Abstract
OBJECTIVE
Examine whether long and short-term sunlight radiation is related to stroke incidence.
METHODS
Fifteen-year residential histories merged with satellite, ground monitor, and model reanalysis data were used to determine sunlight radiation (insolation) and temperature exposure for a cohort of 16,606 stroke and coronary artery disease free black and white participants aged 45+ from the 48 contiguous United States. Fifteen, ten, five, two and one-year exposures were used to predict stroke incidence during follow-up in Cox proportional hazard models. Potential confounders and mediators were included during model-building.
RESULTS
Shorter exposure periods exhibited similar, but slightly stronger relationships than longer exposure periods. After adjustment for other covariates, the previous year’s monthly average insolation exposure below the median gave an HR=1.61 (95% CI: 1.15, 2.26) and the previous year’s highest compared to the second highest quartile of monthly average maximum temperature exposure gave an HR=1.92 (1.27, 2.92).
INTERPRETATION
These results indicate a relationship between lower levels of sunlight radiation and higher stroke incidence. The biological pathway of this relationship is not clear. Future research will show whether this finding stands, the pathway for this relationship, and if it is due to short or long-term exposures.
INTRODUCTION
Cardiovascular health varies with season, weather, and climate.1–4 While temperature variation has been a primary target of investigation, sunlight also varies seasonally and has not been adequately investigated. Sunlight directly alters vitamin D status, but aside from skin cancer there are little data on how sunlight affects human health.5–7 When exposed to ultraviolet B radiation, the skin produces vitamin D.8 Although there are few studies of vitamin D and stroke, there is indication that vitamin D insufficiency may increase vascular event risk factors.5–9 Both northern geographic latitude and lower vitamin D levels have been linked to higher blood pressure and other adverse cardiovascular risks, with potential mechanisms involving the renin-angiotensin system, inflammation, vasculature, or glycemic control.10 Exposure to ultraviolet B radiation has also been shown to be beneficial for blood pressure and other stroke risk factors.8, 10, 11 Current research and recommendations on controllable vitamin D-related risk factors and outcomes almost entirely focus on dietary and supplemental intake. However, since the primary determinant of vitamin D blood levels is sunlight exposure, research is also needed to determine if sunlight exposure is related to the same outcomes.7, 8, 12
We sought to test the hypothesis of whether higher levels of sunlight radiation are associated with lower stroke incidence. To test this hypothesis, we used measures derived from ground, satellite, and model reanalysis data and merged them with the most recent 15-year residential histories of participants in a national longitudinal cohort.
METHODS
Study participants
The REasons for Geographic and Racial Differences in Stroke (REGARDS) is a longitudinal study of United States (US) participants aged 45+ years with oversampling of African Americans and those residing in the Southeast.13 At baseline, 35% of the participants were residing in the stroke belt (a southeastern area of the US with high stroke mortality) and an additional 21% of the participants were sampled from the stroke buckle (an even higher stroke mortality region within the stroke belt). The remaining 44% were from the rest of the nation. The cohort population at baseline was 42% African-American/58% white and 45% male/55% female. Further details on the study are available elsewhere.13 The REGARDS study and the current analysis were approved by the Institutional Review Boards of participating institutions.
Data collection
At baseline, the participant’s self-reported demographic and behavioral factors, and medical history were determined from telephone interviews. Validated interview instruments were used to collect depressive symptoms (the Center for Epidemiological Studies-Depression Scale-4 item version), cognitive status (Six-Item Screener derived from the Mini-Mental State Examination), and physical function (Physical Component Summary score from the SF-12 Health Survey).14–17 Shortly following the telephone call, trained professionals collected blood pressure, height, weight, venipuncture, urine, and conducted an electrocardiogram (ECG) during home visits. Blood and urine were sent to a central repository at the University of Vermont and ECG data were read at Wake Forest University. The examiner left a “Places You Have Lived” questionnaire and the Block98 food frequency questionnaire (NutritionQuest™, Berkeley, CA).
Stroke ascertainment
Between the first in-home visit and last follow-up date prior to this analysis (February 2003 to April 2011), participants were interviewed by telephone every 6 months to determine hospitalization events for potential stroke. A proxy answered questions if the participant was unable to respond. Stroke events were ascertained after examining medical records following report of suspected stroke, transient ischemic attack (TIA), or stroke symptoms. Stroke verification and subtype classification (ischemic or hemorrhagic) were performed by a stroke adjudication committee consisting primarily of stroke neurologists.
Death ascertainment
A death was reported by a proxy when participants were contacted every 6 months or when the proxy called the REGARDS toll-free number. Deaths were also obtained using the Social Security Death Index and restricted-access search engines, such as Lexis-Nexis.
Assessment of sunlight exposure and temperature
We used data from the National Aeronautics and Space Administration (NASA) product of the North American Land Data Assimilation System Phase 2 (NLDAS-2) dataset to determine sunlight radiation and temperature. The NLDAS-2 is based on model reanalysis data as well as remotely-sensed and ground observations, and consists of a grid surface with ~14 km resolution over North America.18 NLDAS-2 solar radiation that was assessed at one-hour intervals was used to calculate a daily integral referred to herein as daily “insolation”. For this study, we merged daily insolation and maximum air temperatures with data from REGARDS’ residential history form, which consists of locations where the participant had lived prior to enrollment into REGARDS, along with age when relocating. Each location the participant recorded was matched to a feature in the US Geological Survey’s Geographic Names Information System using ArcGIS 9.3. We assumed participants moved during July of the indicated moving year. For participants who had a period of missing residential data due to having an unidentifiable location or residence outside of the contiguous 48 United States, we used only the existing residential history to compute environmental exposure averages.
Because our study explored novel environmental measures, there was a need to determine what exposure periods carry important health relationships. Previous studies involving pollution and cardiovascular outcomes have found that 15 years should be an adequate latent period to find a relationship between pollution and cardiopulmonary mortality so this was our main exposure duration.4, 19–21
We calculated each month’s average of daily insolation exposure at each location and determined each participant’s 15-year average exposure, as well as the average exposure at shorter intervals (1, 2, 5, and 10 years) based on these monthly averages. Since our hypothesis was that lower levels of sunlight radiation may have an adverse effect on stroke outcome, the highest quartile of insolation exposure was used as a reference. Similar to insolation exposure methods, monthly averages of the daily temperatures were calculated and used for shorter and longer-term exposures. Because both hot and cold temperatures have been shown to have adverse relationships with stroke, we did not a priori select a temperature quartile to use a reference.22, 23
Statistical Methods
Statistical analyses were performed using SAS version 9.2 (SAS Corporation, Cary, North Carolina). We used Cox proportional hazards models to assess the relationships between environmental variables and first incident stroke. Participants’ data were censored at last available contact or death. Eight percent of the cohort was lost to follow- up. We considered models with all stroke and only ischemic stroke separately. Of the 30,239 participants enrolled at baseline, participants were excluded from the cohort due to data anomalies (n=58), reported stroke or coronary artery disease (CAD) prior to the baseline telephone interview or stroke in between the baseline telephone and in-home interview (n=7,027), or missing residential history (n=3,070). Since many confounders had large numbers of participants missing data, we attempted to minimize selection bias by creating a separate “missing” category for any variable that had more than 1,000 participants missing data. After this, another 3,480 still had missing confounder data. Of the 16,606 participants remaining, 351 participants had at least one stroke: 309 ischemic, 32 hemorrhagic, and 10 unknown.
We first evaluated different climate exposure time periods in models including both a measure of insolation and temperature for each time period (15, 10, 5, 2, or 1-year previous to in-home visit). We then chose the model with the lowest Akaike Information Criterion (AIC), indicating the best model fit, for further model-building.
Covariate adjustment
We built sequential models to examine covariate effects on the main association. We first considered the potential confounders at baseline: age in years, race, region, gender, rurality (based on the US Department of Agriculture’s rural-urban commuting area codes from 2000), education, income, neighborhood poverty, vitamin D intake, exercise, TV/computer time, medication use (aspirin, statins or anti-hypertensives), alcohol, smoking, depressive symptoms (≥4 points out of 12), the Physical Component Summary score, and body mass index. We then also added established cerebrovascular risk factors available in REGARDS as potential mediators of the relationship between sunlight exposure and stroke events: systolic blood pressure, cholesterol, diabetes, atrial fibrillation, previous transient ischemic attack, and presence of stroke symptoms without self-reported stroke at baseline.
Covariate distribution differences by insolation exposure were tested using likelihood ratio chi-square tests and t-tests. Cox regression models results were reported using hazard ratios (HRs) accompanied by 95% profile likelihood confidence intervals and type III chi-square tests to determine p-values. During Cox regression modeling, we used log-log survival plots to determine if hazards were proportional. Statistical significance was assessed at α=0.05. These exploratory analyses were not adjusted for the multiple comparisons that were made.
RESULTS
Climatic exposure variable selection
Analyses of different environmental exposure time periods indicated that when together in a multivariable model, insolation and temperature exposures for the year previous to each participant’s in-home interview carried the lowest AIC. During model building, it was determined that insolation exhibited a threshold effect. In order to obtain more precise confidence intervals, the quartiles were collapsed and insolation exposure was modeled as a being above or below the median.
Descriptives
Among the potential demographic confounders only race was not associated with insolation (Supplemental Table 1). Covariate proportion differences between insolation categories were rather small (under 10%) with the exception of region: those below the median insolation were more likely to be in the non-belt region (57% vs. 32%), less likely to be in the stroke buckle region (10% vs. 33%), but nearly equally likely to be in the belt/non-buckle region (33% vs. 35%). Similarly, temperatures varied significantly by numerous covariates, but only variation by region of residence was particularly large (Supplemental Table 2).
Cox regression models
The Pearson correlation statistic indicated that insolation and temperature measures were highly correlated (r=0.70, p<0.0001). Pearson’s residuals from a model containing insolation to predict time to stroke were included in a separate model containing maximum temperature to predict time to stroke. Both temperature (p<0.0001) and the Pearson’s residuals (p=0.0011) were significant, suggesting that insolation and temperature had independent relationships with stroke incidence. Log-log survival plots indicated time to stroke curves were not proportional by region, so region was controlled through stratification. Also, since age exhibited a curvilinear relationship with stroke, both age and age-squared were included.
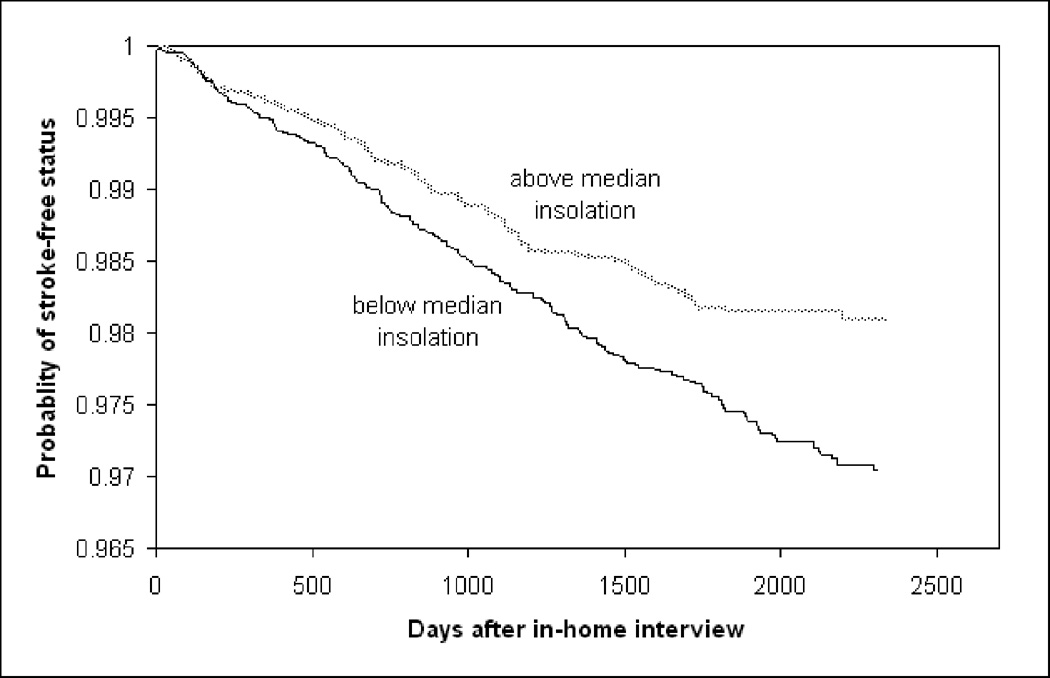
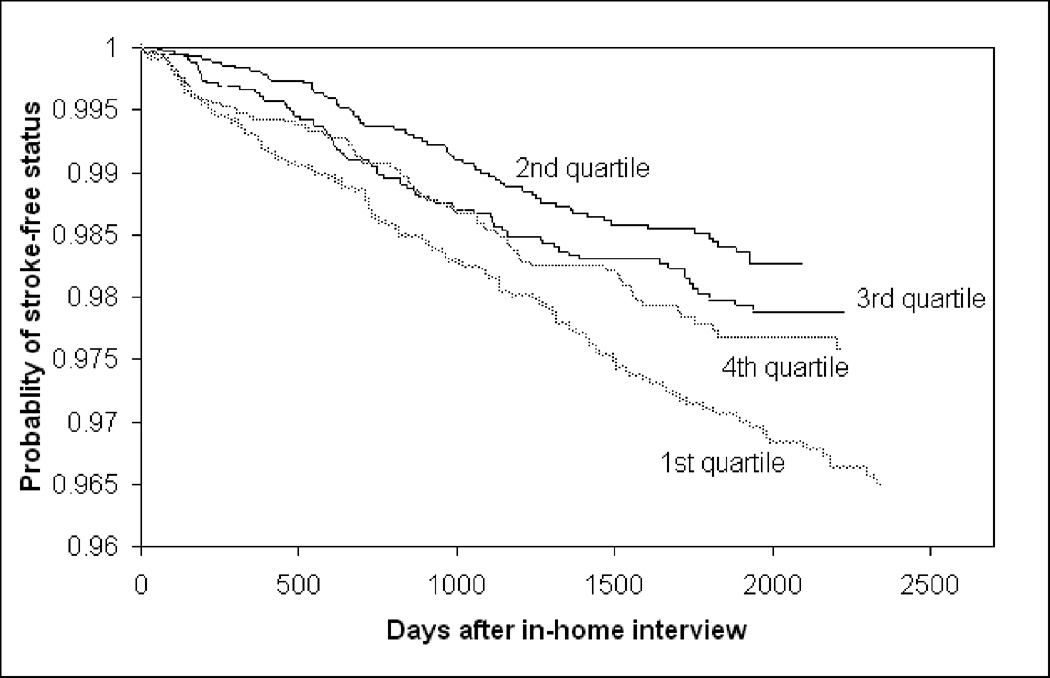
The Kaplan-Meier stroke event curves in Figures 1 and 2 for insolation and temperature show that low insolation exposures, and both high and low temperatures, are associated with higher stroke rates. Time to ischemic stroke as a separate outcome is not shown because the relationships were very similar. Reduced insolation (less sunlight radiation) was associated with increased stroke incidence (Table 1) (HR=1.44; 95% CI: 1.16, 1.79). This association strengthened when temperature was added to the model (HR=1.73; 95% CI: 1.25, 2.41). The addition of the potential confounders indicated in covariate adjustment section of the statistical methods slightly attenuated the HR point estimates, but relationships were generally the same. The addition of the potential mediators indicated in the statistical methods section did not meaningfully affect the results (Table 1), therefore we did not pursue further mediation analyses.
Figure 1.
Survival Curves for Insolation and Time to Stroke
Figure 2.
Survival Curves for Maximum Temperature Quartile and Time to Stroke
Table 1.
Hazards ratios and 95% confidence intervals for associations between insolation and maximum temperature averages for the year previous to in-home interview with stroke incidence, 351 stroke events (n=16,606)
| Model | Below median | Quartile of Maximum Temperature | |||
|---|---|---|---|---|---|
| Insolation (n=8275) |
1st (n=4142) |
2nd (n=4212) |
3rd (n=4210) |
4th (n=4042) |
|
| Insolation Only* | 1.44 (1.16, 1.79) | -- | -- | -- | -- |
| Temperature Only* | -- | 1.86 (1.39, 2.52) | Ref | 1.22 (0.88, 1.69) | 1.36 (0.99, 1.89) |
| Insolation and Temperature† | 1.73 (1.25, 2.41) | 1.68 (1.25, 2.29) | Ref | 1.51 (1.07, 2.14) | 2.05 (1.36, 3.11) |
| Adjusted Model‡ | 1.59 (1.14, 2.23) | 1.49 (1.05, 2.14) | Ref | 1.73 (1.19, 2.51) | 1.95 (1.29, 2.96) |
| Mediation Model§ | 1.61 (1.15, 2.26) | 1.42 (1.00, 2.04) | Ref | 1.70 (1.17, 2.46) | 1.92 (1.27, 2.92) |
Bold values indicated variables with chi-square p-values<0.05
Insolation Only and Temperature Only models each contain only a measure of insolation or temperature with stroke of any subtype as outcome
Insolation and Temperature model contains both measures of insolation or temperature with stroke of any subtype as outcome
Adjusted Model adds age, race, gender, rurality, education, income, neighborhood poverty, vitamin D intake, exercise, TV/computer time, medication use (aspirin, statins or anti-hypertensives), alcohol, smoking, depressive symptoms, Physical Component Summary score, and body mass index to the Insolation and Temperature Model, and controls for region through stratification
Mediation Model adds systolic blood pressure, cholesterol, diabetes, atrial fibrillation, previous transient ischemic attack, and presence of stroke symptoms without self-reported stroke at baseline to the Adjusted Model
DISCUSSION
Stroke exhibits seasonal characteristics, with increased rates in the winter and decreased rates in the summer.1, 4 Various climatic conditions could contribute to this cerebrovascular risk pattern, but only temperature has been extensively investigated.2, 19, 23, 24 When exposed to UVB radiation, the skin produces vitamin D3, which the liver then converts to 25(OH)D. Vitamin D supplements and fortifications often consist of vitamin D2, which is also converted to 25(OH)D. The kidney is the primary site to convert 25(OH)D to its more active form, 1,25(OH)2D. Blood serum levels of 25(OH)D are usually used to determine vitamin D status, thus can be affected by both cutaneous and ingested vitamin D sources.25 Vitamin D status, which until recently was thought to only affect bone metabolism, is increasingly found to be related to various chronic diseases, and there is indication that vitamin D insufficiency may increase vascular event risk.26–29
Our study found the seemingly contradictory result that higher insolation levels were related to lower stroke incidence, which would seem to contradict that there are higher sunlight levels found in the southeastern stroke belt13. However, our study agreed with previous finding that extreme temperatures, and in particular high temperatures, such as those in the southeast, are also associated with higher stroke risk.30, 31 It has been posited that poor autonomic and endothelial function, haemoconcentration, increased levels of cholesterol and fibrinogen and blood viscosity, and hormonal variation contribute to the relationship between temperature and stroke, but the mechanism remains unclear.4, 24 This raises the possibility that the relationship between insolation and stroke may be masked by other meteorological (temperature, air pollution) and non-meteorological factors (demographic variables, access to healthcare). Our results indicate that lower sunlight exposure does not likely affect the risk of stroke through factors that generally do not fluctuate within shorter periods of time, such as diabetes or history of TIA. However, because we did not have repeated measures of our acutely time-varying factors (such as blood pressure) we could not adequately examine these as possible mediating factors. In addition, our inclusion of single measurements of time-varying risk factors might introduce bias into our results, although this is unlikely since the inclusion of these factors did not meaningfully alter the relationships between meteorological factors and stroke risk.
Our study found that time periods with more recent exposures have stronger relationships than exposures including time periods further in the past, suggesting that possible effects of sunlight and temperature on stroke are due to shorter periods (days to months) preceding stroke, rather than long-term exposures (multiple years). However, short and longer-term exposures are highly correlated, so it is still unclear whether high and low temperatures are associated with increased stroke risk due to the acute increases in extreme temperatures modeled in previous studies, or due to the higher yearly average temperatures we modeled in our study.19, 32, 33 Another explanation for stronger short-term associations could be that residential histories more distant in time are subject to less accurate participant recall. Sensitivity analyses, determining relationship differences between participants who had left or never left their current states of residence for the previous 15 years, showed that relationships among the two groups were similar, but slightly stronger among those who had never left (Appendix 1). However, this small difference could also be from regional interactions due to state size differences.
We included a large number of covariates in our analyses to ensure that this newly discovered relationship was not due to confounding. Over-fitting does not appear to be a major concern, since the addition of the covariates did not greatly attenuate the relationship. We performed sensitivity analyses which indicated that selection bias due to exclusions may be present, but found this did not likely account for the entire association (Appendix 1) (Supplemental Table 3, 4). These sensitivity analyses also show that the exclusion of cognitively impaired participants does not alter the results (Appendix 1) (Supplemental Table 4).
This novel use of NASA data in public health allows us to differentiate long-term environmental differences between participants residing in any US location. In previous studies, meteorological differences have often been shown simply by comparison of latitudes or by proxy variables such as air pressure.5, 34, 35 Although our exposure measures provide improved spatial and temporal resolutions, and directly measure individual participants’ sunlight availability, exposure measures were not directly measured via personal monitor for each participant; therefore exposure misclassification still exists as a possible source of bias. This could happen if during the time period of an exposure measurement a participant spent a large amount of time in a climate different from that indicated by the outdoor exposures linked to his or her residence. In addition to exposure misclassification, it is possible that our findings are confounded by spatial autocorrelation, although that is not likely since adding region to the model did not attenuate the relationship. Another potential limitation is that there may be confounders for which we have not accounted, such as air pollution. While we did not account for variables such as cloudiness and altitude, insolation measures represent the sunlight energy received on the ground, so the effects of such variables are taken into account.
Given ultraviolet light’s relationship with skin cancer, current public health messages often recommend simply minimizing sun exposure.36 If future studies confirm that sunlight is protective against cerebrovascular risk, it can be added to other benefits of moderate sun exposure, including possible benefits towards non-skin cancers, bone health, arthritis, mood, and cognitive function. These findings may indicate that public health messages include a positive role for sunlight, while still outlining the negative impacts of excessive exposures.10, 34, 37–40 Future studies regarding the effects of differing long-term and acute sunlight and temperature exposures on various risk factors conceptualized in terms of both disease and reactivity are needed to explore these relationships.
Supplementary Material
ACKNOLWEDGEMENTS
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
The NLDAS hourly data used in this study were acquired as part of the mission of NASA's Earth Science Division and archived and distributed by the Goddard Earth Sciences (GES) Data and Information Services Center (DISC).
This research project is supported by a cooperative agreement U01 NS041588 from the NINDS. Additional funding, data, data processing, and consultation were provided by an investigator-initiated grant from NASA (grant# NNX09AV81G).
REFERENCES
- 1.Wang Y, Levi CR, Attia JR, D'Este CA, Spratt N, Fisher J. Seasonal variation in stroke in the Hunter Region, Australia: a 5-year hospital-based study, 1995–2000. Stroke. 2003 May;34(5):1144–1150. doi: 10.1161/01.STR.0000067703.71251.B6. [DOI] [PubMed] [Google Scholar]
- 2.Alperovitch A, Lacombe JM, Hanon O, et al. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals: the Three-City study. Arch Intern Med. 2009 Jan 12;169(1):75–80. doi: 10.1001/archinternmed.2008.512. [DOI] [PubMed] [Google Scholar]
- 3.Barnett AG. Temperature and cardiovascular deaths in the US elderly: changes over time. Epidemiology. 2007 May;18(3):369–372. doi: 10.1097/01.ede.0000257515.34445.a0. [DOI] [PubMed] [Google Scholar]
- 4.Sobel E, Zhang ZX, Alter M, et al. Stroke in the Lehigh Valley: seasonal variation in incidence rates. Stroke. 1987 Jan-Feb;18(1):38–42. doi: 10.1161/01.str.18.1.38. [DOI] [PubMed] [Google Scholar]
- 5.Wong A. Incident solar radiation and coronary heart disease mortality rates in Europe. Eur J Epidemiol. 2008;23(9):609–614. doi: 10.1007/s10654-008-9274-y. [DOI] [PubMed] [Google Scholar]
- 6.Turner PL, Mainster MA. Circadian photoreception: ageing and the eye's important role in systemic health. Br J Ophthalmol. 2008 Nov;92(11):1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reusch J, Ackermann H, Badenhoop K. Cyclic changes of vitamin D and PTH are primarily regulated by solar radiation: 5-year analysis of a German (50 degrees N) population. Horm Metab Res. 2009 May;41(5):402–407. doi: 10.1055/s-0028-1128131. [DOI] [PubMed] [Google Scholar]
- 8.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011 Jan 20;364(3):248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 9.Makariou SE, Michel P, Tzoufi MS, Challa A, Milionis HJ. Vitamin D and Stroke: Promise for Prevention and Better Outcome. Curr Vasc Pharmacol. 2012 Jun 22; doi: 10.2174/15701611113119990119. [DOI] [PubMed] [Google Scholar]
- 10.Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009 Jul;338(1):40–44. doi: 10.1097/MAJ.0b013e3181aaee91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998 Aug 29;352(9129):709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 12.Webb AR. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006 Sep;92(1):17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 14.Callahan CM, Hendrie HC, Tierney WM. Documentation and evaluation of cognitive impairment in elderly primary care patients. Ann Intern Med. 1995 Mar 15;122(6):422–429. doi: 10.7326/0003-4819-122-6-199503150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educational and Psychological Measurement. 1993;53(4):1117–1125. [Google Scholar]
- 16.Wadley VG, McClure LA, Howard VJ, et al. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke. 2007 Apr;38(4):1143–1147. doi: 10.1161/01.STR.0000259676.75552.38. [DOI] [PubMed] [Google Scholar]
- 17.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Cosgrove B, Lohmann D, Mitchell K, et al. Real-time and retrospective forcing in the North American Land Data Assimilation System (NLDAS) project. J Geophys Res. 2003;108(D22):8842–8854. [Google Scholar]
- 19.Basu R, Ostro BD. A multicounty analysis identifying the populations vulnerable to mortality associated with high ambient temperature in California. Am J Epidemiol. 2008 Sep 15;168(6):632–637. doi: 10.1093/aje/kwn170. [DOI] [PubMed] [Google Scholar]
- 20.Peterson F, Chen L, Knutsen S, Beeson WL, Ghamsary M, Abbey D. Is Risk of Fatal Stroke Associated With Long-Term Exposure To Particulate Ambient Air Pollutants. Results From the Ahsmog Study. Epidemiology. 2003;14(5) Supplement:S67. [Google Scholar]
- 21.Pope CA, 3rd, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004 Jan 6;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 22.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008 Sep 5;134(5):728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong YC, Rha JH, Lee JT, Ha EH, Kwon HJ, Kim H. Ischemic stroke associated with decrease in temperature. Epidemiology. 2003 Jul;14(4):473–478. doi: 10.1097/01.ede.0000078420.82023.e3. [DOI] [PubMed] [Google Scholar]
- 24.Medina-Ramon M, Zanobetti A, Cavanagh DP, Schwartz J. Extreme temperatures and mortality: assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect. 2006 Sep;114(9):1331–1336. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 26.Marniemi J, Alanen E, Impivaara O, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005 Jun;15(3):188–197. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008 Jan 29;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007 Sep 10;167(16):1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 29.Pilz S, Dobnig H, Fischer JE, et al. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke. 2008 Sep;39(9):2611–2613. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 30.Rocklov J, Forsberg B. The effect of temperature on mortality in Stockholm 1998--2003: a study of lag structures and heatwave effects. Scand J Public Health. 2008 Jul;36(5):516–523. doi: 10.1177/1403494807088458. [DOI] [PubMed] [Google Scholar]
- 31.Braga AL, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ Health Perspect. 2002 Sep;110(9):859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XY, Barnett AG, Hu W, Tong S. Temperature variation and emergency hospital admissions for stroke in Brisbane, Australia, 1996–2005. Int J Biometeorol. 2009 Nov;53(6):535–541. doi: 10.1007/s00484-009-0241-4. [DOI] [PubMed] [Google Scholar]
- 33.Green RS, Basu R, Malig B, Broadwin R, Kim JJ, Ostro B. The effect of temperature on hospital admissions in nine California counties. Int J Public Health. 2009 Sep 22; doi: 10.1007/s00038-009-0076-0. [DOI] [PubMed] [Google Scholar]
- 34.Keller MC, Fredrickson BL, Ybarra O, et al. A warm heart and a clear head. The contingent effects of weather on mood and cognition. Psychol Sci. 2005 Sep;16(9):724–731. doi: 10.1111/j.1467-9280.2005.01602.x. [DOI] [PubMed] [Google Scholar]
- 35.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997 Aug;30(2 Pt 1):150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 36.Barysch MJ, Hofbauer GF, Dummer R. Vitamin D, ultraviolet exposure, and skin cancer in the elderly. Gerontology. 2010 Jun;56(4):410–413. doi: 10.1159/000315119. [DOI] [PubMed] [Google Scholar]
- 37.Ding C, Cicuttini F, Parameswaran V, Burgess J, Quinn S, Jones G. Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum. 2009 May;60(5):1381–1389. doi: 10.1002/art.24486. [DOI] [PubMed] [Google Scholar]
- 38.Kent ST, McClure LA, Crosson WL, Arnett DK, Wadley VG, Sathiakumar N. Effect of sunlight exposure on cognitive function among depressed and non-depressed participants: a REGARDS cross-sectional study. Environ Health. 2009;8:34. doi: 10.1186/1476-069X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant WB. In defense of the sun: An estimate of changes in mortality rates in the United States if mean serum 25-hydroxyvitamin D levels were raised to 45 ng/mL by solar ultraviolet-B irradiance. Dermatoendocrinol. 2009 Jul;1(4):207–214. doi: 10.4161/derm.1.4.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godar DE, Pope SJ, Grant WB, Holick MF. Solar UV doses of adult Americans and vitamin D(3) production. Dermatoendocrinol. 2011 Oct;3(4):243–250. doi: 10.4161/derm.3.4.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.