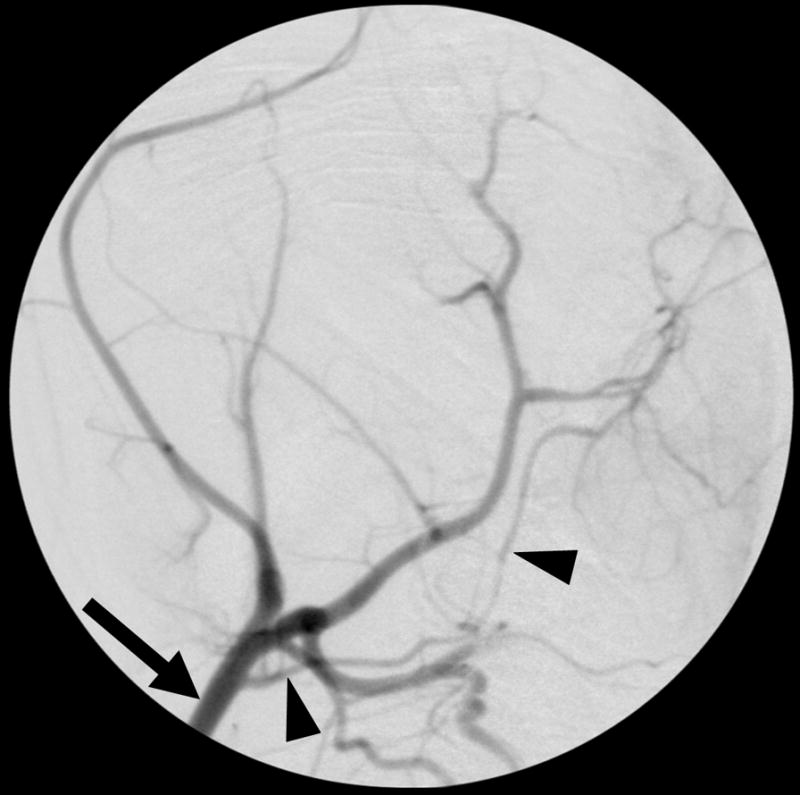
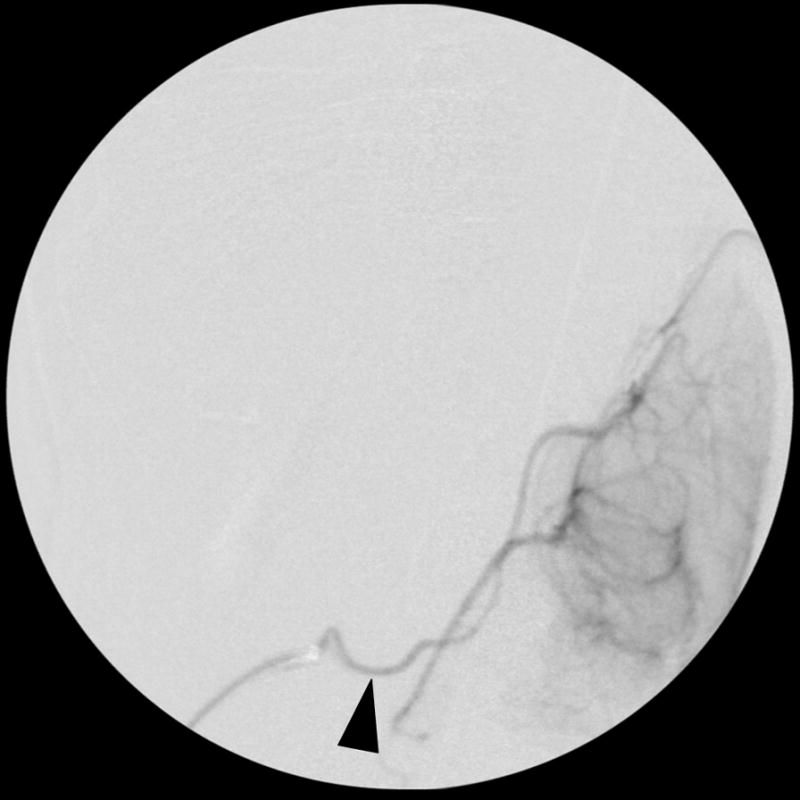
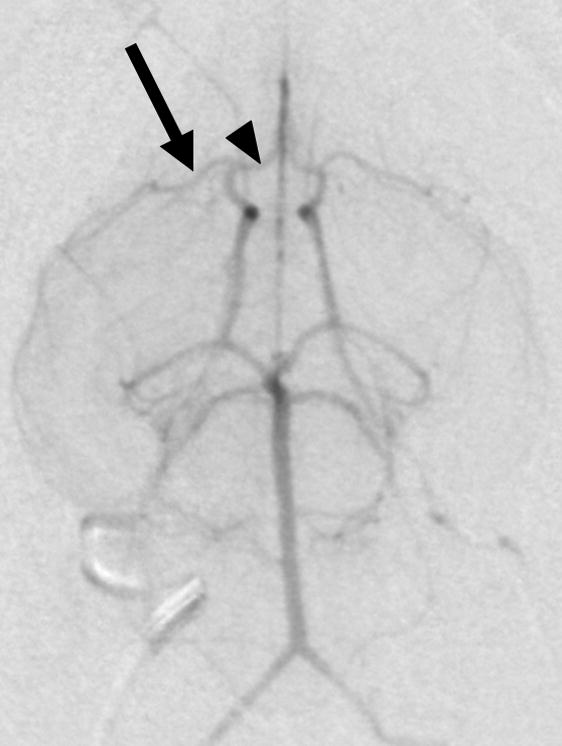
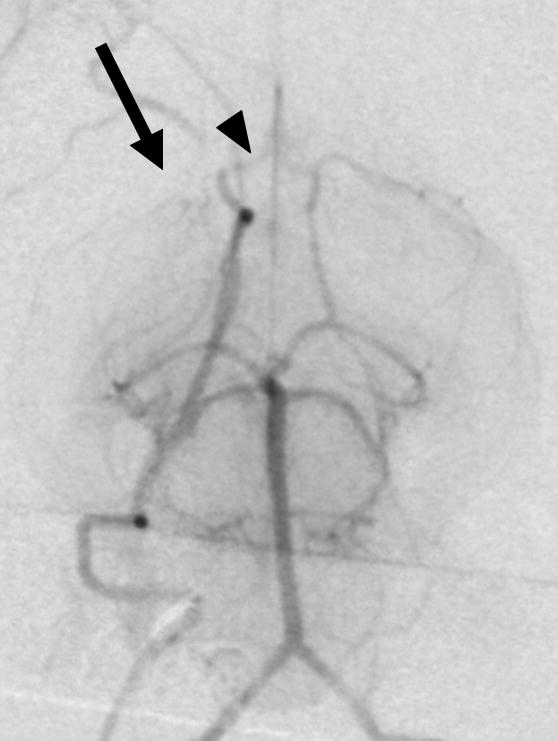
(a) Common carotid selection (arrow), lateral view. The internal carotid artery is seen coursing to the base of the brain (arrowheads). (b) Internal carotid sub-selection (arrowhead) in a lateral view demonstrates filling of the cerebral vasculature. (c) A frontal-view internal carotid angiogram clearly shows the Circle of Willis including the middle cerebral artery (MCA, arrow), and the anterior cerebral artery (ACA, arrowhead). (d) Repeat angiography after injection of three embolic microspheres (700–900 μm) shows the occlusion of the MCA (arrow) and persistent flow in the ACA (arrowhead).
2. Materials and Methods
2.1 Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee. Adult New Zealand White rabbits (N=151 in total, 78 females and 73 males, mean body weight 5.1 ± 0.03 kg) (Myrtles Rabbitry, Thompsons Sta., TN, USA) were used.
2.2 Anatomy
The Circle of Willis (COW) in the New Zealand White rabbit is similar to the human; a complete ring-like network of arteries allows blood flow from any of the supplying vessels to all of the major branches. However, in rabbits the internal carotid arteries (ICA) are often smaller than the vertebral arteries which combine to form the much larger basilar artery. Variations in the COW are common (Caldwell et al., 2011). Most important is duplication of the MCA, which is seen in 29% of cases. This led one investigator (Kirchhof et al., 2002) to not use any animals with duplications and led us to try small emboli which could individually occlude these small vessels.
Other important variations outside the COW include a common trunk origin of occipital artery and ICA, and the occasional absence of the vertebral artery. The vertebral artery approach has been recommended in the past (Pan et al., 1987) but does not specifically select the MCA or ACA and is not discussed here. The complex ICA origin in 16% of rabbits makes its selection and embolization difficult due to geometry or spasm.
2.3 Surgical Technique
A schematic representation of the procedure is in Appendix Figure 1.
2.3.1 Anesthesia
Rabbits were sedated with intramuscular ketamine (30 mg/kg, Ketaset; Fort Dodge, Fort Dodge, IA) and xylazine (3 mg/kg, AnaSed, Lloyd Laboratories, Shenandoah, IA) and maintained under anesthesia by mask ventilation with 1–2% isoflurane (Novaplus; Hospira Inc.; Lake Forest, IL).
2.3.2 Angiography
All imaging was performed on rabbits in a recumbent position using C-arm digital subtraction angiography (DSA) (OEC 9800; GE Healthcare, Salt Lake City, UT). Using sterile technique, a surgical cut down was performed on a common femoral artery and a 3-Fr vascular sheath was used to introduce an angiographic catheter. The customized 65-cm angled-tip 3-Fr catheter (Slip-Cath, modified JB1; Cook Inc., Bloomington, IN) was advanced using fluoroscopic guidance over a 0.025-in. angle-tip guide wire (Glidewire; Terumo Medical Corp., Elkton, MD) to the aortic arch. A photograph of the angled-tip catheter is in Appendix Figure 2. Preliminary arch angiography was performed in a frontal view, using Iodixanol 270 contrast (Visipaque; GE Healthcare, Salt Lake City, UT) to image a subtracted fluoroscopy of the arch and its branches. This subtracted fluoroscopy was used for selection of a common carotid artery (CCA), usually the right. The small catheter does not compromise CCA blood flow. The image intensifier was then rotated from the frontal view to a lateral view. Another subtracted fluoroscopy injection identified the ICA for selection. Once engaged, the small catheter usually compromised ICA blood flow until withdrawn, therefore time here was intentionally limited (Fig 1a–b) (Culp et al., 2007). Angiograms using hand injections of 1–2 ml of contrast in both projections showed the detailed anatomy of the entire COW (Fig 1b–c).
The 3-Fr catheter was small enough to select the ICA or vertebral artery and could accommodate a 0.025-in. guide wire. This wire, larger than used in most microcatheters, aided in sub-selection and the catheter lumen was large enough for injection of emboli without excessive fragmentation. Standard 80-cm long catheters were cut to 65-cm, new hubs applied, and various curves steamed into their tips. A simple angled tip was useful for right common carotid, ICA, and vertebral selections. Sharply curved variations were occasionally helpful in left common carotid selection at the aortic arch. Excellent technique is required to avoid vascular spasm or introduction of air bubbles which can cause infarcts. Standard angiographic techniques and skills transfer rapidly from human to rabbit anatomy.
2.3.3 Embolization
The selected embolus was gently injected with 0.7 ml to 2.0 ml of saline flush slowly following it down the catheter. A follow-up angiogram confirmed embolus arrival in the COW or a branch and localized the arterial occlusion (Fig 1d). The catheter was then pulled back into the CCA and normal ICA flow resumed. Delayed common carotid angiograms were obtained as desired but reselection of the ICA was compromised by frequent spasm and, thus, avoided. The catheter and sheath were eventually removed, the femoral artery ligated and skin sutured.
2.4 Emboli
Embolus model selection depends on specific experiment requirements. Clot can be fresh (3 to 6 hours) and easy to thrombolyse, or aged (3 days) and resistant to thrombolysis (Murray et al., 2010). Single or multiple emboli can be injected, and size can vary from small (1.0-mm) to represent small clots, or longer (4.0-mm) pieces to model large clots. Insoluble emboli using synthetic microspheres can be very small, less than 100 μm in diameter for modeling small peripheral infarcts similar to those from debris penetrating protective devices in carotid stent placement, or much larger, 700 to 900 μm for modeling insoluble atheromata that occlude major vessels such as the MCA, ACA, or distal ICA.
2.4.1 Fresh Clot Model
For fresh clot emboli an arterial rabbit blood sample (2–3 mL) was obtained and immediately transferred into 30.5-cm lengths of butterfly pediatric infusion sets (No. 4506; Abbot Hospitals, Inc., North Chicago, IL) with an internal diameter of 1.0-mm and allowed to clot at 37°C for 3 to 6 hours. The cylindrical clot was ejected into a dish containing physiological saline and precisely cut into 1.0-mm lengths with a diameter of approximately 0.6-mm after incubation induced shrinkage. For uniformity, segments were cut from the center of the red clot. A single clot piece was drawn into a 3.0-mL syringe with physiological saline for embolization and traversed the catheter without visible fragmentation.
2.4.2 Aged Clot Model
For clots more resistant to thrombolysis, and to develop larger strokes, a larger aged clot was used (Murray et al., 2010). Fresh rabbit blood was placed into a 1.5-mm inner diameter glass tube (Natelson Blood Collecting Tube; Fisher Scientific, Waltham, MA), incubated at 37°C for 6 hours and then refrigerated at 4°C for 3 days. These were much firmer, pliable enough for injection through the catheter without visible fragmentation, and resistant to autolysis. The cylindrical clot was 4.0-mm in length and averaged 1.2-mm in diameter. Additional preliminary lengths and numbers of clots were also investigated (Appendix Table 1).
2.4.3 Microsphere Model
Embolic spheres (Embosphere Microspheres, BioSphere Medical Inc., Rockland, MA) of various sizes and numbers modeled permanent insoluble emboli. Additionally, this model was used to investigate the effect of different sacrifice times on endpoint measurements. The 700–900 μm microspheres deformed enough to traverse the catheter and provided consistent vessel occlusion when two or three were used. Additional preliminary sizes and numbers of microspheres were also investigated (Appendix Table 2).
2.5 Endpoint Measurements
2.5.1 Brain Sections
The most practical stroke evaluation and measurement is performed on the harvested brain following euthanasia. The calvaria was removed, immediately followed by the brain. Embolus locations were noted on gross examination and photographed (Fig 2). The brain was chilled immediately in an iced saline bath and in one hour was firm enough for sectioning in a chilled brain matrix mold (RBM-7000C; ASI Instruments Inc.; Warren, MI). While 2.0-mm slices were possible in fixed tissue using this device, fresh infarcted tissue often fragmented when cut in 2.0-mm slices, thus all measurements were made on coronal brain sections at 4.0-mm intervals.
Figure 2.
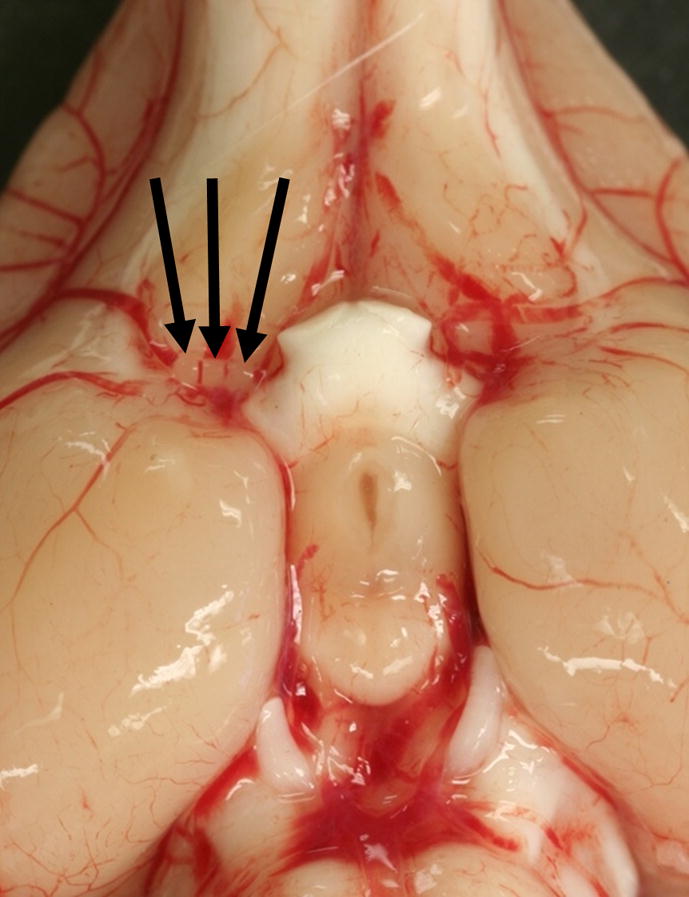
Gross image of the ventral surface of the rabbit brain shows three 700–900 μm microsphere emboli (arrows) located in the middle cerebral artery origin at the connection with the internal carotid artery.
Brain sections (n=8, 4.0-mm brain sections/rabbit) were immediately placed in 1% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich; St. Louis, MO) for 45 minutes at 37°C. This vital tissue stain shows viable tissue as red or pink and infarct as pale areas (Fig 3). Each section was photographed and the total area of brain and the area(s) of infarct were quantitated digitally using Image J software (NIH) and multiplied by section thickness to obtain volumes. Infarct volume percent was calculated as a percentage of total brain volume. The sections were then fixed in 10% formalin and received histological examination using standard hematoxylin and eosin (H&E) stains. These measured intracranial hemorrhage and also confirmed infarct areas. Pathologic examinations and infarct volume measurements were performed by a blinded observer.
Figure 3. Infarct area in a rabbit brain section.
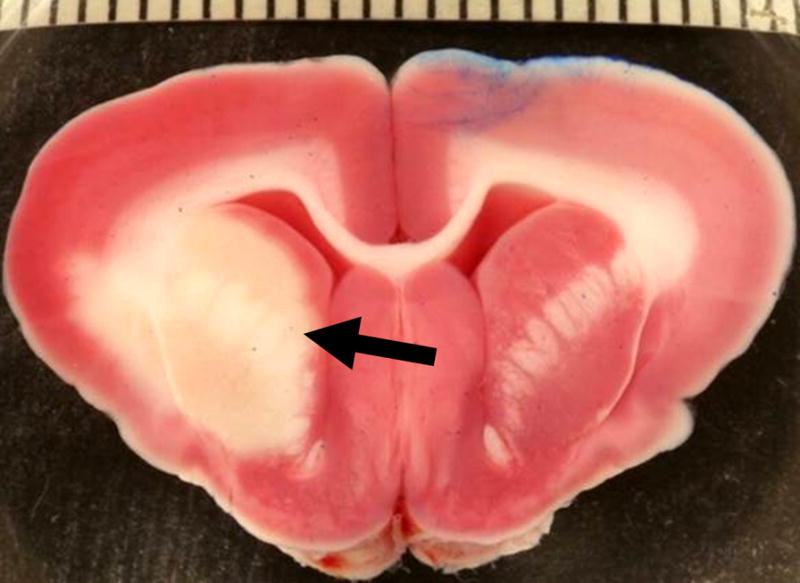
2,3,5-triphenyltetrazolium chloride (TTC) stain at 24 hours after embolization with three 700–900 μm microspheres shows viable tissue as red or pink, and a pale infarct area (arrow) in the distribution of the right middle cerebral artery. The scale bar represents millimeters and the blue color is a laterality marker indicating the left hemisphere.
2.5.2 Neurological Assessment Score (NAS)
The NAS is a composite score comprised of motor, sensory, balance, and reflex measures utilizing the wryneck test which ranges from 1 to 10 and was scored by a blinded observer after the animal awoke. Higher scores indicate more severe neurological injury (Zhao et al., 2001; Zhao et al., 2002).
2.5.3 Other
Additional endpoints were used for stroke diagnosis, characterization, and measurement, but are beyond the scope of the present study. Serum biomarkers, MMP-9 and S-100B, were measured as a correlative to infarct volume (Woods et al., 2011). Imaging techniques included MRI using a clinical 3T machine with a wrist coil or a dedicated high field 7T animal MRI, and clinical CT images and were repeated in a subgroup out to 80 days post-embolization, N=3.
2.6 Statistical Analysis
Infarct volume percent values were compared among experimental groups using analysis of variance methods. If significant evidence of non-normal distributions was demonstrated, the non-parametric Kruskal-Wallis procedure based on ranks was used. The correlation coefficient was used to assess the relationship between infarct volume percent and NAS. Comparisons were made by using the software package StatXact (Cytel, Cambridge, MA).
3. Results
3.1 Animals
A total of N=151 rabbits completed the study. The three principal stroke models included N=105 rabbits (Table 1). Alternative clot experiments, N=26, are listed in Appendix Table 1, and alternative microsphere experiments, N=20, in Appendix Table 2.
Table 1. Principal embolization techniques.
Comparison of the effects of various emboli, characteristics of emboli, and sacrifice time on neurological assessment score (NAS) and percent infarct volume. Data are reported as mean ± standard error.
| Embolus | N | No. of emboli | Embolus size | Sacrifice time (h) | NAS | Infarct Volume (%) |
|---|---|---|---|---|---|---|
| Aged Clot | 11 | 1 | 4.0 mm | 24 | 2.1 ± 0.5 | 3.52 ± 1.31 |
| Fresh Clot | 50 | 1 | 1.0 mm | 24 | 1.9 ± 0.3 | 0.45 ± 0.14 |
| Microsphere | 7 | 2 or 3 | 700–900 μm | 4 | 3.4 ± 1.2 | 3.66 ± 1.38 |
| 6 | 2 or 3 | 700–900 μm | 7 | 2.3 ± 0.8 | 3.88 ± 1.40 | |
| 16 | 2 or 3 | 700–900 μm | 24 | 3.2 ± 0.8 | 3.39 ± 1.04 |
3.2 Anatomy
Complex origins of the ICA were seen in 16% of all cases (24/151). Other variations reported previously include MCA duplications, vertebral artery absence, and posterior vessel asymmetries (Caldwell et al., 2011).
3.3 Overall Neurological Assessment Scores (oNAS)
An analysis including rabbits with posterior vessel occlusions yielded mean oNAS values that positively correlated to percent infarct volume, r=0.50, 0.65, and 0.62 for fresh clot, aged clot, and microsphere emboli, respectively (p<0.001). Cumulative mean oNAS values of all models indicate a strong positive relationship r=0.61 (p<0.0001).
3.4 Complications
In all models, animals with abnormal vessel occlusions, excessive spasm, anomalous and obstructive access, or severe symptoms leading to early sacrifice or death were excluded. In the fresh clot model these totaled 5 cases, the aged clot model had 2, and the microsphere model had 8. Thus 86% (90/105) cases were successful in the three principal stroke models (Table 1). The distribution of complications was posterior vessel occlusions (7), other angiographic difficulties such as spasm or air bubbles (5), or severe symptoms precluding survival (3). While overall NAS values (oNAS, above) included posterior vessel occlusions, the remainder of this study will exclude those and other complications to more closely mimic MCA strokes in humans. These selected values are signified by “NAS.”
3.5 Fresh Clot Model
The series of n=50 rabbits embolized with one fresh (3–6 hr) cylindrical clot (1.0-mm length × 0.6-mm diameter) had a mean infarct volume at 24 hours of 0.45±0.14% (Table 1). Angiography: 54% of cases (27/50) had angiographic evidence of MCA occlusion or MCA branch occlusion, 34% (17/50) had incomplete occlusions on angiography with only subtle changes in localized flow, and 12% (6/50) had no angiographic evidence of occlusion. None had angiographic occlusion of posterior arteries. Infarct Volume %: 60% of cases (30/50) had 0% measured infarct volume and presumably experienced prompt autolysis and recanalization or had adequate vasodilatation or collateral development to prevent infarction. This occurred in 74% of MCA occlusions (20/27), 41% of incomplete occlusions (7/17), and 50% of cases with no visible occlusion (3/6). Severe vasospasm of the ICA occurred in 6% of cases (3/50). NAS: the mean was 1.9±0.3. The NAS was significantly correlated with infarct volume percent measurements: r=0.437, p=0.0015. A variation of the fresh clot model is listed in Appendix Table 1.
3.6 Aged Clot Model
The series of N=11 rabbits embolized with one aged (3-day) cylindrical clot (4.0-mm length × 1.2-mm diameter) had a mean infarct volume at 24 hours of 3.52±1.31% (Table 1). Eighty-two % had MCA occlusion (9/11), 45% also had ACA occlusion (5/11), and 18% had no visible occlusion (2/11). None had ICA vasospasm. Other variations of the aged clot model are listed in Appendix Table 1. The mean NAS was 2.1±0.5 and was not well correlated with infarct volume percent measurements: r=0.129, p=0.705.
3.7 Microsphere Model
3.7.1
The 24 hour series of N=16 rabbits embolized with two or three 700–900 μm microspheres had a mean infarct volume of 3.39±1.04% (Table 1). All had MCA occlusion (16/16) and 38% also had ACA occlusion (6/16). None had ICA vasospasm. The mean NAS was 3.2±0.8 and was significantly correlated with infarct volume percent measurements: r=0.593, p=0.0155.
3.7.2
The 7 hour series of N=6 rabbits embolized with two or three 700–900 μm microspheres had a mean infarct volume of 3.88±1.41% (Table 1). 100% had MCA occlusion (6/6) and 67% also had ACA occlusion (4/6). None had ICA vasospasm. The mean NAS was 2.3±0.8 and was not well correlated with infarct volume percent measurements: r=0.343, p=0.505.
3.7.3
The 4 hour series of N=7 rabbits embolized with two or three 700–900 μm microspheres had a mean infarct volume of 3.66±1.38% (Table 1). All had MCA occlusion (7/7) and 71% also had ACA occlusion (5/7). Three had mild ICA vasospasm. The mean NAS was 3.4±1.2 and was not well correlated with infarct volume percent measurements: r=−0.058, p=0.902. Other variations of the microsphere model are listed in Appendix Table 2.
4. Discussion
Animal choice and variations of technique in stroke research provide different pathophysiologies and wide-ranging potential for understanding mechanisms and developing therapeutic approaches of variable relevance (Hossmann, 2012). Zivin’s classic rabbit stroke model successfully paved the way to tPA as the standard of current human care. Donnan and others show differences in brain size and ratio of white to gray matter may be crucial (Donnan, 2008, Zhang et al., 2000). Mechanisms of injury and optimum therapies differ in gray and white matter. The rabbit is much higher in white matter proportion than mice and rats, supporting its selection as a second model, but the proportion remains well below that in humans (Table 2). Pigs are an appealing next animal up the scale towards humans, but the rete mirabilis interferes with angiographic stroke modeling in pigs. Prior to future human trials, demonstrations of efficacy in a second animal model, the rabbit or other larger animals, should be obtained (Heiss et al., 2012).
Table 2. Brain weight and white matter proportions in various species.
The most common species used in stroke research, mice and rats, have the largest differences in brain weight and white matter proportion compared to humans. Since white and gray matter involve different mechanisms of injury and neuroprotection, matching human proportions may be important. Scale issues of brain size may also be critical.
| Species | Brain Weight (g) | White Matter % |
|---|---|---|
| Mouse | 0.4 | 10.6 |
| Rat | 2.0 | 12.2 |
| Rabbit | 10–13 | 17.6 |
| Cat | 30 | 23.4 |
| Pig | 180 | 27.7 |
| Human | 1300–1400 | 40.6 |
We chose to use our angiographic skills and equipment to provide single embolic occlusions to major intracerebral arteries. The object was to mimic the standard human stroke that is usually studied, the MCA embolic stroke. Therefore we used ICA injections which provided flow to the MCA and ACA.
We tried other approaches including carotid cut downs but preferred to have our operative site remote from the test site to minimize local spasm and flow disruption due to the surgery. These factors can greatly change flow and confound the intended effect of embolization. When using the carotid cut down technique, the flush of emboli with the prescribed amount of saline, 4.0-ml, or that volume of contrast, resulted in visible reflux into the aortic arch and wide distribution of the emboli throughout the animal in most cases. If the origin of the common carotid was ligated or clamped, this volume caused flow through the ICA into the Circle of Willis, with reflux back through the vertebrals, and into the arch.
After using 5-Fr, 4-Fr, 3-Fr, and combinations of these catheters with microcatheters down to 1.3-Fr, several points became clear. Although we could successfully traverse an ICA or vertebral artery approach to the ophthalmic artery or MCA or ACA with the smallest flow-directed microcatheters, and we could deposit emboli there, the very high cost and fragility of these systems was not appropriate for a large series of animals. In addition, spasm often compromised these demanding angiographic expeditions even with the administration of intravascular nitroglycerine. Therefore, super-selective MCA embolization was discounted as a potential standard model.
While others inject clots through a previously placed CCA catheter into conscious rabbits and then immediately assess neurological defects (Lapchak et al., 2004; Lapchak et al., 2007; Lapchak et al., 2011), our ICA angiographic study must be performed in anesthetized animals. Therefore, neurological assessment was done after recovery. Anesthesia can influence stroke formation, yet the use of identical control groups provides good comparison with test groups.
Our models deliver emboli to the ICA, where flow carries them to the MCA and/or ACA in almost 90% of cases. The small fresh clots enter deeper into the MCA in most animals, since their size and pliable nature allow them to fit even into duplicated MCA vessels. This can be important since 29% of animals have duplication of the MCA resulting in smaller individual vessels (Kirchhof et al., 2002, Caldwell et al., 2011). However, the small size and relatively weak fibrin network of these fresh emboli lead to easy lysis, either autolysis as occurs in human transient ischemic attacks, or thrombolysis as occurs in tPA and similar therapies (Murray et al., 2010). This model results in very small average infarcts, so small that successful therapy can produce only limited improvements as judged by any end point system. This narrow range of potential improvement results in statistical challenges requiring large numbers of animals to resolve and limits usefulness of this model.
The larger aged clots, 4.0-mm long cylinders, are more resilient and tolerant of flexing. The internal bonds in 3-day-old clot are unlikely to autolyse in time to prevent infarction and usually require an active therapy to break (Murray et al., 2010). The 4.0-mm clot is physically too large to enter a duplicated MCA but usually occludes both by covering their origins. This large occlusion leads to larger infarcts. However, the proximity to the posterior communicating artery leads to occasional fragments entering a posterior cerebral artery or superior cerebellar artery and causing non-standard strokes and severe symptoms requiring euthanasia.
Microspheres model insoluble emboli, atheromata, and are very stable once delivered. Distribution is very similar to aged clot as are infarct volumes. While this cannot model reperfusion like clot lysis models, it is very appropriate as a permanent occlusion and can also apply to collateral circulation studies.
Multiple emboli tend to plug vessels in sequence as flow directs them, first to the largest vessel, usually the MCA. Then, with obstruction there, flow will divert and the following emboli will proceed to the next vessel with good flow, and so on. Therefore, with multiple injections or even moderate delay between arrivals of emboli, the large anterior vessels will be occluded first and trailing emboli will travel to posterior vessels (Appendix Table 2). Here occlusions cause severe symptoms and non-standard strokes, unusable for some of our comparisons though they may be appropriate for some investigations and produce higher overall NAS values.
All embolic vessel occlusions are a combination of embolus and fresh clot formed in the vessel around the embolus. Fresh clot will form in stagnant blood upstream to the next proximal branch with good flow and downstream to the first good collateral flow. Initially the new clot will be very fragile, but eventually it will become firm and organize unless autolysis or thrombolysis intervene. Therefore even a tiny embolus may be associated with a much larger volume of clot. This suggests that clot propagation and autolysis or thrombolysis factors will affect all three models, regardless of the size and makeup of the original embolus.
Others have used injection of thrombin into the MCA via a microcatheter. The nature of the resulting clot is apparently not a small embolus but rather a larger distribution of intravascular clots (Kirchhof et al., 2002; Jahan et al., 2008). The impact of distal collateral flow in this model is uncertain and may be compromised by distal small vessel occlusions. Other surgical ligations or occlusions with a suture and subsequent removal may cause intravascular changes related to the surgery instead of a pure embolic abnormality (Wexler et al., 2002; Aronowski et al., 1996). Removal of the obstruction may not remove adjacent thrombus and restore flow in some cases.
The fresh clot model coupled small change from normal with fairly broad overall variation when using percent infarct volume measurements. Therefore many animals were required to prove any significant change. The aged clot model and microsphere model increased the magnitude of change. This reduced the required number of animals to demonstrate group differences in therapeutic experiments (Culp et al., 2011).
Since swelling of the brain can shift midline structures and change hemisphere measurements, we chose to use the entire brain as the basis of measuring percent of brain infarct. Some others use percent of a single hemisphere in smaller animals, effectively doubling the infarct volume value (Wexler et al., 2002; Aronowski et al., 1996).
To define the minimum time required for our model to yield good measurements of infarct volume percent, the same type of microsphere embolization was done with groups sacrificed at 24 hours, 7 hours, and 4 hours. The mean infarct volume percent was very similar in each, 3.39%, 3.88%, and 3.66%, respectively. While we have not defined the minimum time required, these all can be used effectively.
Typically, other rabbit studies do not use infarct volumes, which have recently been proven to be excellent indicators of long-term human outcomes (Yoo et al., 2012; Vogt et al., 2012), but more commonly use a simple behavioral test, the presence or absence of any behavior or neurologic defect. This system, successfully used by Zivin and Lapchak, does not consider severity, just the presence or absence of objective findings. Their analysis, based on differing clot dose requirements to produce any detectable neurological abnormality (including deaths), requires sophisticated dose curve computations (Zivin et al., 1985; Zivin et al., 1992; Lapchak et al., 2004; Lapchak et al., 2007; Lapchak et al., 2011). Using widely scattered emboli, the posterior vessels will likely be involved and symptoms will be more severe even with extremely small or histologically undetectable infarcts (Brown et al., 2010).
In our model, more reflective of common human MCA strokes, posterior occlusions are much less likely. When early death or non-standard angiographic findings including severe spasm, air bubbles, or posterior vessel occlusion are seen, the animals can be discarded. However, some may include posterior vessel occlusions, which are not rare when using microspheres. Our model excluding posterior vessel occlusions creates more uniform infarcts that are appropriate for volume measurements and allows long term survival, more than 80 days. In various test groups (data not presented), nine animals did well clinically until sacrifice at more than 24 hours, one at 20 days and 8 at 80 days, including the 3 controls animals presented here (Appendix Table 2).
Study limitations include the relatively high cost of the models compared to rats and the demanding nature of subselective angiography. When anterior circulation infarcts, infarcts in a more silent region of the brain, are involved, a basic weakness in NAS values becomes apparent. Without including posterior occlusions, our NAS scores are not dramatically changed following embolization in this model, even though percent infarct volumes may meet criteria for statistical difference (Culp et al., 2011). In the fresh clot model, we attribute the low but significant positive correlation between NAS and percent infarct volume to the similar distributions between relatively normal and abnormal rather than a strong linear relationship. Additionally, the limitations of TTC staining as an endpoint include the possibility of failing to detect microinfarction or apoptosis (Liszczak et al., 1984; Bederson et al., 1986).
In summary, stroke model characterization depends primarily on emboli. These include a mild stroke or transient ischemic attack model with frequent autolysis (1.0-mm fresh clot embolus), and moderate to severe strokes with 4.0-mm 3-day-old clot embolus (soluble) or permanent occlusion with spheres (insoluble). In all of these, animals may survive long term and measurement of infarct volume percent in anterior infarcts is a sensitive end point.
Supplementary Material
Comparison of the effects of additional clot emboli and characteristics of emboli on neurological assessment score (NAS) and percent infarct volume. Data are reported as mean±standard error.
Comparison of the effects of additional microsphere emboli, characteristics of emboli, and sacrifice time on neurological assessment score (NAS) and percent infarct volume. Data are reported as mean±standard error. *Bilateral access to both internal carotid arteries (ICA) and embolization in each with two microspheres. †Sequential injections of emboli into one ICA, with a pause between injections. **Long-term follow up of these infarcts, up to 80 days, shows decreasing infarct volume percent as infarct becomes scar and adjacent ventricles dilate. While we have not fully defined this curve, MRI findings show this well. NAS findings also appear to decrease with time, making all measurements less dramatic with age. The optimum sensitivity for determining differences in therapy will therefore be in the first few hours or days.
Schematic representation of the rabbit angiographic stroke model. (TTC = triphenyltetrazolium chloride)
The straight-tip catheter is formed to an angle using a wire and applying steam. Various degrees of “hockey-stick” curve (including the one pictured here and others with more or less curve) were effective for internal carotid artery selection.
Highlights.
Cerebral angiography techniques in the rabbit are described.
Three embolic models of acute ischemic stroke are developed.
Endpoints include infarct volume percent and neurological assessment score.
Stroke models vary by embolus type and can be modified to fit research needs.
Acknowledgments
This work is supported in part by:
NIH R01 HL082481; NIH R01 CA99178; NIH 1UL1RR029884
UAMS Grants:
Hornick; COM Pilot Grant; COM Bridging Grant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronowski J, Samways E, Strong R, Rhoades HM, Grotta JC. An alternative method for the quantitation of neuronal damage after experimental middle cerebral artery occlusion in rats: analysis of behavioral deficit. J Cereb Blood Flow Metab. 1996;16:705–713. doi: 10.1097/00004647-199607000-00022. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Invest Radiol. 2011;46:202–207. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AT, Skinner RD, Flores R, Hennings L, Borrelli MJ, Lowery J, et al. Stroke location and brain function in an embolic rabbit stroke model. J Vasc Interv Radiol. 2010;21:903–909. doi: 10.1016/j.jvir.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Flores R, Lowery J, Brown AT, Culp WC. Variations in the circle of Willis in the New Zealand white rabbit. J Vasc Interv Radiol. 2011;22:1188–1192. doi: 10.1016/j.jvir.2011.01.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp BC, Brown AT, Erdem E, Lowery J, Culp WC. Selective intracranial magnification angiography of the rabbit: basic techniques and anatomy. J Vasc Interv Radiol. 2007;18:187–192. doi: 10.1016/j.jvir.2006.12.720. [DOI] [PubMed] [Google Scholar]
- Culp BC, Culp WC. Rabbit subselective angiography stroke model. Stroke. 2008;39:e165. doi: 10.1161/STROKEAHA.108.526335. [DOI] [PubMed] [Google Scholar]
- Culp WC, Flores R, Brown AT, Lowery JD, Roberson PK, Hennings LJ, et al. Successful microbubble sonothrombolysis without tissue-type plasminogen activator in a rabbit model of acute ischemic stroke. Stroke. 2011;42:2280–2285. doi: 10.1161/STROKEAHA.110.607150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp WC, Woods SD, Skinner RD, Brown AT, Lowery JD, Johnson JL, et al. Dodecafluoropentane emulsion decreases infarct volume in a rabbit ischemic stroke model. J Vasc Interv Radiol. 2012;23:116–121. doi: 10.1016/j.jvir.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnan GA. The 2007 Feinberg Lecture: a new road map for neuroprotection. Stroke. 2008;39:242–248. doi: 10.1161/STROKEAHA.107.493296. [DOI] [PubMed] [Google Scholar]
- Flores R, Hennings LJ, Lowery JD, Brown AT, Culp WC. Microbubble-augmented ultrasound sonothrombolysis decreases intracranial hemorrhage in a rabbit model of acute ischemic stroke. Invest Radiol. 2011;46:419–424. doi: 10.1097/RLI.0b013e31820e143a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Brainin M, Bornstein NM, Tuomilehto J, Hong Z for the Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators. Cerebrolysin in Patients With Acute Ischemic Stroke in Asia: Results of a Double-Blind, Placebo-Controlled Randomized Trial. Stroke. 2012;43:630–636. doi: 10.1161/STROKEAHA.111.628537. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32:1310–6. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan R, Stewart D, Vinters HV, Yong W, Vinuela F, Vandeberg P, et al. Middle cerebral artery occlusion in the rabbit using selective angiography: application for assessment of thrombolysis. Stroke. 2008;39:1613–1615. doi: 10.1161/STROKEAHA.107.507376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof K, Welzel T, Zoubaa S, Lichy C, Sikinger M, de Ruiz HL, et al. New method of embolus preparation for standardized embolic stroke in rabbits. Stroke. 2002;33:2329–2333. doi: 10.1161/01.str.0000027436.82700.73. [DOI] [PubMed] [Google Scholar]
- Kong LQ, Xie JX, Han HB, Liu HD. Improvements in the intraluminal thread technique to induce focal cerebral ischaemia in rabbits. J Neurosci Methods. 2004;137:315–9. doi: 10.1016/j.jneumeth.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Schubert DR, Maher PA. Delayed treatment with a novel neurotrophic compound reduces behavioral deficits in rabbit ischemic stroke. J Neurochem. 2011;116:122–131. doi: 10.1111/j.1471-4159.2010.07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic stroke in rabbit. Stroke. 2004;25:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- Liszczak TM, Hedley-Whyte ET, Adams JF, Han DH, Kolluri VS, Vacanti FX, et al. Limitations of tetrazolium salts in delineating infarcted brain. Acta Neuropathol. 1984;65:150–7. doi: 10.1007/BF00690469. [DOI] [PubMed] [Google Scholar]
- Murray V, Norrving B, Sandercock PA, Terént A, Wardlaw JM, Wester P. The molecular basis of thrombolysis and its clinical application in stroke. J Intern Med. 2010;267:191–208. doi: 10.1111/j.1365-2796.2009.02205.x. [DOI] [PubMed] [Google Scholar]
- Pan G, Wright KC. Clot embolic stroke in the vertebrobasilar system of rabbits: a transfemoral angiographic technique. Cardiovasc Intervent Radiol. 1987;10:285–290. doi: 10.1007/BF02578011. [DOI] [PubMed] [Google Scholar]
- Vogt G, Laage R, Shuaib A, Schneider A VISTA Collaboration. Initial lesion volume is an independent predictor of clinical stroke outcome at day 90: an analysis of the Virtual International Stroke Trials Archive (VISTA) database. Stroke. 2012;43:1266–72. doi: 10.1161/STROKEAHA.111.646570. [DOI] [PubMed] [Google Scholar]
- Wexler EJ, Peters EE, Gonzales A, Gonzales ML, Slee AM, Kerr JS. An objective procedure for ischemic area evaluation of the stroke intraluminal thread model in the mouse and rat. J Neurosci Methods. 2002;113:51–58. doi: 10.1016/s0165-0270(01)00476-9. [DOI] [PubMed] [Google Scholar]
- Woods SD, Flores R, Roberson PK, Lowery JD, Skinner RD, Culp WC. Decreased serum levels of S-100B protein reflect successful treatment effects in a rabbit model of acute ischemic stroke. Open Neurol J. 2011;5:55–57. doi: 10.2174/1874205X01105010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AJ, Chaudhry ZA, Nogueira RG, Lev MH, Schaefer PW, Schwamm LH, Hirsch JA, González RG. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke. 2012;43:1323–30. doi: 10.1161/STROKEAHA.111.639401. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, Suzuki Y, Kondo K, Kawano K, Ikeda Y, Umemura K. Cerebral hemorrhage due to heparin limits its neuroprotective effects: studies in a rabbit model of photothrombotic middle cerebral artery occlusion. Brain Res. 2001;902:30–39. doi: 10.1016/s0006-8993(01)02285-5. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Suzuki Y, Kondo K, Kawano K, Ikeda Y, Umemura K. A novel MCA occlusion model of photothrombotic ischemia with cyclic flow reductions: development of cerebral hemorrhage induced by heparin. Brain Res Brain Res Protoc. 2002;9:85–92. doi: 10.1016/s1385-299x(01)00124-6. [DOI] [PubMed] [Google Scholar]
- Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- Zivin JA, Waud DR. Quantal bioassay and stroke. Stroke. 1992;23:767–773. doi: 10.1161/01.str.23.5.767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the effects of additional clot emboli and characteristics of emboli on neurological assessment score (NAS) and percent infarct volume. Data are reported as mean±standard error.
Comparison of the effects of additional microsphere emboli, characteristics of emboli, and sacrifice time on neurological assessment score (NAS) and percent infarct volume. Data are reported as mean±standard error. *Bilateral access to both internal carotid arteries (ICA) and embolization in each with two microspheres. †Sequential injections of emboli into one ICA, with a pause between injections. **Long-term follow up of these infarcts, up to 80 days, shows decreasing infarct volume percent as infarct becomes scar and adjacent ventricles dilate. While we have not fully defined this curve, MRI findings show this well. NAS findings also appear to decrease with time, making all measurements less dramatic with age. The optimum sensitivity for determining differences in therapy will therefore be in the first few hours or days.
Schematic representation of the rabbit angiographic stroke model. (TTC = triphenyltetrazolium chloride)
The straight-tip catheter is formed to an angle using a wire and applying steam. Various degrees of “hockey-stick” curve (including the one pictured here and others with more or less curve) were effective for internal carotid artery selection.


