Abstract
Advanced age is associated with alterations in innate and adaptive immune responses, which contribute to an increased risk of infection in elderly patients. Coupled with this immune dysfunction, elderly patients demonstrate impaired wound healing with elevated rates of wound dehiscence and chronic wounds. To evaluate how advanced age alters the host immune response to cutaneous wound infection, we developed a murine model of cutaneous Staphylococcus aureus wound infection in young (3–4 month) and aged (18–20 month) BALB/c mice. Aged mice exhibit increased bacterial colonization and delayed wound closure over time compared to young mice. These differences were not attributed to alterations in wound neutrophil or macrophage TLR2 or FcγRIII expression, or age-related changes in phagocytic potential and bactericidal activity. To evaluate the role of chemotaxis in our model, we first examined in vivo chemotaxis in the absence of wound injury to KC, a neutrophil chemokine. In response to a subcutaneous injection of KC, aged mice recruited fewer neutrophils at increasing doses of KC compared to young mice. This paralleled our model of wound infection, where diminished neutrophil and macrophage recruitment was observed in aged mice relative to young mice despite equivalent levels of KC, MIP-2 and MCP-1 chemokine levels at the wound site. This reduced leukocyte accumulation was also associated with lower levels of ICAM-1 in wounds from aged mice at early time points. These age-mediated defects in early neutrophil recruitment may alter the dynamics of the inflammatory phase of wound healing, impacting macrophage recruitment, bacterial clearance and wound closure.
Introduction
An estimated 25 billion in US health care expenditure is spent on care of chronic, non-healing wounds (1). One patient sub-population, the elderly, account for a major portion of patients afflicted by chronic and infected wounds (1–3). Aging is associated with a decline in immune function which can elevate rates of wound infection and delay wound closure in these patients (4–6). Advanced age is associated with a general decline in innate and adaptive immune function which contributes to an increased susceptibility to opportunistic bacterial and viral infections in the elderly (2, 7–9). Underlying this elevated susceptibility to infection are deficits in host recognition, phagocytosis, migration and activation of a pathogen-specific adaptive immune response (10–13). In addition to the immunosenescence that accompanies aging, advanced age is also marked by heightened levels of circulating pro-inflammatory mediators in the absence of an inciting stimulus, a state referred to as “inflamm-aging (14, 15).” Together, these phenomena are thought to contribute to poor outcomes following infection or trauma in clinical and animal models of aging (2, 16–20).
Alongside playing a critical role in eradication of foreign pathogens, the innate immune response is also crucial to tissue repair, another physiologic response compromised by aging. Cutaneous wound repair is a complex process, marked by immune cell infiltration and inflammation, fibroblast and keratinocyte proliferation, angiogenesis, and remodeling of the extracellular matrix to ensure restoration of the normal skin barrier (5, 21–24). Natural and succinct progression through these interdependent stages of wound healing is required to ensure rapid and adequate repair of epidermal and dermal architecture. Clinical observations reveal that elderly patients have an impaired response to cutaneous injury, resulting in elevated rates of wound dehiscence and subsequent chronic wounds (1, 25). Non-healing wounds in this patient population have an increased risk of infectious complications. This risk is associated with higher morbidity and mortality, and infectious spread in long-term care facilities (26). Moreover, older patients are more susceptible to post-surgical infections and subsequent problematic wound closure with costly prolonged hospital stays (2, 25).
Laboratory studies using murine models of cutaneous injury have substantiated the aforementioned clinical findings as advanced age has been shown to alter the dynamics of all stages of the wound repair process (27–30). Evidence suggests age-associated differences in the magnitude and duration of neutrophil and macrophage infiltration into the wound bed during the inflammatory phase of wound healing (27, 29). These phagocytes are instrumental in limiting infectious spread and regulation of the temporal progression through the early phases of wound closure (22, 31, 32). Despite compromised immune function and increased rates of infectious wound complications in the elderly, the impact of advanced age on the host immune response to cutaneous wound infection has been neglected. Staphylococcus aureus (S. aureus) is a common dermatopathogen that accounts for at least 75% of skin and soft tissue infections and 30% of surgical-site infections, with a dramatic increase in S. aureus-associated surgical site infections to 50% in patients over 65 (2, 33, 34). We developed a cutaneous excisional S. aureus wound infection model in young (3–4 month) and aged (18–20 month) BALB/c mice. Using this model, we are the first to demonstrate that advanced age is associated with heightened bacterial colonization and delayed wound closure due to reduced innate immune cell recruitment.
Materials and Methods
Animal Model
3–4 month old (young) and 18–20 month old (aged) BALB/c mice (Charles River/NIA, Kingston Facility, Stony Ridge, NY) were utilized to determine age-dependent differences in response to cutaneous wound injury and infection. All animal studies were approved and performed with strict accordance to the regulations established by the Loyola University Chicago Animal Care and Use Committee. Following acclimation at Loyola’s Animal Care Facility, young and aged mice were subjected to dorsal excisional cutaneous injury as previously described (35). Briefly, mice were administered 100 mg/kg ketamine and 10 mg/kg xylazine i.p. followed by i.p. saline to ensure systemic distribution of the anesthetic. Once the mice no longer responded to firm pressure applied to their hind limb, their dorsum’s were shaved and cleansed with ethanol pads. Mice were then subjected to 6 dorsal full-thickness (skin and panniculus carnosa) cutaneous wounds with a 3 mm dermal punch biopsy (Acuderm, Ft. Lauderdale, FL). Immediately after injury, mice received ~103 CFU/10uL of S. aureus and were returned to their cages on heating pads. Following arousal from anesthesia after injury and infection, no overt difference in grooming, mobility, activity and nutritional status were observed between young and aged animals. A low inoculum of bacteria was chosen to prevent sepsis which is known to negatively impact wound healing (36). S. aureus Newman strain was grown overnight in tryptic soy broth (TSB) at 37°C under constant agitation. The next day, 1 mL of S. aureus in TSB was resuspended in 2mL fresh TSB and incubated at 37°C for 2 hours to ensure mid-logarithmic growth at the time of application to cutaneous wounds. Bacterial concentration (CFU/mL) was determined by absorbance at 600 nm and the final inoculum confirmed by back-plating on mannitol salt agar (MSA; BD Diagnostics, Sparks, MD).
Mice were sacrificed at days 1, 3, 7 and 10 after injury and infection. The pelt was removed and photographed to measure wound size as described below. A larger 5 mm punch biopsy was used to remove the 3 mm wounds. One-two wounds from each animal were used to examine for bacterial colonization, ex vivo phagocytosis, flow cytometric analysis of wound immune infiltrate and cytokine and chemokine analysis as detailed below. Blood was obtained via cardiac puncture for flow cytometric analysis, as well as the bactericidal assay.
Bacterial Colonization
Skin, spleen and kidney were each homogenized in 1 mL sterile PBS and 10-fold serial dilutions to 106 were plated on MSA plates (BD Diagnostics, Sparks, MD). Heparinized whole blood was directly plated onto MSA plates. Plates were incubated at 37°C for 24–48 hours and colonies were counted to determine levels of bacterial colonization. Significant bacterial dissemination to spleen, kidney and blood was not observed (data not shown). For all wound colonization data, one wound from each individual animal was used to represent an N of one and experiments were repeated 3–4 times at each time point.
Wound Size
Wound size was evaluated by digital photography and image analysis as previously described (35). Briefly, at days 1, 3, 7 and 10 all six wounds per animal were photographed with a Canon EOS SLR digital camera. Each pelt was photographed at a fixed distance of 20 cm with a ruler placed within the frame of each photograph. Photoshop 7.0 (Adobe Systems Inc., San Jose, CA) was used to determine the number of pixels in the open wound area using the magic wand tool, with zoom at 100% and a tolerance setting of 60. Separate animals were sacrificed immediately following wound injury and wound size was determined to represent day 0. Wound areas at each time point were compared with day 0 wounds: (pixels at days1–10/pixels at day 0)×100 were used to determine the percent open wound area at each time point. The % open area of the 6 wounds from each animal were averaged to give one value for each animal, such that the average of 6 wound is an N of 1 individual animal. Data represents 3–4 replicate experiments.
Skin and Whole Blood Flow Cytometry
Single cell suspensions of wound cells for flow cytometry were generated as previously described (37). At days 1–7 after injury and infection, animals were euthanized, pelts removed and wounds excised using a 5 mm punch biopsy. Two wounds were used to achieve an adequate total cell yield and represent an N of one individual animal. Diced wounds were incubated overnight at 4°C in RPMI 1640 culture media containing 10% FBS (Hyclone, Logan, UT), 2 mM L-glutamine (Gibco, Grand Island, NY), 1% penicillin/streptomycin (Gibco, Grand Island, NY), 2 mg gentamycin sulfate (Mediatech Inc, Manassas, VA) and 0.3 mg dispase II (Roche Diagnostics, Indianapolis, IN). The next day, tissue pieces were removed and subjected to further enzymatic digestion with 1 mg collagenase from Clostridium histolyticum type 1A (Sigma-Aldrich, St. Louis, MO), 1.2 mg DNAse I from bovine pancreas Grade II (Roche Diagnostics, Indianapolis, IN), 1 mg hyaluronidase from bovine testes type 1-S (Sigma-Aldrich, St. Louis, MO), in RPMI 1640 with 5% FBS, 2 mM L-glutamine (Gibco, Grand Island, NY), 1% penicillin/streptomycin (Gibco, Grand Island, NY), 2 mg gentamycin sulfate (Mediatech Inc, Manassas, VA) and magnesium chloride hexahydtrate for 2 hours at 37°C. After two hours, these solutions were combined and debris removed by filtration with a 70μm filter. Cells that remained adherent to the tissue culture plastic were treated with Accutase (eBioscience, San Diego, CA) for 5–8 minutes at 37°C followed by vigorous pipetting. Cells were washed and adjusted to 1×106/mL. Cells were blocked for 20 minutes with FcBlock (anti-CD16/CD32, eBioscience) and rat IgG (Jackson Immuno), and then stained with PE-Cy7-F4/80 (eBioscience, San Diego, CA), FITC-Gr-1 (eBioscience, San Diego, CA), APC-CD3 (eBioscience, San Diego, CA) or APC-TLR2 (eBioscience, San Diego, CA). Cells were washed twice and then resuspended in flow buffer (1% BSA, 0.1% sodium azide and 2mM EDTA in PBS). For blood analysis, heparinized whole blood was collected and stained per the manufacture’s protocol (Leinco Technologies). Whole blood (100 μL) was blocked for 20 minutes with FcBlock and rat IgG and then stained with PE-Cy7-F4/80, FITC-Gr-1 and PE-CXCR2. Following staining, erythrocytes were lysed with 2 mL Easy Lyse Solution (Leinco Technologies) for 11.5 minutes. Lysis was terminated by addition of 2 mL ice cold Wash Buffer (Leinco Technologies). Cells were washed twice in flow buffer and resuspended in 500μL flow buffer. Both skin and blood samples were collected on the FACSCanto I and FACS LSR Fortessa (BD Bioscience, San Jose, CA) and data were analyzed by FlowJo Software (Tree Star Inc, Ashland, OR). Fluorescence minus one control staining and single color controls were used to determine positive staining.
Phagocytosis and FcγRIII Staining
Following isolation, wound cells were subjected to pHrodo-S. aureus phagocytosis as previously described (37). Cells were resuspended to 1×106 cells/mL in Phagocytosis Uptake Buffer (HBSS (Gibco, Grand Island, NY), with 20 mM HEPES, pH 7.4) per the manufacturer’s instructions (Invitrogen, Carlsbad, CA). pHrodo-S. aureus BioParticles (Invitrogen, Carlsbad, CA) were reconstituted to 1 mg/mL in Phagocytosis Uptake Buffer and then opsonized with rabbit polyclonal IgG antibodies (Invitrogen, Carlsbad, CA) for 1 hour at 37°C. Control tubes for each animal were placed on ice (4°C) and experimental tubes placed at 37°C for 15 minutes to allow for temperature equilibration. pHrodo-S. aureus was then added at a ratio of 30:1 bacteria particles to cell. Samples were incubated for 0–60 minutes at 4°C (control) or 37°C (experimental) after which phagocytosis was stopped by addition of 2 mL ice cold Phagocytosis Uptake Buffer and placement on ice. Cells were blocked with rat IgG and anti-FcγRII (R&D Systems, Minneapolis, MN), to block FcγRI and FcγRII, respectively, for 20 minutes at 4°C. Samples were then stained with PE-anti-FcγRIII (R&D Systems, Minneapolis, MN) for 30 minutes followed PE-Cy7-anti-F4/80 and FITC-anti-Gr1 for 30 minutes at 4°C (38). Cells were washed twice, resuspended in flow buffer, and phagocytosis and FcγRIII expression on wound neutrophils and macrophages were examined by flow cytometry. Data were acquired on the FACSCanto1 (BD Bioscience, San Jose, CA) and analyzed by FlowJo Software (Tree Star Inc, Ashland, OR).
Chemokine Analysis
One wound from each animal was homogenized in 1mL BioRad Cell Lysis buffer (BioRad, Hercules, CA) supplemented with Factor 1, Factor 2 and PMSF per the manufacture’s instructions (BioRad, Hercules, CA). Homogenates were sonicated at 30%, syringe-filtered (25 μm) and chemokines (KC, MIP-2 and MCP-1) as well as adhesion molecules (ICAM-1) were analyzed by ELISA (R&D Systems, Minneapolis, MN) (39).
In Vivo Chemotaxis
Young and aged mice were administered 100 mg/kg ketamine and 10 mg/kg xylazine followed by saline to ensure systemic distribution of the anesthetic. Once the mice no longer responded to firm pressure applied to their hind limb, their dorsum’s were shaved and cleansed with ethanol pads. In the dorsal subscapular midline, mice were then injected s.c. with saline vehicle (50μL), or 100 or 1000 pg of recombinant mouse KC in a volume of 50μL. Mice were sacrificed 8 hours after injections. The injection site and surrounding tissue were excised with an 8 mm punch biopsy and subjected to digestion as outlined above (37). Neutrophil recruitment to the injection site was evaluated by flow cytometry as described above. Data are expressed as fold change of baseline neutrophil recruitment after saline injections.
Statistical analysis
Data are shown as mean ± SEM of each group. One wound, or an average of wounds as described above, from each animal was used to reach an N of 1, such that an N of 12 represents 12 individual animals. Data were analyzed by Student’s t test or one- way or two-way ANOVA with Tukey’s or Bonferroni post-hoc tests, respectively, where appropriate using GraphPad Prisim 5 (GraphPad, La Jolla, CA). A value of p ≤0.05 was considered significant.
Results
Bacterial colonization and wound closure is impaired with advanced age
To determine the impact of advanced age on the innate immune response following cutaneous injury and S. aureus infection, we developed a model of cutaneous wound infection in young and aged mice to evaluate bacterial colonization and wound size at days 1, 3, 7 and 10 after injury and infection. Bacterial colonization in wounds from aged mice were elevated over the time course examined as compared to young mice, with post-hoc significance observed at day 3 (Figure 1, p<0.05). Levels of wound bacterial burden were unaltered in aged animals at day 7 as compared to day 3, whereas gradual reduction in wound bacterial content was observed in young animals by day 7. Moreover, by day 10, wounds from young mice averaged 103 CFU/mL, compared to 106 CFU/mL in aged animals. In parallel to these studies, wound size was evaluated by digital photography and image analysis (Figure 2A). Compared to young mice, wounds from aged mice had a larger percent open wound area remaining at days 3 and 7 following injury and infection (Figure 2B, p<0.05), with incomplete closure at day 10. At day 3, wounds in aged animals remained ~80% open as compared to ~66% in young mice (Figure 2B, p<0.05). By day 7, an ~ 2-fold increase in percent open wound area was observed in aged mice as compared to young (Figure 2B, p<0.05). The persistent open wound bed at day 10 in aged animas (~11% verse ~0.9% in young mice) could result in subsequent infectious complications in aged mice. Together, these findings recapitulate clinical observations of increased infectious complications and delayed healing with advanced age.
Figure 1. Wound bacterial colonization at days 1 through 10 following cutaneous injury and S. aureus infection.
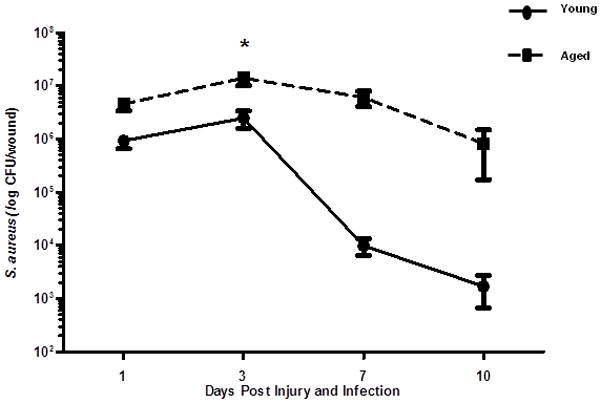
Young BALB/c (3–4 months, black circles and solid line) and aged BALB/c (18–20 months, black squares and dotted line) received six, 3mm dorsal cutaneous wounds followed by ~103 CFU S. aureus/wound. At days 1, 3, 7 and 10 after injury and infection, mice were sacrificed and bacterial colonization was determined by growth on MSA plates. Data are shown on a log scale as mean ± SEM, *p<0.001 compared to young at same time point by two-way ANOVA; N=8–20 individual animals per group at each time point. Data are cumulative of 3–4 replicate experiments at a given time point.
Figure 2. Wound size at days 1 through 10 following cutaneous wound infection with S. aureus.
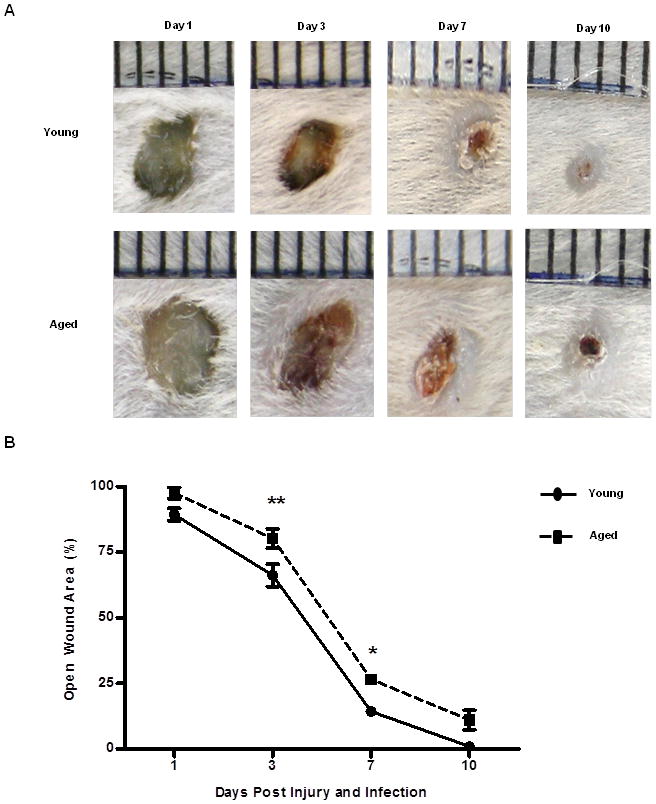
(A) Representative images of wound from young (top panel) and aged (bottom panel) mice are days 1, 3, 7, and 10 after injury and infection. (B) Wound size expressed as percent open wound area relative to time zero at days 1, 3, 7 and 10 after cutaneous wound infection in young (black circle and solid line) and aged (black squares and dotted line) mice. The 6 wounds from each animal were averaged to give one value for each animal. N=7–15 individual animals per group at each time point. Data are shown as mean ± SEM, **p<0.01 and *p<0.05 compared to young at same time point by two-way ANOVA; N=7–15 individual animals per group at each time point. Data are cumulative of 3 replicate experiments at a given time point.
Host recognition is not altered following injury and infection
Previous studies have reported decreased TLR expression in various innate immune cell subsets with advanced age (reviewed in (6, 10)). TLR-1, -2 and -6 play critical roles in early host recognition of Gram-positive pathogens, and loss of TLR2 or downstream mediators of TLR signaling, such as MyD88, results in increased susceptibility to S. aureus infection (40–43). Given differences in bacterial colonization at day 3, we chose to evaluate TLR2 expression on resident tissue macrophages (F4/80+Gr-1− cells from uninjured skin) as well as on infiltrating wound leukocytes in aged mice relative to young (Figure 3A). In aged mice, a reduction in the absolute number of TLR2+ resident tissue macrophages (Figure 3B, p<0.05) was observed compared to young mice. Following injury and infection, there were no age-dependent changes in the frequency of TLR2+ infiltrating macrophages and neutrophils (Figure 3C–D). Moreover, aging did not alter the expression of TLR2 on infiltrating leukocytes after injury and infection (Table 1). The changes in number of TLR2+ resident tissue macrophages with age may increase susceptibility to infection; however, following injury and infection, TLR2 expression did not contribute to prolonged bacterial infection in aged animals.
Figure 3. Expression of TLR2 by cutaneous neutrophils and macrophages.
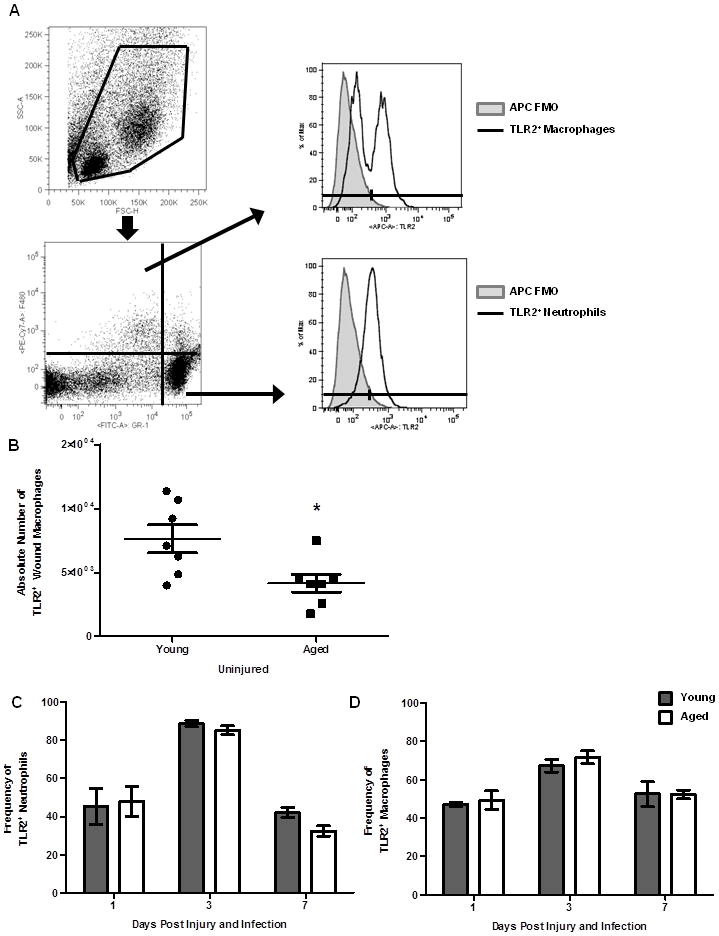
The number of neutrophils or macrophages expressing TLR2 was examined by flow cytometry. (A) Gating strategy for TLR2+ resident and infiltrating leukocytes. Live cells were gated on Gr-1 and F4/80; F4/80−Gr-1+ (neutrophils), F4/80+Gr-1− (macrophages). Neutrophil and macrophage populations were then evaluated for TLR2 positivity (dotted black line). (B) Absolute number of TLR2+ macrophages in uninjured skin from young and aged mice, *p<0.02 by Student’s t-test, N=7 per group. (C) Frequency of TLR2+ wound macrophages at day 1 and day 3 in young (gray bars) and aged (white bars) mice. (D) Frequency of TLR2+ neutrophils at day 1 and day 3 in young and aged mice. Data are shown as mean ± SEM; N=8–17 individual animals per group and are cumulative of 3 replicate experiments per time point. Data are not significant.
Bacterial clearance is not affected by advanced age
In addition to recognition of bacteria by the host immune system, neutrophils and macrophages that are recruited to the wound site are required to phagocytose and clear invading organisms. In particular, deficiencies in neutrophil and macrophage phagocytosis, as well as bactericidal potential, have been associated with reduced bacterial clearance as well as chronic or repeated infections (26, 44–46). A major phagocytic pathway in these cells is the FcγR pathway (47). Interestingly, reduced expression of FcγRIII (CD16) and phagocytosis have been observed in neutrophils from aged humans (46). Similar studies in macrophage populations suggest a reduced phagocytic potential with advanced age (48–50). Thus, we sought to determine if differences in phagocytosis and FcγRIII expression may contribute to delayed resolution of bacterial clearance in our model. Initially, we examined FcγRIII expression in peripheral blood neutrophils in our young and aged animals pre- and post-infection. In line with studies by Butcher et al., there was a reduction in the frequency of FcγRIII+ neutrophils in aged mice following injury and infection (Supplementary Figure 1)(46). Butcher et al. had documented that reduced FcγRIII expression was associated with reduce phagocytic potential in neutrophils isolated from the periphery of elderly subjects (46). Interestingly, few studies have examined the impact of the wound microenvironment on phagocytic cell function in the setting of advanced age. Given these data, we examined the phagocytic potential and the expression of FcγRIII pre-and post- phagocytosis in wound cells isolated from young and aged mice. Phagocytosis of pHrodo-S. aureus bioparticles by wound neutrophils were not different in young and aged mice (Figure 4A–B). Despite no observed changes in phagocytosis, there were fewer FcγRIII+ neutrophils pre- and post- phagocytosis in aged animals and the MFI of FcγRIII was diminished (Figure 4C–D, p<0.05). Similar to these findings, no age-dependent changes in phagocytosis by wound macrophages were observed (Figure 5A–B). In both young and aged mice, the frequency of FcγRIIIhi macrophages was reduced post-phagocytosis as compared to cells from age-matched controls (Figure 5C, p<0.05). However, there were no age-dependent differences in the frequency of total FcγRIII+ macrophages between young and aged mice pre- or post-phagocytosis (Figure 5C). Moreover, there was a reduction in the MFI of FcγRIII in aged mice compared to young regardless of exposure to pHrodo-S. aureus (Figure 5D, p<0.05).
Figure 4. Wound neutrophil phagocytosis of S. aureus and FcγRIII expression.
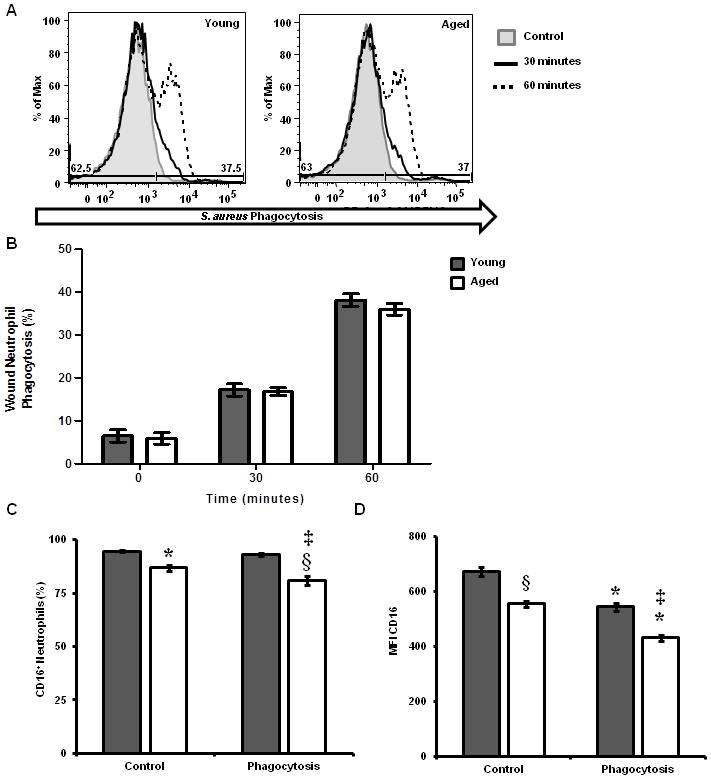
Wound leukocyte suspensions were allowed to phagocytose pHrodo-S. aureus particles for 0–60 minutes and then stained for FcγRIII expression. Phagocytosis and FcγRIII were assayed by flow cytometry. (A) Representative histograms (shaded gray-4°C, control; black-37°C, 30 minutes; and black dotted-37°C, 60 minutes) of phagocytosis by wound neutrophils (F4/80−Gr-1+S. aureus+). (B) Percentage of wound neutrophils that phagocytosed pHrodo-S. aureus particles in young (gray bars) and aged (white bars) mice. Data are not significant. (C) Percentage of wound neutrophils expressing FcγRIII in young and aged mice; *p<0.05 verse young control, ‡p<0.001 verse young phagocytosis, §p<0.05 verse young and aged controls by one-way ANOVA. (D) MFI of FcγRIII on wound neutrophils from young and aged mice; §p<0.001 verse young control, ‡p<0.001 verse young control and phagocytosis,*p<0.001 verse aged matched control by one-way ANOVA. Data are shown as mean ± SEM; N=9–12 individual animals per group for phagocytosis assays and are cumulative of 3 replicate experiments. N=4–8 individual animals per group for FcγRIII studies and are cumulative of 2 replicate experiments.
Figure 5. Wound macrophage phagocytosis of S. aureus and FcγRIII expression.
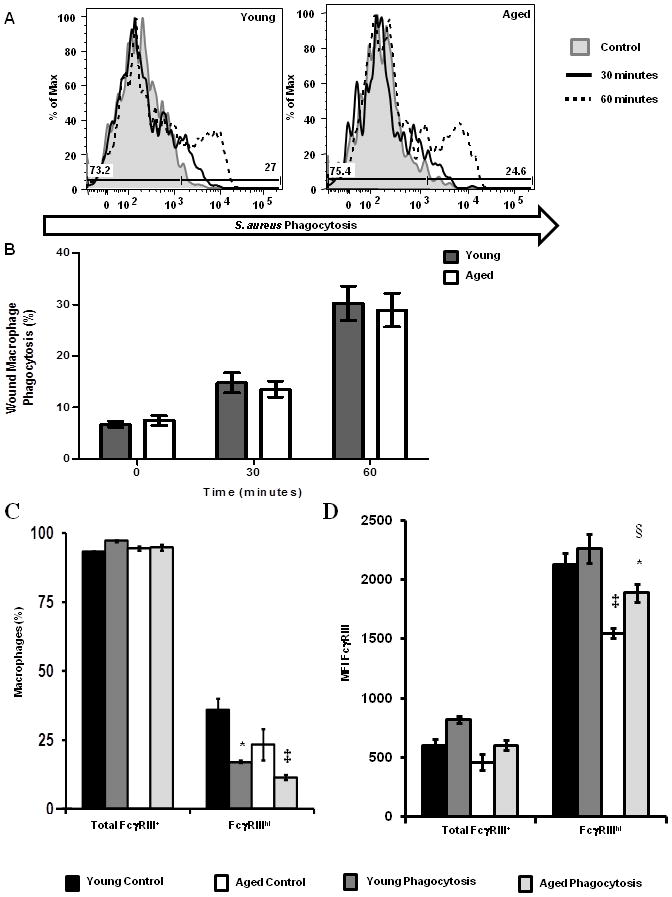
Wound leukocyte suspensions were allowed to phagocytose pHrodo-S. aureus particles for 0–60 minutes and then stained for FcγRIII expression. Phagocytosis and FcγRIII were assayed by flow cytometry. (A) Representative histograms (shaded gray-4°C, control; black-37°C, 30 minutes; and black dotted-37°C, 60 minutes) of phagocytosis by wound macrophages (F4/80+Gr-1−S. aureus+). (B) Percentage of wound macrophages that phagocytosed pHrodo-S. aureus particles in young (gray bars) and aged (white bars) mice. Data are not significant. (C) Percentage of wound macrophages expressing FcγRIII in young and aged mice; *p<0.05 verse young control, ‡p<0.01 verse aged control by one-way ANOVA. (D) MFI of FcγRIII on wound macrophages from young and aged mice; ‡p<0.01 verse young control,*p<0.05 verse young phagocytosis, §p<0.05 verse aged control by one-way ANOVA. Data are shown as mean ± SEM; N=9–12 individual animals per group for phagocytosis assays and are cumulative of 3 replicate experiments. N=4–8 individual animals per group for FcγRIII studies and are cumulative of 2 replicate experiments. *p<0.05 verse young control, §p<0.05 verse young control and young phagocytosis, ‡p<0.05 verse age control.
Though age did not impact the phagocytic potential of isolated wound neutrophils or macrophages ex vivo, decreased FcγR expression has been shown to play a role in bactericidal activity via Rac-2 mediated formation of the NADPH oxidase complex (47, 51). To evaluate if the observed reduction in FcγRIII impaired the ability of neutrophils to kill ingested organism, isolated peripheral blood neutrophils were subjected to a modified bactericidal assay (52). Neutrophils were allowed to phagocytose opsonized pHrodo-S. aureus for 15 minutes, and percent killing of ingested bacteria was evaluated at 45 and 75 minutes after the end of the phagocytosis phase. No age-related differences in bactericidal activity were observed at either time point (Supplementary Figure 2).
Aging impairs in vivo chemotaxis to dorsal cutaneous skin
Previously, our laboratory has demonstrated that peripheral blood neutrophils from aged animals demonstrate a basal hyperchemokinesis with a reduced directional migration toward a chemotatic stimulus in vitro (53). Given no difference in parameters of bacterial clearance, we extended these findings to determine if age-related differences in leukocyte migration in vivo may contribute to heightened bacterial colonization and delayed wound closure in aged mice. Following s.c. injection of 100 pg of KC, a potent neutrophil chemokine and the murine homolog of human IL-8, neutrophil recruitment to the dorsal skin in aged mice was reduced as compared to young (Figure 6, p<0.05). To determine if this could be overcome by higher doses of a chemotactic agent, we evaluated neutrophil recruitment after injection of 1,000 pg of KC. At a ten-fold higher dose, aged mice still had impaired neutrophil recruitment as compared to their younger counterparts given the same dose (Figure 6, p<0.05). However, neutrophil recruitment in aged mice at a dose of 1000 pg was similar to that observed in young mice at a dose of 100 pg. These data suggest that aging impairs chemotaxis to cutaneous tissue with advanced age in response to a direct chemotatic stimulus.
Figure 6. In vivo neutrophil chemotaxis in response to KC.
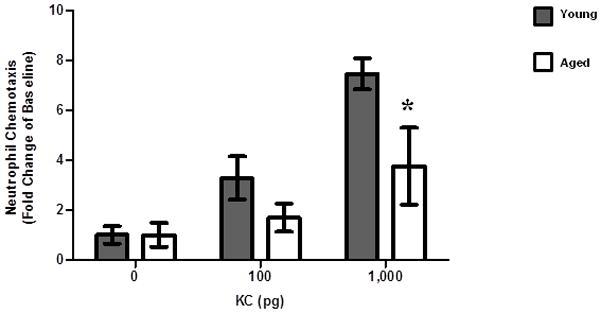
Young and aged mice were injected s.c. with increasing doses of KC (0, 100 and 1000 pg) and neutrophil recruitment to the skin was examined by flow cytometry. Data are shown as mean ± SEM, N=3–6 individual animals per group and are cumulative of 2 replicate experiments. *p<0.05 verses young at same time point by two-way ANOVA.
Peak leukocyte infiltration is attenuated in aged mice
Once we established that neutrophil recruitment in aged mice was diminished in vivo in response to a lone chemotatic stimulus, we sought to determine if we observed reduced leukocyte accumulation at the wound site in our model. Previous studies have documented that advanced age can alter the inflammatory cell infiltrate in non-infected wounds of aged mice (27, 29) as well as other tissues(54, 55); however, the impact of cutaneous infection on leukocyte recruitment with age has not been examined. Wound leukocyte recruitment was assayed by flow cytometry at days 1–7 after injury and infection (Figure 7). At day 1, a similar number of wound neutrophils and macrophages were observed in young and aged mice (Figure 7A–B). However, at day 3, there was a reduction in neutrophil and macrophage numbers isolated from wounds from aged mice. (Figure 7A–B, p<0.05). Numbers of wound neutrophils and macrophages were comparable in young and aged mice at day 7, despite persistent infection in aged animals. No difference in T cell recruitment was noted at any time point (Figure 7C). The delay in neutrophil and macrophage recruitment at day 3 in aged animals may impair early bacterial clearance while the inability to recruit elevated numbers of leukocytes in aged mice at later time points may contribute to the persistent infection observed in these animals. Moreover, this altered leukocyte recruitment may perturb wound healing kinetics, delaying transition from the inflammatory to the proliferative phase and ultimately impairing wound closure and restoration of the dermal matrix.
Figure 7. Time course of cutaneous leukocyte accumulation following injury and infection.
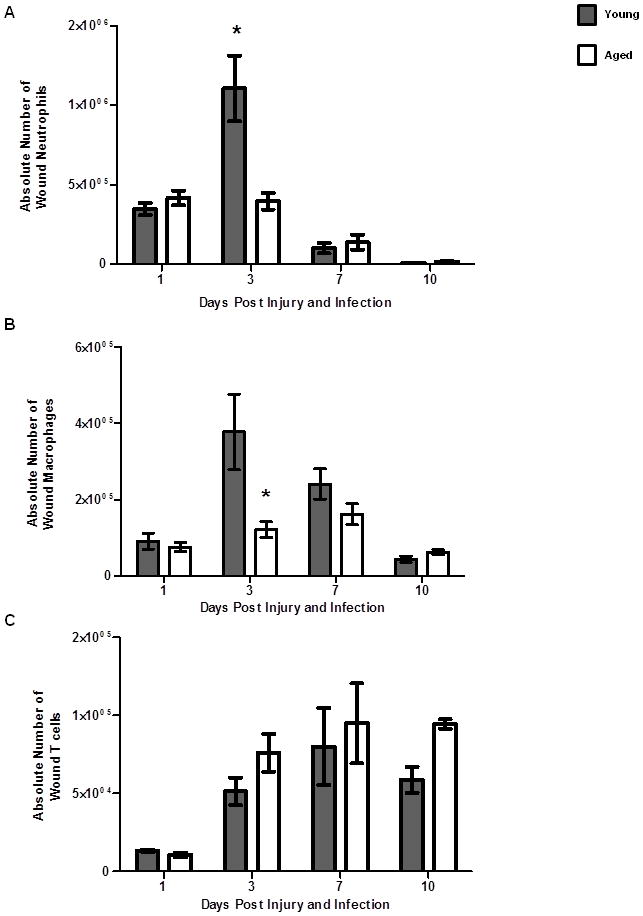
Leukocyte infiltration to the wound and surrounding tissue area of young (gray bars) and aged (white bars) was assessed by flow cytometry at days 1 through 10 post cutaneous wound infection. (A) Gating strategy for wound neutrophils (F4/80−/Gr-1+), wound macrophages (F4/80+/Gr-1−) and wound T cells (CD3+) by flow cytometry. (B) Absolute number of wound neutrophils; *p<0.01 compared to young at same time point by two-way ANOVA. Data are shown as mean ± SEM; N=8–19 per group at days 1–7, N=3–6 at day 10. (C) Absolute number of wound macrophages. Data are shown as mean ± SEM; N=8–19 individual animals per group at days 1–7, N=3–6 individual animals per group at day 10. Data are cumulative of 3–4 replicate experiments at days 1–7 and are cumulative of 2 replicate experiments at day 10. (D) Absolute number of wound T cells. Data are shown as mean ± SEM; N=3–9 individual animals per group and are cumulative of 2 replicate experiments. Data are not significant by two-way ANOVA.
Enhanced chemokine secretion in wounds from aged animals
Important to neutrophil and macrophage recruitment are the chemotatic stimuli generated following tissue injury by keratinocytes and resident tissue leukocytes (56, 57). To determine if the decreased neutrophil and macrophage numbers were due to reduced chemoattractant production, we evaluated the wound chemokine milieu. Interestingly, at day 1 following injury and infection, wounds from aged mice had elevated levels of neutrophil chemokines KC and MIP-2 (Figure 8A–B, p<0.05). Furthermore, wounds from aged mice had increased levels of MCP-1, a chemokine that helps recruit and differentiate circulating monocytes (Figure 8C, p<0.05). However, these elevated levels of neutrophil and macrophage chemokines were associated with similar neutrophil and macrophage recruitment at day 1. At day 3, when neutrophil and macrophage numbers were significantly reduced in aged animals, levels of these three chemotatic mediators were similar in young and aged mice (Figure 8, p<0.05). In conjunction with reduced leukocyte accumulation, these findings paralleled our results observed following subcutaneous injection of KC, suggesting that a stronger chemotatic stimulus may be required to mediate a similar chemotatic response with advanced aged.
Figure 8. Neutrophil and macrophage chemokine levels in wound homogenates.
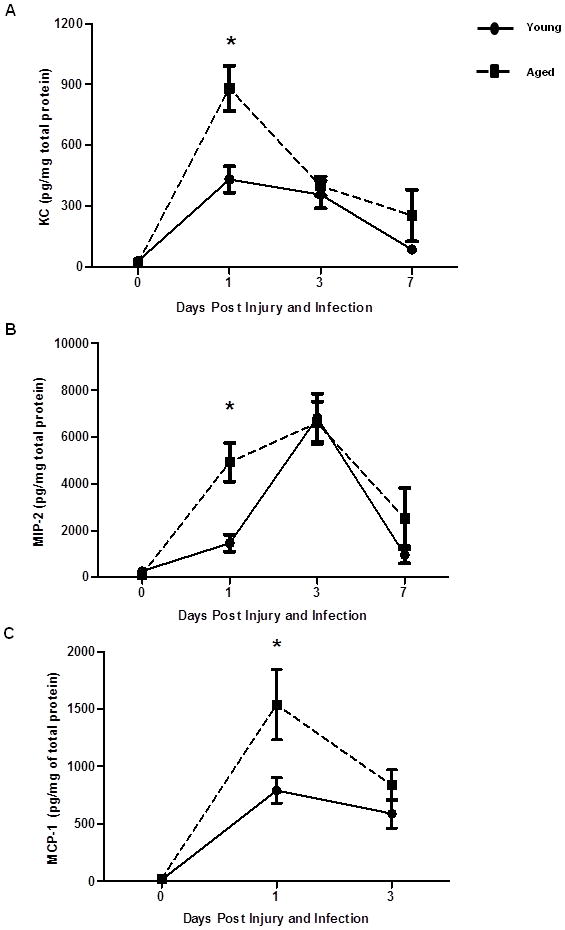
Neutrophil and macrophage chemokine levels in wounds of young (black circles and solid line) and aged (black squares and dotted line) mice present at the site of wound infection were measured by ELISA. (A) KC, *p<0.0001 compared to young at same time point by two-way ANOVA. (B) Macrophage inflammatory protein-2 (MIP-2), *p<0.05 compared to young at same time point by two-way ANOVA. (C) Macrophage chemoattractant protein-1 (MCP-1), *p<0.05 compared to young at same time point by two-way ANOVA. Data are shown as mean ± SEM; N=8–22 individual animals per group (days 0–3) and N=3–6 individual animals per group (day 7). Data are cumulative of 3–4 replicate experiments at days 0–3 and cumulative of 2 replicate experiments at day 7.
Elevated CXCR2 expression in peripheral blood neutrophils of aged animals following injury
KC and MIP-2 are ligands for the chemotatic receptor CXCR2 present on circulating neutrophils (58). Ligation of CXCR2 results in upregulation of selectins and integrins which allow neutrophils to roll, adhere and subsequently transmigrate across endothelial walls to reach the site of injury or infection (59–62). Considering a strong chemotatic stimulus was present at the wound site in aged animals, we examined the peripheral neutrophil pool to determine if age-dependent differences in CXCR2 expression could account for reduced leukocyte recruitment with age. Following injury and infection at days 1 and 3, the frequency of neutrophils in circulation was elevated in aged mice compared to young mice at both time points (Figure 9A–B, p<0.05). There was no difference in the frequency of CXCR2+ neutrophils between young and aged mice prior to or following injury (Figure 9C). Following cutaneous wound infection, downregulation of CXCR2 expression was observed in mice of both age groups, however the MFI of CXCR2 on neutrophils from aged mice was significantly elevated (Figure 9D). This elevation persisted out to day 3, where CXCR2 expression remained increased in aged mice relative to young mice. These data suggest that mediators downstream of CXCR2 may contribute to decreased neutrophil recruitment to the wound site in aged animals.
Figure 9. Peripheral blood neutrophil frequency and chemokine receptor expression.
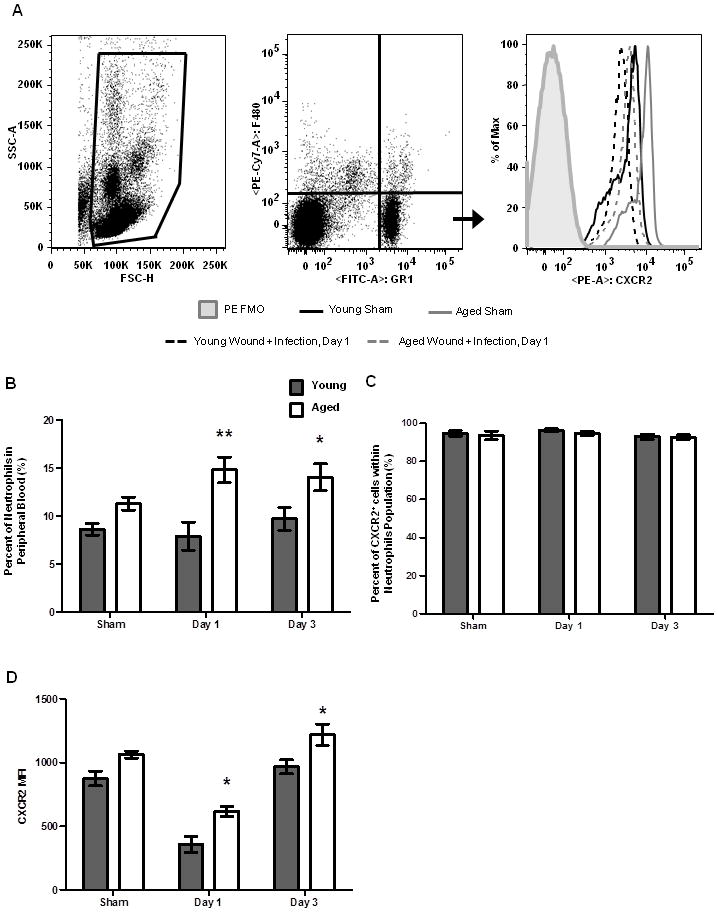
Whole blood was analyzed by flow cytometry to determine the percentage of circulating neutrophils and expression of the chemotatic receptor CXCR2. (A) Gating strategy for neutrophils in whole blood by flow cytometry. (B) Percentage of circulating neutrophils in peripheral blood in young (gray bars) and aged (white bars) mice. (C) Frequency of CXCR2+ neutrophils in peripheral blood. (D). Expression of CXCR2 on peripheral blood neutrophils. Data shown as mean ± SEM, N=9–18 individual animals per group and are cumulative of 3–4 replicate experiment. *p<0.05 and **p<0.001 verses young at same time point by two-way ANOVA.
ICAM-1 is reduced in infected wounds from aged mice
In addition to the role of KC and MIP-2 mediated neutrophil upregulation of selectins and integrin via CXCR2, secretion of these chemokines as well as pro-inflammatory cytokines like TNF-α, promote upregulation of adhesion molecules like ICAM-1 on vascular endothelial cells to promote rolling and firm adhesion of neutrophils along the vascular endothelium (63–65). Perturbation of the upregulation of adhesion molecules would impair neutrophil adherence and diapedesis into the infected wound tissue. Thus, we hypothesized that ICAM-1 may fail to be upregulated in aged mice as compared to young (Figure 10). Prior to wound infection, levels of ICAM-1 were comparable between young and aged mice. Following S. aureus wound infection at day 1, levels of ICAM-1 trend downwards in both age cohorts. Interesting, at day 3 when neutrophil and macrophage recruitment is significantly reduced in aged mice, levels of ICAM-1 are reduced in aged mice as compared to young (Figure 10, *p<0.05). These data suggest that decreased ICAM-1 in aged mice may contribute to reduced neutrophil trafficking into tissue. Moreover, elevated levels of chemokines like KC and MIP-2 in the absence of relative increases in ICAM-1 suggest that there may be a dysregulation of the signaling pathways in endothelial cells that act to upregulate ICAM-1 in response to these chemokines.
Figure 10. ICAM-1 levels in wound homogenates.
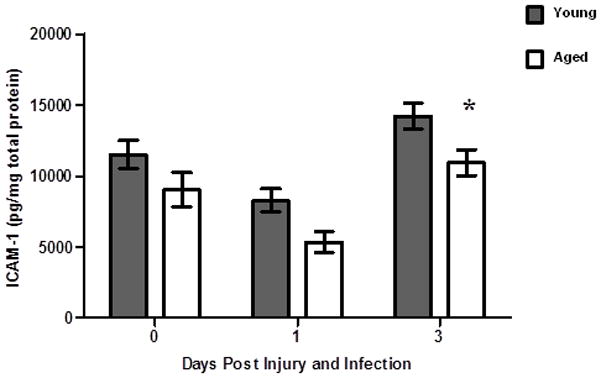
ICAM-1 levels in wounds of young (gray bars) and aged (white bars) mice present at the site of wound infection were measured by ELISA. Data are shown as mean ± SEM; N=8–12 individual animals per group. Data are cumulative of 2 replicate experiments. *p<0.05 verses young at same time point by two-way ANOVA.
Discussion
In review, we have developed a murine model of cutaneous wound infection with S. aureus which demonstrate elevated bacterial colonization and delayed wound closures in aged mice at early time points, which persists out to day 10 post wound infection. These findings parallel clinical observations in elderly patients with elevated S. aureus infection rates and prolonged, costly hospital stays (2, 25, 34). Other murine models of infection, such as pneumonia, also report an increased bacterial burden in aged animals (66). Moreover, we observed that wound closure was protracted in aged animals. Studies in murine models of uninfected wounds report similar delays in wound closure with advanced age (28, 29); however, the time to closure was reduced as compared to infected wounds from our animals.
An important factor in mediating the host response to infection is early recognition of foreign pathogens by host immune cells by pathogen-associated molecular patterns via pattern recognition receptors (PRRs) such as TLR2 (68). Loss of TLR2 or MyD88, a mediator of TLR signaling, have been associated with increased susceptibility to S. aureus infection and heightened levels of S. aureus bacteremia (40, 42, 43, 69). In our model, we observed an age-dependent decrease in the absolute number of TLR2+ resident tissue macrophages, however, no differences in expression or frequency of TLR2+ infiltrating wound neutrophils or macrophages were observed. Studies demonstrate variable reduction of TLRs macrophage expression with age (55, 66, 70), while previous studies from our own laboratory have demonstrated no change in peritoneal or splenic macrophage TLR2 and TLR4 expression (71). In circulating neutrophil populations, human studies suggest that expression of TLR2 and TLR4 are unaltered with age, though membrane-associated MyD88 was decreased with age after exogenous stimulation with LPS (11). Though not evaluated in our study, age-associated alterations in TLR2 signaling pathways, such as MyD88, may dampen TLR2 responses in aged individuals and contribute to delayed resolution of bacterial infection (72–74).
Previous studies have reported that aging negatively impacts the ability of both neutrophils and macrophages to phagocytosis pathogens (26, 27, 46, 48, 49), though few have evaluated the phagocytic potential of leukocytes following recruitment in response to tissue injury. Furthermore, reduced FcγRIII expression in circulating human neutrophils from elderly donors was correlated with impaired phagocytosis (46). Given that our observed differences in FcγRIII did not contribute to functional changes in FcγRIII-dependent phagocytosis or bactericidal activity, we did not further evaluate additional phagocytic mechanisms and pathways. However, that lack of age-related differences in neutrophil phagocytosis is in concert with other murine studies that do not recapitulate the phagocytosis deficits observed in circulating human neutrophils (55). Our study extends these findings in rodent studies, demonstrating that neutrophil phagocytosis is not impaired by advanced age following recruitment to a specific tissue microenvironment.
We next chose to examine if age-associated alterations in chemotaxis could contribute to impaired resolution of wound infection in our aged mice as previous studies highlight reduced migration of peripheral blood neutrophils isolated from healthy, elderly humans and mice (11, 29, 54, 55). Specifically, following sterile cutaneous wound injury, neutrophil peak infiltration has been shown to be attenuated in aged C57BL/6 mice as compared to young (29). This parallels studies of oral infection with Salmonella Typhimurium (54) or peritoneal challenge with Candida albicans (55), were aged mice demonstrated reduced neutrophil chemotaxis to these respective compartments. Alternatively, neutrophil trafficking to the lung after trauma or environmental insults is enhanced with age, highlighting the unique and delicate microenvironment of the aging lung (18, 78, 79).
Recently, we reported that peripheral blood neutrophils from unmanipulated young and aged mice have a basal hyperchemokinesis but lack directional migration ex vivo in response to KC (53). We extend these findings to show that at increasing doses of KC in vivo, neutrophils from aged mice had a diminished migratory response to cutaneous tissue as compared to young. These data mirrored neutrophil recruitment in response to cutaneous S. aureus wound infection in our model as elevated levels of neutrophil chemokines were required to mediate a similar chemotatic response in aged mice as compared to young. This reduction in neutrophil accumulation was associated with delayed resolution of wound infection in our aged animals. Clinically, patients with reduced neutrophil function or numbers are at a heightened risk for chronic and repeated infections with catalase-positive bacteria, like S. aureus (44, 45, 80). However, the accepted dogma of efficient wound healing implies that gratuitous neutrophil accumulation can result in excessive ROS and elastase production, exacerbating tissue damage and impeding wound healing (5, 81). In wound healing models using fetal or young mice show that a paucity of neutrophil recruitment is correlated with reduced scar formation (82, 83) and that neutrophil depletion may accelerate aseptic wound closure (84). Despite these findings, studies in the aging literature have demonstrated that even in the setting of sterile wound healing, ablation of the neutrophil population delays wound closure in aged animals (29). Importantly, increasing neutrophil numbers via i.p. administration of G-CSF enhanced rates of wound closure in aged mice to those observed in young mice (29). In the context of an infected wound, the necessity of neutrophil recruitment becomes increasingly paramount. Thus, the decreased absolute numbers of neutrophils and macrophages in our model may not only contribute to bacterial colonization differences observed with age, but may also play a role in delayed wound resolution in aged animals.
While we saw blunted chemotaxis to the CXCR2 ligand KC, we observed elevated CXCR2 expression on circulating neutrophils from aged animals, suggesting that despite adequate expression of CXCR2, mediators of CXCR2 signaling may be impaired with age. For example, recruitment of β-arrestin 2 to CXCR2 has been shown to be required for CXCR2 activation (60) and has been reported to be decreased in brain tissue of elderly humans (85). Others have documented that high levels of KC promote CXCR2 receptor desensitization and impaired chemotaxis (86). Thus, it is plausible that the early peak in KC levels observed in aged mice acts to attenuate neutrophil recruitment despite persistent infection in our aged animals. Moreover, we demonstrate that ICAM-1 expression is reduced at day 3 in aged mice as compared to young. ICAM-1 is critical in neutrophil adhesion and subsequent transmigration from the vasculature to the target tissue. Previously we have shown that following burn trauma, lung endothelial ICAM-1 is elevated and associated with prolonged neutrophilia in lungs from aged mice as compared to young (53). The differences between these studies are likely attributable to different models of injury and the unique microenvironment of the lung as discussed previously.
Given these data, it is reasonable to suggest that enhancing neutrophil recruitment in aged mice may reduce bacterial colonization and wound closure. Interestingly, G-CSF is not only an important factor in granulocyte development in the bone marrow and survival in circulation (87–89), but it also serves to enhance CXCR2 mediated chemotaxis (90, 91), improve integrin-adhesion molecule interactions (92) and upregulate ICAM-1 (93). Specifically, G-CSF has been shown to upregulate CXCR2 via STAT3, enhance signaling downstream of CXCR2 (90), restore chemotatic defects observed in aged rats (77), induce endothelial ICAM-1 expression (93) and promote neutrophil adhesion to ICAM-1 (92, 94). Together, these suggest a plausible interconnection between G-CSF, CXCR2 and ICAM-1 and elucidating these interactions may provide insight and novel therapeutic approaches to age-related impaired chemotaxis and immune cell dysfunction. However, caution should be taken when considering altering the distribution of leukocytes, in particular in the elderly, as they are highly susceptible to pneumonia.
As bacterial strains, in particular S. aureus, continually evade our current antibiotic treatments, understanding how advanced age impairs the host immune response may allow us to target immunomodulatory mechanisms that will improve outcomes following cutaneous wound injury in elderly patients. Future work examining methods to enhance leukocyte migration to the wound bed, such as local or systemic growth factors, may allow for modulation of the host immune system to enhance its response to invading pathogens following tissue injury in immunocompromised patients.
Supplementary Material
Table I.
Mean fluorescent intensity of TLR2 by cutaneous neutrophils and macrophages.
| Macrophages (F4/80+/Gr-1−/TLR2+) | Neutrophils (F4/80−/Gr-1+TLR2+) | ||||
|---|---|---|---|---|---|
| Uninjured | Day 1 | Day 3 | Day 1 | Day 3 | |
| Young | 518.7±22.2 | 678.3±48.1 | 603.0±17.0 | 612.1±39.1 | 451.6±15.9 |
| Aged | 586.3± 42.0 | 627.5±42.3 | 642.2±41.5 | 576.0±30.5 | 441.6±13.2 |
No difference was observed in the MFI of TLR2 in macrophages isolated from uninjured skin, or in the MFI of TLR2 in neutrophils or macrophages isolated from infected wounds. No significant differences between young and aged at each time point were observed. Data are shown as mean ± SEM; N=7–11 per group.
Acknowledgments
The authors would like to thank Anita Zahs, Shegufta Mahbub, Jessica L. Palmer, Stewart R. Carter, Sara Hlavin, and Patricia Simms for their technical assistance and thoughtful critique of this manuscript. The authors would also like to thank Pamela L. Witte, PhD as head of the Immunology and Aging Program at Loyola University Medical Center and for her support on the Institutional training grant.
Footnotes
Funding
This work was supported by grants from the NIH/NIA R01 AG018859 (EJK), T32 AG031780 (PLW), the Ralph and Marian C. Falk Medical Research Trust (EJK) and the Loyola University Chicago Stritch School of Medicine MD/PhD Program.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye KS, Anderson DJ, Sloane R, Chen LF, Choi Y, Link K, Sexton DJ, Schmader KE. The effect of surgical site infection on older operative patients. J Am Geriatr Soc. 2009;57:46–54. doi: 10.1111/j.1532-5415.2008.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. J Tissue Viability. 2009;18:13–19. doi: 10.1016/j.jtv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–6. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker AL, Schneider DF, Kovacs EJ. Neutrophils and natural killer T cells as negative regulators of wound healing. Expert Rev Dermatol. 2011;6:5–8. doi: 10.1586/edm.10.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brubaker AL, Palmer JL, Kovacs EJ. Age-related Dysregulation of Inflammation and Innate Immunity: Lessons Learned from Rodent Models. Aging Dis. 2011;2:346–360. [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 9.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahbub S, Brubaker AL, Kovacs EJ. Aging of the Innate Immune System: An Update. Curr Immunol Rev. 2011;7:104–115. doi: 10.2174/157339511794474181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O, Khalil A, Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–26. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 12.Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol. 2011;186:3441–3451. doi: 10.4049/jimmunol.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira LF, Duarte de Souza AP, Borges TJ, Bonorino C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: Decreased stimulation by aged dendritic cells. Mech Ageing Dev. 2011;132:187–194. doi: 10.1016/j.mad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 2011;74:2313–23. doi: 10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E, Franceschi C. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:3161–71. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- 16.Gomez CR, Nomellini V, Baila H, Oshima K, Kovacs EJ. Comparison of the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury: scald injury versus I.p. LPS administration. Shock. 2009;31:178–84. doi: 10.1097/SHK.0b013e318180feb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomellini V, Gomez CR, Gamelli RL, Kovacs EJ. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2009;31:11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol. 2008;83:1493–501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental Effects of Aging on Outcome from Traumatic Brain Injury: A Behavioral, Magnetic Resonance Imaging, and Histological Study in Mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- 20.Sharma OP, Oswanski MF, Sharma V, Stringfellow K, Raj SS. An appraisal of trauma in the elderly. Am Surg. 2007;73:354–8. [PubMed] [Google Scholar]
- 21.Kim MH, Curry FR, Simon SI. Dynamics of neutrophil extravasation and vascular permeability are uncoupled during aseptic cutaneous wounding. Am J Physiol Cell Physiol. 2009;296:C848–56. doi: 10.1152/ajpcell.00520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radek KA, Baer LA, Eckhardt J, DiPietro LA, Wade CE. Mechanical unloading impairs keratinocyte migration and angiogenesis during cutaneous wound healing. J Appl Physiol. 2008;104:1295–1303. doi: 10.1152/japplphysiol.00977.2007. [DOI] [PubMed] [Google Scholar]
- 24.Roy S, Khanna S, Rink C, Biswas S, Sen CK. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics. 2008;34:162–184. doi: 10.1152/physiolgenomics.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Butcher SK, Killampalli V, Chahal H, Kaya Alpar E, Lord JM. Effect of age on susceptibility to post-traumatic infection in the elderly. Biochem Soc Trans. 2003;31:449–51. doi: 10.1042/bst0310449. [DOI] [PubMed] [Google Scholar]
- 27.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–35. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 28.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479–87. [PubMed] [Google Scholar]
- 29.Nishio N, Okawa Y, Sakurai H, Isobe K. Neutrophil depletion delays wound repair in aged mice. Age (Dordr) 2008;30:11–19. doi: 10.1007/s11357-007-9043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51:1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- 31.Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, Liu FT, Isseroff RR, Simon SI. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008;128:1812–1820. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daley JM, Reichner JS, Mahoney EJ, Manfield L, Henry WL, Jr, Mastrofrancesco B, Albina JE. Modulation of macrophage phenotype by soluble product(s) released from neutrophils. J Immunol. 2005;174:2265–2272. doi: 10.4049/jimmunol.174.4.2265. [DOI] [PubMed] [Google Scholar]
- 33.Saadatian-Elahi M, Teyssou R, Vanhems P. Staphylococcus aureus, the major pathogen in orthopaedic and cardiac surgical site infections: a literature review. Int J Surg. 2008;6:238–245. doi: 10.1016/j.ijsu.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 35.Schneider DF, Palmer JL, Tulley JM, Kovacs EJ, Gamelli RL, Faunce DE. Prevention of NKT Cell Activation Accelerates Cutaneous Wound Closure and Alters Local Inflammatory Signals. J Surg Res. 2010;171:361–73. doi: 10.1016/j.jss.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rico RM, Ripamonti R, Burns AL, Gamelli RL, DiPietro LA. The effect of sepsis on wound healing. J Surg Res. 2002;102:193–7. doi: 10.1006/jsre.2001.6316. [DOI] [PubMed] [Google Scholar]
- 37.Brubaker AL, Schneider DF, Palmer JL, Faunce DE, Kovacs EJ. An improved cell isolation method for flow cytometric and functional analyses of cutaneous wound leukocytes. J Immunol Methods. 2011;373:161–166. doi: 10.1016/j.jim.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karavitis J, Murdoch EL, Deburghgraeve C, Ramirez L, Kovacs EJ. Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcgammaR-mediated phagocytosis. Cell Immunol. 2012;274:61–71. doi: 10.1016/j.cellimm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bird MD, Morgan MO, Ramirez L, Yong S, Kovacs EJ. Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice. J Burn Care Res. 2010;31:652–660. doi: 10.1097/BCR.0b013e3181e4c58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 Mediates Neutrophil Recruitment Initiated by IL-1R but Not TLR2 Activation in Immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 43.Jann NJ, Schmaler M, Ferracin F, Landmann R. TLR2 enhances NADPH oxidase activity and killing of Staphylococcus aureus by PMN. Immunol Lett. 2010;135:17–23. doi: 10.1016/j.imlet.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Harrison CJ. Innate immunity as a key element in host defense against methicillin resistant Staphylococcus aureus. Minerva Pediatr. 2009;61:503–514. [PubMed] [Google Scholar]
- 45.Guide SV, Stock F, Gill VJ, Anderson VL, Malech HL, Gallin JI, Holland SM. Reinfection, rather than persistent infection, in patients with chronic granulomatous disease. J Infect Dis. 2003;187:845–853. doi: 10.1086/368388. [DOI] [PubMed] [Google Scholar]
- 46.Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, O’Mahony D, Lord JM. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70:881–6. [PubMed] [Google Scholar]
- 47.Anderson KE, Chessa TA, Davidson K, Henderson RB, Walker S, Tolmachova T, Grys K, Rausch O, Seabra M, Tybulewicz VL, Stephens LR, Hawkins PT. PtdIns3P and Rac direct the assembly of the NADPH oxidase on a novel, pre-phagosomal compartment during FcR-mediated phagocytosis in primary mouse neutrophils. Blood. 2010;116:4978–4989. doi: 10.1182/blood-2010-03-275602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de la Fuente M, Hernanz A, Guayerbas N, Alvarez P, Alvarado C. Changes with age in peritoneal macrophage functions. Implication of leukocytes in the oxidative stress of senescence. Cell Mol Biol (Noisy-Le-Grand) 2004;50 Online Pub: OL683–90. [PubMed] [Google Scholar]
- 49.Guayerbas N, Puerto M, Alvarez P, de la Fuente M. Improvement of the macrophage functions in prematurely ageing mice by a diet supplemented with thiolic antioxidants. Cell Mol Biol (Noisy-Le-Grand) 2004;50 Online Pub: OL677–81. [PubMed] [Google Scholar]
- 50.Izgut-Uysal VN, Agac A, Karadogan I, Derin N. Peritoneal macrophages function modulation by L-carnitine in aging rats. Aging Clin Exp Res. 2004;16:337–41. doi: 10.1007/BF03324561. [DOI] [PubMed] [Google Scholar]
- 51.Ming W, Li S, Billadeau DD, Quilliam LA, Dinauer MC. The Rac effector p67phox regulates phagocyte NADPH oxidase by stimulating Vav1 guanine nucleotide exchange activity. Mol Cell Biol. 2007;27:312–323. doi: 10.1128/MCB.00985-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong CW, Kim TK, Ham HY, Nam JS, Kim YH, Zheng H, Pang B, Min TK, Jung JS, Lee SN, Cho HJ, Kim EJ, Hong IH, Kang TC, Lee J, Oh SB, Jung SJ, Kim SJ, Song DK. Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine. GlyR alpha 2/TRPM2/p38 MAPK signaling. J Immunol. 2010;184:4401–4413. doi: 10.4049/jimmunol.0902814. [DOI] [PubMed] [Google Scholar]
- 53.Nomellini V, Brubaker AL, Mahbub S, Palmer JL, Gomez CR, Kovacs EJ. Dysregulation of Neutrophil CXCR2 and Pulmonary Endothelial ICAM-1 Promotes Age-Related Pulmonary Inflammation. Aging and Disease. 2012;3:234–47. [PMC free article] [PubMed] [Google Scholar]
- 54.Ren Z, Gay R, Thomas A, Pae M, Wu D, Logsdon L, Mecsas J, Meydani SN. Effect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 mice. J Med Microbiol. 2009;58:1559–1567. doi: 10.1099/jmm.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murciano C, Yanez A, O’Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol Med Microbiol. 2008;53:214–21. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 56.Dipietro LA, Reintjes MG, Low QE, Levi B, Gamelli RL. Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound Repair Regen. 2001;9:28–33. doi: 10.1046/j.1524-475x.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 57.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 58.Hillyer P, Mordelet E, Flynn G, Male D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin Exp Immunol. 2003;134:431–41. doi: 10.1111/j.1365-2249.2003.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J Exp Med. 2004;200:935–9. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molteni R, Crespo CL, Feigelson S, Moser C, Fabbri M, Grabovsky V, Krombach F, Laudanna C, Alon R, Pardi R. {beta}-Arrestin 2 is required for the induction and strengthening of integrin-mediated leukocyte adhesion during CXCR2-driven extravasation. Blood. 2009;114:1073–1082. doi: 10.1182/blood-2008-10-183699. [DOI] [PubMed] [Google Scholar]
- 61.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Bruyn KMT, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. The Small GTPase Rap1 Is Required for Mn2+- and Antibody-induced LFA-1- and VLA-4-mediated Cell Adhesion. Journal of Biological Chemistry. 2002;277:29468–29476. doi: 10.1074/jbc.M204990200. [DOI] [PubMed] [Google Scholar]
- 63.Basit A, Reutershan J, Morris MA, Solga M, Rose CE, Jr, Ley K. ICAM- 1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am J Physiol Lung Cell Mol Physiol. 2006;291:L200–7. doi: 10.1152/ajplung.00346.2005. [DOI] [PubMed] [Google Scholar]
- 64.Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16:31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 66.Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ashcroft GS, Horan MA, Ferguson MW. Aging is associated with reduced deposition of specific extracellular matrix components, an upregulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Dermatol. 1997;108:430–7. doi: 10.1111/1523-1747.ep12289705. [DOI] [PubMed] [Google Scholar]
- 68.Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35:555–562. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- 69.Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, Hoebe K, Du X, Rutschmann S, Jiang Z, Bigby T, Nizet V, Zouboulis CC, Beutler B. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun. 2005;73:4512–21. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 71.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–13. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 74.Li Y, Howell EA, Lagoo AS, Kuchibhatla M, Pan H, Cohen HJ, Lagoo SA. Differential gene expression of interleukin-1 receptor associated kinase-1 and interleukin-1 receptor associated kinase-M in peripheral blood mononuclear cells of young and aged rats following preconditioning with endotoxin. Shock. 2009;31:55–63. doi: 10.1097/SHK.0b013e3181778ab2. [DOI] [PubMed] [Google Scholar]
- 75.Fossati G, Moots RJ, Bucknall RC, Edwards SW. Differential role of neutrophil Fcgamma receptor IIIB (CD16) in phagocytosis, bacterial killing, and responses to immune complexes. Arthritis Rheum. 2002;46:1351–1361. doi: 10.1002/art.10230. [DOI] [PubMed] [Google Scholar]
- 76.Syam S, Mero P, Pham T, McIntosh CA, Bruhns P, Booth JW. Differential recruitment of activating and inhibitory Fc gamma RII during phagocytosis. J Immunol. 2010;184:2966–2973. doi: 10.4049/jimmunol.0900016. [DOI] [PubMed] [Google Scholar]
- 77.Yoshino T, Tamura M, Kawabe M, Nomura H, Imai N, Ono M. Effects of recombinant human granulocyte colony-stimulating factor on neutrophil functions in aged animals. Br J Haematol. 1992;82:664–670. doi: 10.1111/j.1365-2141.1992.tb06941.x. [DOI] [PubMed] [Google Scholar]
- 78.Moriyama C, Betsuyaku T, Ito Y, Hamamura I, Hata J, Takahashi H, Nasuhara Y, Nishimura M. Aging enhances susceptibility to cigarette smoke-induced inflammation through bronchiolar chemokines. Am J Respir Cell Mol Biol. 2010;42:304–311. doi: 10.1165/rcmb.2009-0025OC. [DOI] [PubMed] [Google Scholar]
- 79.Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–51. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 80.Lanoix JP, Pluquet E, Lescure FX, Bentayeb H, Lecuyer E, Boutemy M, Dumont P, Jounieaux V, Schmit JL, Dayen C, Douadi Y. Bacterial infection profiles in lung cancer patients with febrile neutropenia. BMC Infect Dis. 2011;11:183. doi: 10.1186/1471-2334-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost. 2004;92:275–80. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 82.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Satish L, Kathju S. Cellular and Molecular Characteristics of Scarless versus Fibrotic Wound Healing. Dermatol Res Pract. 2010;2010:790234. doi: 10.1155/2010/790234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–55. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 85.Grange-Midroit M, Garcia-Sevilla JA, Ferrer-Alcon M, La Harpe R, Walzer C, Guimon J. G protein-coupled receptor kinases, beta-arrestin-2 and associated regulatory proteins in the human brain: postmortem changes, effect of age and subcellular distribution. Brain Res Mol Brain Res. 2002;101:39–51. doi: 10.1016/s0169-328x(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 86.Wiekowski MT, Chen SC, Zalamea P, Wilburn BP, Kinsley DJ, Sharif WW, Jensen KK, Hedrick JA, Manfra D, Lira SA. Disruption of neutrophil migration in a conditional transgenic model: evidence for CXCR2 desensitization in vivo. J Immunol. 2001;167:7102–7110. doi: 10.4049/jimmunol.167.12.7102. [DOI] [PubMed] [Google Scholar]
- 87.Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23:33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 88.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–61. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 89.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen-Jackson H, Panopoulos AD, Zhang H, Li HS, Watowich SS. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood. 2010;115:3354–63. doi: 10.1182/blood-2009-08-240317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolach B, van der Laan LJ, Maianski NA, Tool AT, van Bruggen R, Roos D, Kuijpers TW. Growth factors G-CSF and GM-CSF differentially preserve chemotaxis of neutrophils aging in vitro. Exp Hematol. 2007;35:541–550. doi: 10.1016/j.exphem.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 92.Hakansson L, Hoglund M, Jonsson UB, Torsteinsdottir I, Xu X, Venge P. Effects of in vivo administration of G-CSF on neutrophil and eosinophil adhesion. Br J Haematol. 1997;98:603–611. doi: 10.1046/j.1365-2141.1997.2723093.x. [DOI] [PubMed] [Google Scholar]
- 93.Fuste B, Mazzara R, Escolar G, Merino A, Ordinas A, Diaz-Ricart M. Granulocyte colony-stimulating factor increases expression of adhesion receptors on endothelial cells through activation of p38 MAPK. Haematologica. 2004;89:578–585. [PubMed] [Google Scholar]
- 94.Chakraborty A, Hentzen ER, Seo SM, Smith CW. Granulocyte colony-stimulating factor promotes adhesion of neutrophils. Am J Physiol Cell Physiol. 2003;284:C103–10. doi: 10.1152/ajpcell.00165.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


