Abstract
Objective
To determine whether peripheral blood stem cell transplant (PBSCT) result in engraftment of donor stem cells in the recipient uterus.
Design
Prospective clinical and laboratory research.
Setting
Translational medicine research hospital.
Patient(s)/Animal(s)
Macaque and human bone marrow transplant recipients.
Intervention(s)
Rhesus macaques received autologous transduced immunoselected cytokine-mobilized CD34+ cells after total body irradiation. Vector constructs expressed green fluorescent protein. In the human subjects, prior PBSCT subjects underwent endometrial biopsy and bone marrow aspiration. Macaque and human endometrial and bone marrow cells were isolated and cultured. Fluorescent microscopy, flow cytometry, and polymerase chain reaction (PCR) were used to evaluate for the presence of donor-derived cells.
Main Outcome Measure(s)
Presence of donor cells in recipient endometrium and bone marrow stroma.
Result(s)
The macaque endometrial cells did not exhibit evidence of green fluorescent protein labeling. Human endometrial cells were cultured and the absence of donor blood contamination was verified. The PCR evaluation of the human endometrial cells did not demonstrate evidence of donor short tandem repeats.
Conclusion(s)
The PBSCT did not result in engraftment of donor-derived cells in the endometrium.
Keywords: Stem cells, peripheral blood stem cell transplant, endometrium, bone marrow
It is possible that cellular therapies using bone marrow-derived cells could provide novel treatment of endometrial diseases affecting women. If transplanted stem cells (like bone marrow cells) engraft in the uterus, this well-established clinical therapy for benign and malignant hematologic conditions could improve our understanding of common diseases of women related to endometrial pathology, such as recurrent miscarriage, endometriosis, heavy menstrual bleeding, infertility, and intrauterine adhesions. Alternatively, modulation of endometrial stem cell function represents a novel target for pharmacologic therapies.
In addition, undifferentiated white blood cells play an important role in diverse range of endometrial function such as immune tolerance of implantation and pregnancy (1, 2). Specifically, a growing body of literature has increased our understanding of natural killer (NK) cells in uterine function (3). Very little is known about cellular subpopulations from the bone marrow that are active in uterine function. As a first step to understand the bone marrow contribution to the endometrium, we characterized endometrial cell derivation in both a human peripheral blood stem cell transplant (PBSCT) model and non-human primate model after long-term, stable engraftment.
Patients who have had whole bone marrow transplantations have been reported to have donor-derived endometrial cells using immunohistochemical staining for HLA expression in the stroma and glands specific to the donor (4), fluorescent in-situ hybridization (FISH) to detect Y chromosomes in both the glands and stroma of females receiving male donor bone marrow cells (5), and in the endometrial epithelial cells of a term placenta using FISH and polymerase chain reaction (PCR) (6). These therapies likely transplant a wide variety of cell types into the recipient and the exact cellular subpopulation responsible for endometrial engraftment is not known. Mobilized peripheral blood stem cells (i.e., hematologic progenitors) collected after granulocyte colony stimulating factor mobilization have become the favored source of hematopoietic stem cells. The PBSCTs are used to treat hematologic conditions specifically, and are well established in our group in both animal (7) and human models (8). However, it is unknown whether patients who have undergone PBSCT protocols display the same ability of the donor cells to engraft in the recipient uterus. The objective of this study was to characterize the hematopoietic stem cell contribution to the endometrium.
MATERIALS AND METHODS
Macaque Model
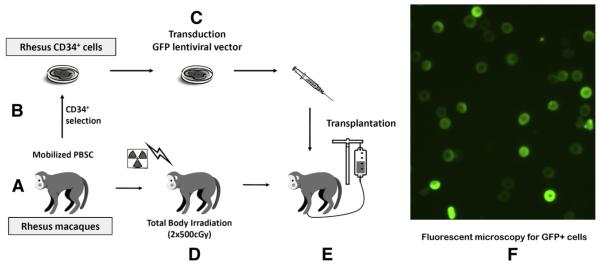
Non-human primate experiments were performed under a research protocol approved by the Animal Care and Use Committee (H0136). Rhesus macaques that had previously undergone an autologous PBSCT (n=4) were used to study endometrial engraftment, which is summarized in Figure 1 (7, 9). Briefly, mobilized hematopoietic stem cells were collected by leukapheresis (7, 10). Peripheral CD34+ cells were isolated from the blood using immunomagnetic bead selection and labeled with an integrating lentiviral vector encoding green fluorescent protein (GFP). During this time, Rhesus underwent pretransplant conditioning with total body irradiation (500 cGy × 2). The CD34+ cells with GFP marking were then back into the autologous macaque to partially reconstitute hematopoiesis. In this Rhesus macaque model, 2.49E+07 CD34+ cells/kg were transplanted into the monkey used for cell culture and fluorescence-activated cell sorter (FACS) analysis (n = 1). The 1.03E+07 CD34+ cells/kg were transplanted the monkey used for immunohistochemical experiments (n = 1). Animals (n = 2) used for fresh digestion and cell sorting for GFP+ cells had 2E+07 and 8E+08 CD34+ cells transplanted to partially reconstitute the bone marrow. An untransplanted monkey was used as a control (n = 1). Monkeys were monitored by vaginal swabbing for evidence of menstrual blood for 3 months before hysterectomy. Hysterotomies were then performed on one transplanted animal 15 months after transplant and one control animal to harvest endometrial tissue during the luteal phase, which has been reported as critical for generation of primary cell cultures in non-human primates (11). For the immunohistochemical experiments, hysterectomy was performed 13 months after PBSCT. For two animals used for fresh digestion and sorting for GFP+ cells, uteri were harvested at necropsy 11 months and 64 months after PBSCT. Bone marrow samples were collected by aspiration into heparin-coated syringes.
FIGURE 1.
Summary of the Rhesus macaques who underwent bone marrow transplantation with autologous green fluorescent protein (GFP)-labeled CD34+ cells after total body irradiation preconditioning resulting in long-term stable mixed bone marrow chimerism of granulocyte, lymphocyte, red blood cell, and platelet lineages (7, 9). (A) Hematopoietic stem cells were mobilized into the peripheral circulation and mononuclear cells were collected by leukapheresis. (B) Peripheral CD34+ cells were isolated from the leukapheresis product. (C) The CD34+ cells were transduced with an integrating lentiviral vector expressing GFP. (D) The macaque underwent pretransplant conditioning total body irradiation (2 × 500 cGy). (E) The CD34+ cells with GFP marking were then autotransplanted back into the macaque. (F) Fluorescent microscopy was performed on endometrial and bone marrow stromal cells cultures to detect the presence of GFP+ cells (indicating donor engraftment).
Human Model
Patients who have previously undergone PBSCT for malignant or nonmalignant conditions were studied. Endometrial biopsies were performed in reproductive aged women under an approved Institutional Review Board protocol (National Institutes of Health protocol 12-CH-0016). Bone marrow aspirates were obtained from patients under approved Institutional Review Board protocol (National Institutes of Health protocol 03-H-0170). Written, informed consent was obtained from the human subjects.
Stromal Cultures
Human endometrial biopsies (n = 10) were enzyme digested and adherent stromal cells cultured as previously described (12). Bone marrow aspirates (n = 5) were cultured using similar conditions with media in plastic flasks at 37°C in a humidified chamber (5% CO2 and ambient O2) and allowed to replicate to confluence. Rhesus macaque endometrium (1 control and 1 transplanted) and bone marrow stromal cells (1 control and 1 transplanted) were cultured using minimal essential medium (MEM) alpha (GIBCO, Invitrogen) with 20% fetal bovine serum and 1% antibiotic antimycotic (GIBCO, Invitrogen).
Fluorescent Microscopy to Detect Donor Cells
For macaque samples, adherent stromal cell cultures from endometrium and bone marrow were imaged with fluorescent microscopy to detect GFP positivity.
Flow Cytometry
Freshly digested endometrium and adherent stromal cultures were trypsinized to form single cell suspensions. Cells were incubated with the following antibodies: PE CD146 (BD Pharmingen catalogue 550315), PE CD 140b (i.e., PDGFRβ BD Pharmingen catalogue 558821), and FITC CD45 (BD Pharmingen catalogue 555482) and compared to PE Mouse IgG1k (BD Pharmingen catalogue 555749) and FITC Mouse IgG1k (BD Pharmingen catalogue 555748) isotype negative controls. Cells were analyzed on BD FACS Caliber and analyzed with BD FACS software (BD Biosciences). Macaque samples were analyzed for the presence of GFP positivity to denote transduced hematopoietic stem cell derivation.
PCR to Detect Donor Short Tandem Repeats
For human samples, adherent stromal cell cultures from endometrium and bone marrow were trypsinized and collected into cell pellets. DNA was extracted using QIAmp DNA Mini Kit (Qiagen). Donor chimerism was then determined by fragment analysis, using the GeneMapper (Applied Biosystems).
Statistics
Paired t-test was used to compare the amount of donor microchimerism in transplant patients’ endometrial or bone marrow samples and peripheral blood. A P value <.05 was considered statistically significant.
RESULTS
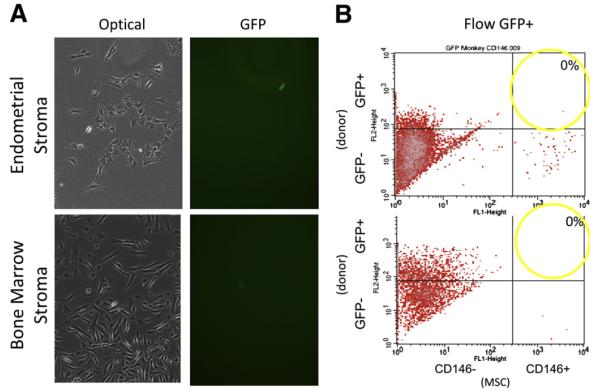
In a Rhesus macaque model (7), peripheral blood images demonstrating GFP+ blood cells have been reported elsewhere (10) from monkeys with long-term stable gene marking in blood cells after transplantation with autologous CD34+ cells labeled with GFP (Fig. 1). A hysterotomy was performed to obtain endometrial tissue and generate adherent stromal cell cultures. Rare GFP+ cells (indicating transduced hematopoietic stem cell derivation) were observed in adherent stromal culture from transplanted animals, but not controls; however, none were double positive for the mesenchymal stem cell marker CD146 (Fig. 2). Similarly, when bone marrow was examined from this autologous Rhesus model, rare GFP+ cells (indicating transduced hematopoietic stem cell derivation) were observed in stromal cell culture. However, they were not CD146 double positive (Fig. 2), indicating that the transplanted hematopoietic stem cell only engrafted in the hematopoietic compartment, but not the stromal compartment of the bone marrow.
FIGURE 2.
(A) Optical imaging of endometrial and bone marrow stromal cell cultures from the macaque. Fluorescent imaging of the endometrial and bone marrow stromal cell cultures showed rare positive green fluorescent protein (GFP+) cells. (B) However, none of these endometrial (top) and bone marrow (bottom) mesenchymal stem cells (MSC) were double positive for the endometrial stem cell marker CD146 and GFP+ (transplant derived) in this macaque model of peripheral blood stem cell transplant.
Two additional animals were used for a fresh endometrial digestion and FACS analysis, but none of the GFP+ sorted cells were able to establish a primary cell culture, whereas GFP- sorted fraction readily established primary cultures (data not shown).
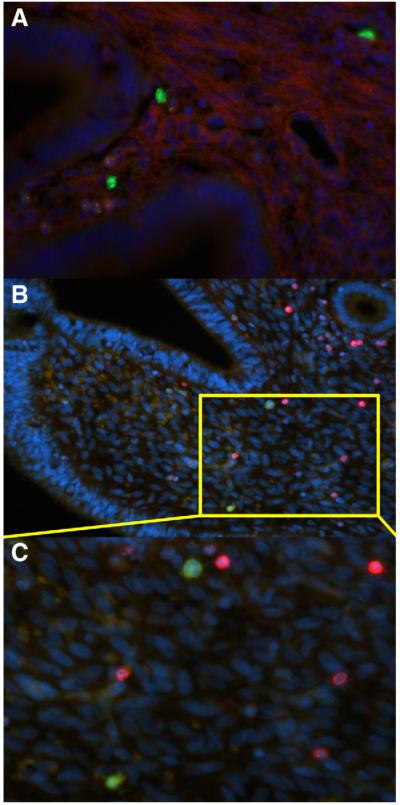
One transplanted animal was used for immunohistochemical, PDGFrβ (CD140b), an endometrial stem cell marker, did not colocalize with transplanted (GFP) hematopoietic stem cell in the endometrium (Fig. 3A). GFP+ cells residing within the endometrium were not CD45+, which is consistent with previous findings (Fig. 3B and C) (13).
FIGURE 3.
Uteri from Rhesus macaque transplanted with positive green fluorescent protein (GFP+) hematopoietic stem cells (HSC) were collected for immunofluorescent. The HSC-derived cells residing in the GFP+ cells (green) do not colocalize with staining for the endometrial stem cell marker PDGFrβ (red) shown in low (A) and high power (B). The HSC-derived cells (green) residing within the endometrium are not CD45+ (red) (C).
In humans, endometrium was collected from different patients than the bone marrow samples, except for one patient who donated both an endometrial and bone marrow sample. Adherent stromal cells cultures were effectively established from nine endometrial and five bone marrow samples. One endometrial sample from an amenorrheic woman did not grow in culture. Histograms of representative FACS analysis for CD146, PDGFrβ, and CD45 are shown in Supplemental Figure 1B. Endometrial and bone marrow stromal cells exhibited high levels of mesenchymal stem cell surface markers CD146 and PDGFrβ (Supplemental Fig. 1). As fresh endometrial and bone marrow samples have blood contamination (which is known to be donor derived), microchimerism testing on these samples would result in a false positive result for donor cells. To verify that culturing stromal cells from endometrium and bone marrow cultures had low levels of donor blood cell contamination, we determined the percentage of cells that were positive for the white blood cell (WBC) marker CD45 by FACS analysis (Supplemental Fig. 1B, available online).
After confirming that culturing the samples purified the stromal compartment of interest, with low levels of WBC contamination (Supplemental Fig. 2, available online), samples were analyzed for donor microchimerism using PCR to detect percentage of donor and recipient short tandem repeat sequences (Table 1). There was no donor chimerism of the stromal cells from the endometrial samples compared with 91.2% chimerism from the peripheral blood cells (P<.001) (Table 1). Similarly, in the bone marrow cohort, there was no donor microchimerism of the stromal cells from the bone marrow samples compared with 97.3% microchimerism from the peripheral blood cell (positive control) samples (P<.001) (Table 1).
Table 1.
Percentage of cells with short tandem repeat sequences specific to donor.
| % Donor by PCR | |||
| Patient | Endometrium | Peripheral blood | Diagnosis |
| A | 0 | 100% whole blood | Aplastic anemia |
| B | 0 | 100% myeloid, 100% CD3 | Lymphoma |
| C | 0 | 100% myeloid, 100% CD3 | ALL |
| D | 0 | 47% myeloid, 9% CD3 | Sickle cell disease |
| E | 0 | 100% myeloid, 45% CD3 | Sickle cell disease |
| F | 0 | 100% myeloid, 60% CD3 | Sickle cell disease |
| G | 0 | 81% myeloid, 24% CD3 | Sickle cell disease |
| H | 0 | 100% myeloid, 100% CD3 | Multiple myeloma |
| I | 0 | 100% myeloid, 100% CD3 | Aplastic anemia |
| Mean ± SEM | 0 ± 0 | 91.2% ± 8.8% | |
| % Donor by PCR | |||
| Patient | Bone marrow | Peripheral blood | Diagnosis |
| G | 0 | 89% myeloid, 29% CD3 | Sickle cell disease |
| J | 0 | 100% myeloid, 41% CD3 | Sickle cell disease |
| K | 0 | 100% myeloid, 28% CD3 | Sickle cell disease |
| L | 0 | 20% myeloid, 3% CD3 | Sickle cell disease |
| M | 0 | 100% myeloid, 2% CD3 | Sickle cell disease |
| Mean ± SEM | 0 ± 0 | 97.3% ± 2.5% | |
Note: ALL = acute lymphocytic leukemia; PCR = polymerase chain reaction.
Wolff. Endometrial stem cell derivation. Fertii Sterii 2012.
DISCUSSION
Several lines of evidence support the existence of pluripotent, or stem cell-like, function of endometrial cells (14). Stem cells are defined at the most fundamental level as being able to self-replicate and differentiate into mature cell phenotypes. At the organ level, the endometrium is one of the most impressive clinical examples of these characteristics in medicine. Not only is the frequency of endometrial regeneration remarkable due to monthly menstruation, but also the magnitude and divergence of endometrial function that occurs in the setting of placental implantation and physiologic changes of pregnancy and birth. Only a cell population with highly dynamic properties and immense proliferative potential could meet these demands.
The identification of endometrial cells with clonogenic activity (15), bromodeoxyuridine (BrdU) label-retaining characteristics (16), and side population characteristic capacity to extrude the DNA-binding dye (Hoechst 33342) through the adenosine triphosphate (ATP) binding cassette transporter protein ABCG2/Bcrp1 provided the first evidence for the existence of cells with stem cell properties residing within the endometrium (17). Functional ability of these cells to differentiate into chondrocytes (12), adipocytes, osteocytes, myocytes (18-20), and neurons (21) has been previously shown by us (12, 21) and other investigators, lending further support to the existence of stem cell-like properties of these cells. Based on these findings, human endometrial-derived stem cells exceed the minimum criteria published by the International Society of Cellular Therapy to be defined as multipotent mesenchymal stromal cells (also known as mesenchymal stem cells) of [1] plastic adherence in cell culture, [2] the ability to differentiate into adipocytes, osteocytes, and chondrocytes in vitro, and [3] must express CD105, CD73, and CD90 and lack expression of CD45, CD34, CD14 or CD11b, CD70α or CD19, and HLA-DR surface molecules (22).
Little is known about the origin of these tissue progenitor cells in the endometrium. One possibility is that these cells arrive in the endometrium during embryogenesis, where they remain in a resting, undifferentiated state until required for regeneration in times of tissue injury. Alternately, this stem cell population could be replenished by constant influx of progenitor cells through the circulation from a bone marrow origin. Cells from the bone marrow have been reported to reconstitute the endometrium in bone marrow recipients in animal (23) and human models (5, 6), but it is unknown whether these donor-derived cells express stem cell markers, exhibit stem cell characteristics, or retain stem cell plasticity.
A notable difference from previous reports of endometrial engraftment and this study is the type of bone marrow transplant regimen used. In the bone marrow, there are two main types of stem cells: [1] hematopoietic stem cells, which give rise to blood cells and [2] bone marrow mesenchymal cells, which generate the supported connective tissues of the bone marrow and have the capability to differentiate into bone, fat, and cartilage (24). In previous reports, patients with donor-derived cells in their endometriumhad undergone whole bone marrow transplant (4), which likely contains a much more heterogeneous population of cells. Specifically, whole bone marrow transplants are also likely to include some of the most primitive hematopoietic stem cells, quietly resting in bone marrow niches, as well as the second large population of stem cells found in the bone marrow–mesenchymal stem cells. Conversely, peripheral blood stem cell transplantations likely consist of a much more homogenous, selected population of hematopoietic stem cells. Only those cells that can respond to granulocyte colony stimulating factor mobilization successfully exit the bone marrow, circulate in the peripheral blood, are collected by leukapheresis, and then successfully reconstitute a recipient’s bone marrow are present in the recipients. Specifically, the difference in engraftment may be due to a lack of bone marrow mesenchymal stem cells in PBSCT, which are present in whole bone marrow transplants. In fact, bone marrow recipients have engraftment of bone marrow mesenchymal cells, whereas PBSCT recipients do not typically exhibit donor mesenchymal cell engraftment of the bone marrow (25, 26). Bone marrow mesenchymal stem cells are more closely related to endometrial stem cells than hematopoietic stem cells. Human endometrium contains a small population of mesenchymal stem cells, which may be responsible for its cyclic growth (27). It is possible that mesenchymal stem cells are a critical component of bone marrow transplantation regimens necessary to effect uterine engraftment. Other investigators have reported similar lack of engraftment of the stromal compartment of the bone marrow after PBSCT (28). Further studies are needed on the outcomes of cellular therapies involving mesenchymal stem cell transplantation.
Our results suggest that hematopoietic stem cells do not contribute to the endometrial stem cell pool. These findings are supported by a recent study by Cervello et al. (29), who reported on five patients receiving transplants of bone marrow-derived cells (4 whole bone marrow and 1 PBSCT) in whom FISH was used to detect the presence of XY donor-derived cells in the endometrium of XX recipients. They demonstrated XY donor-derived cells in the endometrium in a range of 1.7%–2.6% of the cells; however, there were no donor-derived cells with the stem cell characteristic of side population activity (29). These data are consistent with our stromal cell culture FACS analysis. In a similar study, Ikoma et al. (5) analyzed three female patients receiving PBSCT from XY male donors. Using FISH to detect the presence of XY cells in endometrial samples in tissue sections, they also identified donor-derived cells in 0.6%–8.4% of epithelial cells and 8.2%–9.8% of stromal cells (5). This study, however, did not look at any stem cell markers, which could suggest monthly regeneration or in vitro stem cell characteristics. It is notable that neither study was able to identify a correlation between time from transplant and the percent of donor engraftment in the endometrium (5, 23). Thus, differences in time from transplant to tissue sampling are unlikely to have affected our results. Another possible explanation for the lack of engraftment in the present study is the use of more stringent molecular methods of identifying donor- (or hematopoietic stem cell) derived cells (e.g., PCR) rather than FISH, which is known to be susceptible to false positivity due to artifacts such as cell fusion and overlapping imaging. These results are further strengthened by the use of this novel Rhesus macaque model, which allowed for the transplantation of GFP+ hematopoietic stem cell to track uterine engraftment.
In summary, PBSCT did not result in the engraftment of hematopoietic stem cell-derived endometrial stromal cells. Our findings support recent studies demonstrating that bone marrow cells do not contribute to cell populations with stem cell characteristics residing in the endometrium.
Supplementary Material
Acknowledgments
The authors thank the National Institutes of Health veterinary and laboratory animal staff at 5 Research Court and Poolesville for maintaining the animals used in this study and William DeGraff and Dr. Jim Mitchell of the Radiation Branch, National Cancer Institute for the use of the Eldorado Cobalt-60 irradiator to perform the transplants.
Supported by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute and in part by the Program in Reproductive and Adult Endocrinology, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD.
Footnotes
E.F.W. has nothing to disclose. N.U. has nothing to disclose. R.E.D. has nothing to disclose. M.E.M. has nothing to disclose. M.M.H. has nothing to disclose. L.L.L. has nothing to disclose. M.J.H. has nothing to disclose. J.F.T. has nothing to disclose.
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
REFERENCES
- 1.Scherjon S, Lashley L, van der Hoorn ML, Claas F. Fetus specific T cell modulation during fertilization, implantation and pregnancy. Placenta. 2011;32(Suppl 4):S291–7. doi: 10.1016/j.placenta.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Laird SM TE, Cork BA, Linjawi S, Blakemore AIF, Li TC. A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update. 2003;9:163–74. doi: 10.1093/humupd/dmg013. [DOI] [PubMed] [Google Scholar]
- 3.Hatta K, Macleod RJ, Gerber SA, Croy BA. Emerging themes in uterine natural killer cell heterogeneity and function. Am J Reprod Immunol. 2012;68:282–9. doi: 10.1111/j.1600-0897.2012.01160.x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor H. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–5. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201:608.e1–8. doi: 10.1016/j.ajog.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Mints M, Jansson M, Sadeghi B, Westgren M, Uzunel M, Hassan M, et al. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod. 2008;23:139–43. doi: 10.1093/humrep/dem342. [DOI] [PubMed] [Google Scholar]
- 7.Uchida N, Washington K, Hayakawa J, Hsieh M, Bonifacino A, Krouse A, et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J Virol. 2009;83:9854–62. doi: 10.1128/JVI.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh M, Kang E, Fitzhugh C, Link M, Bolan C, Kurlander R, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–17. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida N, Bonifacino A, Krouse AE, Metzger ME, Csako G, Lee-Stroka A, et al. Accelerated lymphocyte reconstitution and long-term recovery after transplantation of lentiviral-transduced rhesus CD34+ cells mobilized by G-CSF and plerixafor. Exp Hematol. 2011;39:795–805. doi: 10.1016/j.exphem.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida N, Hargrove PW, Lap CJ, Evans ME, Phang O, Bonifacino AC, et al. High-efficiency transduction of Rhesus hematopoietic repopulating cells by a modified HIV1-based lentiviral vector. Mol Ther. 2012;20:1882–92. doi: 10.1038/mt.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazleabas AT. A baboon model for simulating pregnancy. Methods Mol Med. 2006;121:101–10. doi: 10.1385/1-59259-983-4:099. [DOI] [PubMed] [Google Scholar]
- 12.Wolff E, Wolff A, Du H, Taylor H. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci. 2007;14:524–33. doi: 10.1177/1933719107306896. [DOI] [PubMed] [Google Scholar]
- 13.Bratincsak A, Brownstein MJ, Cassiani-Ingoni R, Pastorino S, Szalayova I, Toth ZE, et al. CD45-positive blood cells give rise to uterine epithelial cells in mice. Stem Cells. 2007;25:2820–6. doi: 10.1634/stemcells.2007-0301. [DOI] [PubMed] [Google Scholar]
- 14.Sasson I, Taylor H. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106–15. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargett C. Identification and characterisation of human endometrial stem/progenitor cells. Aust N Z J Obstet Gynaecol. 2006;46:250–3. doi: 10.1111/j.1479-828X.2006.00582.x. [DOI] [PubMed] [Google Scholar]
- 16.Chan R, Gargett C. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–38. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 17.Kato K, Yoshimoto M, Adachi S, Yamayoshi A, Arima T, Asanoma K, et al. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22:1214–23. doi: 10.1093/humrep/del514. [DOI] [PubMed] [Google Scholar]
- 18.Schwab K, Gargett C. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 19.Rogers J, Young H, Adkison L, Lucas P, Black AJ. Differentiation factors induce expression of muscle, fat, cartilage, and bone in a clone of mouse pluripotent mesenchymal stem cells. Am Surg. 1995;61:231–6. [PubMed] [Google Scholar]
- 20.Gargett C, Schwab K, Zillwood R, Nguyen H, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–45. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff E, Gao X, Yao K, Andrews Z, Du H, Elsworth J, et al. Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J Cell Mol Med. 2011;15:747–55. doi: 10.1111/j.1582-4934.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23.Du H, Taylor H. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25:2082–6. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 24.Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503–15. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieger K, Marinets O, Fietz T, Korper S, Sommer D, Mucke C, et al. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol. 2005;33:605–11. doi: 10.1016/j.exphem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Dickhut A, Schwerdtfeger R, Kuklick L, Ritter M, Thiede C, Neubauer A, et al. Mesenchymal stem cells obtained after bone marrow transplantation or peripheral blood stem cell transplantation originate from host tissue. Ann Hematol. 2005;84:722–7. doi: 10.1007/s00277-005-1067-8. [DOI] [PubMed] [Google Scholar]
- 27.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 28.Cilloni D, Carlo-Stella C, Falzetti F, Sammarelli G, Regazzi E, Colla S, et al. Limited engraftment capacity of bone marrow-derived mesenchymal cells following T-cell-depleted hematopoietic stem cell transplantation. Blood. 2000;96:3637–43. [PubMed] [Google Scholar]
- 29.Cervello I, Gil-Sanchis C, Mas A, Faus A, Sanz J, Moscardo F, et al. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One. 2012;7:e30260. doi: 10.1371/journal.pone.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.