Summary
Elevation of the larynx is critical to swallowing function, an observation supported by the fact that radiation therapy-induced dysphagia is associated with reduced laryngeal elevation. We investigated muscles underlying hyolaryngeal elevation by using muscle functional MRI. We acquired scans from 11 healthy subjects to determine whole-muscle T2 signal profiles pre-swallowing, post-swallowing, and after performing swallowing exercises. Results demonstrate muscles essential to laryngeal elevation and exercises that target them.
Purpose
Reduced hyolaryngeal elevation, a critical event in swallowing, is associated with radiation therapy. Two muscle groups that suspend the hyoid, larynx, and pharynx have been proposed to elevate the hyolaryngeal complex: the suprahyoid and longitudinal pharyngeal muscles. Thought to assist both groups is the thyrohyoid, a muscle intrinsic to the hyolaryngeal complex. Intensity modulated radiation therapy guidelines designed to preserve structures important to swallowing currently exclude the suprahyoid and thyrohyoid muscles. This study used muscle functional magnetic resonance imaging (mfMRI) in normal healthy adults to determine whether both muscle groups are active in swallowing and to test therapeutic exercises thought to be specific to hyolaryngeal elevation.
Methods and Materials
mfMRI data were acquired from 11 healthy subjects before and after normal swallowing and after swallowing exercise regimens (the Mendelsohn maneuver and effortful pitch glide). Whole-muscle transverse relaxation time (T2 signal, measured in milliseconds) profiles of 7 test muscles were used to evaluate the physiologic response of each muscle to each condition. Changes in effect size (using the Cohen d measure) of whole-muscle T2 profiles were used to determine which muscles underlie swallowing and swallowing exercises.
Results
Post-swallowing effect size changes (where a d value of >0.20 indicates significant activity during swallowing) for the T2 signal profile of the thyrohyoid was a d value of 0.09; a d value of 0.40 for the mylohyoid, 0.80 for the geniohyoid, 0.04 for the anterior digastric, and 0.25 for the posterior digastric-stylohyoid in the suprahyoid muscle group; and d values of 0.47 for the palatopharyngeus and 0.28 for the stylopharyngeus muscles in the longitudinal pharyngeal muscle group. The Mendelsohn maneuver and effortful pitch glide swallowing exercises showed significant effect size changes for all muscles tested, except for the thyrohyoid.
Conclusions
Muscles of both the suprahyoid and the longitudinal pharyngeal muscle groups are active in swallowing, and both swallowing exercises effectively target muscles elevating the hyolaryngeal complex. mfMRI is useful in testing swallowing muscle function.
Introduction
Radiation-induced swallowing dysfunction is a widely recognized comorbidity associated with cancer treatment (1). The mechanism of swallowing is extremely complex and not fully understood; however, impaired hyolaryngeal excursion and subsequent inadequate opening of the upper esophageal sphincter are most commonly attributed to postradiation therapy dysphagia (2). Intensity modulated radiation therapy (IMRT) promotes tissue sparing by shaping doses of radiation to avoid salivary glands and oropharyngeal structures thought to be essential to swallowing, where possible (3). Current IMRT recommendations focus on sparing the pharyngeal constrictor muscles and the larynx but exclude muscles potentially important to hyolaryngeal elevation (3-6). The purpose of this study was to advance the current understanding of muscles essential to hyolaryngeal elevation in normal swallowing and to explore how these findings compare with current IMRT guidelines.
Elevation of the hyolaryngeal complex is central to the intricate set of movements required to transfer a bolus from the oral cavity through the hypopharynx and into the esophagus. The hyolaryngeal complex consists of the hyoid muscle, the laryngeal cartilages, and the muscles intrinsic to the hyoid and larynx, including the thyrohyoid. The cricopharyngeus, the most inferior portion of the pharyngeal constrictor muscles, attaches to the hyolaryngeal complex and forms the upper esophageal sphincter (7). Proper hyolaryngeal elevation draws the airway anterior to the trajectory of an oncoming bolus and stretches open a relaxed upper esophageal sphincter. Inadequate hyolaryngeal elevation can result in aspiration or bolus retention, putting the patient at risk for inadequate oral intake, the need for an altered diet, and pneumonia.
Anatomical research has determined that morphologically, 2 extrinsic muscle groups have the greatest potential for hyolaryngeal elevation: the suprahyoid muscles, consisting of the mylohyoid, geniohyoid, anterior digastric, posterior digastric, and stylohyoid muscles, and the longitudinal pharyngeal muscles, consisting of the stylopharyngeus, salpingopharyngeus, and palatopharyngeus muscles (Fig. 1) (8). In addition, the thyrohyoid muscle (intrinsic to the hyolaryngeal complex) is thought to assist in approximating the larynx and hyoid (8). Of these muscles, current IMRT parameters protect the longitudinal pharyngeal muscles (except for their proximal attachments) but not the suprahyoid muscles or the thyrohyoid (3, 6). These exclusions are based on structural and not physiological findings (3). Importantly, swallowing research argues that the suprahyoid and thyrohyoid muscles are primarily responsible for hyolaryngeal elevation and, therefore, the opening of the upper esophageal sphincter (9). While anatomical studies propose that both muscle groups are important for hyolaryngeal elevation, the functional data verifying that these muscles are active in human swallowing are incomplete.
Fig. 1.
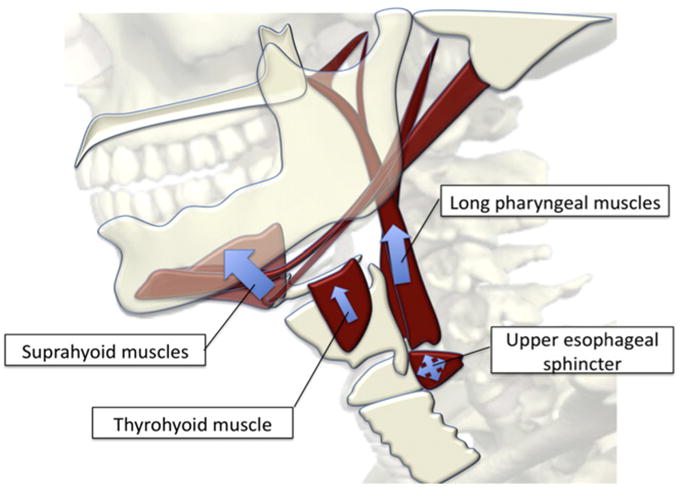
Illustration of muscles underlying hyolaryngeal elevation in swallowing. This mechanism stretches open an inhibited cricopharyngeus, which in turn forms the upper esophageal sphincter. The suprahyoid muscles comprise an anterior group that suspends the hyoid bone, and the long pharyngeal muscles form a posterior group that suspends the larynx. The thyrohyoid muscle is intrinsic to the hyolaryngeal complex and is thought to approximate the larynx and hyoid synergistically with the long pharyngeal muscles.
Electromyography is the standard method for measuring muscle function. One electromyography study verified muscle activity from the suprahyoid muscle group (geniohyoid and mylohyoid) and the longitudinal pharyngeal muscles (palatopharyngeus) in human swallowing (10). As deep muscles such as the stylopharyngeus are inaccessible to intramuscular electrodes, all the muscles of interest in this study have not been verified as active during human swallowing in any single study. Muscle functional magnetic resonance imaging (mfMRI) overcomes this limitation by allowing for a quantitative measurement of muscle activity after an episode of muscle use for any muscle that can be reliably segmented (11). This technique takes advantage of the fact that metabolic byproducts of muscle activity create changes in intracellular water concentration. These changes are measured with mfMRI as changes in transverse relaxation time (T2 signal recorded in milliseconds), which increases as a result of muscle activity.
In a clinical trial, swallowing exercises promoted less structural deterioration of swallowing muscles as determined by muscle thickness measurements and T2 relaxation times, using MRI (12). However the salience of swallowing exercises to all potential muscles underlying laryngeal elevation has not been fully determined as some muscles are difficult to measure with surface or intramuscular electrodes. The aims of this study were to use mfMRI to determine the relative involvement of muscles underlying hyolaryngeal elevation during swallowing and to evaluate the specificity of 2 swallowing exercises in targeting muscles that elevate the hyolaryngeal complex. Our hypotheses were that: (1) after swallowing, the suprahyoid and longitudinal pharyngeal muscles will show significant effect size changes in whole-muscle T2 signal profiles; and that (2) the Mendelsohn maneuver and effortful pitch glide (EPG) exercises will effectively target muscles that elevate the hyolaryngeal complex as demonstrated by significant effect size changes in whole-muscle T2 signal profiles.
Methods and Materials
Eleven young, healthy subjects including 6 males and 5 females, 22-30 years old (mean age, 25), judged to have normal swallowing ability by a speech language pathologist (S.L.) were included in this study under a research protocol approved by the Boston University Medical Campus Institutional Review Board. Subjects were trained under biofeedback by a swallowing specialist (S.L.) to perform the Mendelsohn maneuver and the EPG swallowing exercises during fiber optic endoscopic examination of swallowing (FEES). Subjects learned and performed exercises in the supine position, as required by the MRI setup.
Four separate mfMRI sequences were acquired for each subject: prior to swallowing, after 8 repeated swallows, following 8 consecutive Mendelsohn maneuvers, and following 8 consecutive EPGs. Multiple contiguous 4-mm axial images from the cricoid to the hard palate were acquired without intravenous contrast using a 3-Tesla MRI scanner (Achieva; Philips) with a 16-channel neurovascular coil. Images were acquired using spin-echo sequences with a repetition time (TR) of 2500 ms and dual echo times (TE) of 17.8 and 80 ms. Structural T1-weighted scans were acquired in 3 planes for use as anatomical cross-reference. Axial 1-mm images were acquired using a fast-field echo sequence with TE/TR values of 2.3/15 ms. Sagittal and coronal 3.3-mm images with a multiple slice turbo spin-echo sequences with TE/TR times of 9.2/453.8 ms were also acquired. Two-planar (coronal and sagittal) dynamic MRI scans (T1-weighted fast gradient echo sequence with TE/TR of 0.9/2.4 ms, 10-mm slice thickness, temporal resolution of 8.3 fps) were acquired to confirm adequate performance of swallowing exercises.
Timed visual cues administered via slides (PowerPoint; Microsoft) projected onto a mirror inside the magnet instructed the subjects to rest, swallow, or perform a specific swallowing exercise. The bolus for repetitive swallowing tasks was thin liquid administered via tubing from which the subject drew a self-selected volume of liquid. From all 11 subjects, scans were acquired before and after repeated swallowing. From 9 of 11 subjects, scans were acquired after 2 sets of swallowing exercises with an interval of 20 minutes' rest between tasks. As a positive control, the remaining 2 subjects performed a prolonged bite (80 seconds) to demonstrate that the change in T2 signal does localize to active muscle (13).
Two independent investigators determined mean signal intensities of long and short TE images by segmenting the thyrohyoid, mylohyoid, geniohyoid, anterior digastric, posterior digastric/stylohyoid complex, palatopharyngeus, stylopharyngeus, and sternocleidomastoid (control) muscles. The masseter was segmented in 2 subjects as a positive control. Both investigators independently segmented all muscles in a slice-by-slice fashion using the semiautomated process detailed below.
An automated segmentation algorithm of digital imaging and communication in medicine (DICOM; Osirix) software was used to segment muscles (http://www.osirix-viewer.com). The process began by manually selecting a single seed point within the muscle body of interest on each slice. Next, the algorithm automatically calculated the means and standard deviations of signal intensities within 1 voxel surrounding the seed point and automatically selected adjacent voxels that fell within the interval of a standard deviation level set by the investigator (Fig. 2). Additional points were manually selected, and a growing region of interest was automatically generated. The investigator accepted or rejected each automated selection based on its visual agreement with the anatomical boundaries of each muscle. This selection process produced a region of interest representing the mean signal intensity of a muscle at a particular slice level. Once a region of interest was determined on one TE slice, it was copied and imported to the second, identical TE slice. The process was repeated on all slice levels for each muscle.
Fig. 2.
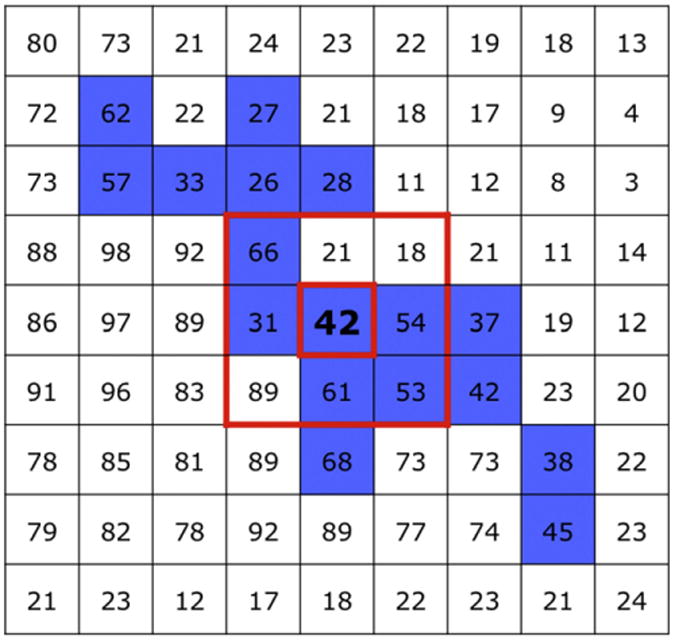
Method for semiautomated muscle segmentation. The Osirix “growing region of interest” interface uses dynamic thresholding by averaging the 8 voxels immediately surrounding a seed point (red box) selected by the investigator (in bold). The algorithm determines the means and standard deviations of signal intensities of these 9 voxels and then selects all neighboring voxels falling within a standard deviation of the mean (in blue). In principle, tissue types that match the signal intensity of the seed point are segmented while other voxels are excluded.
An anatomist with expertise in head and neck anatomy (W.P.) and a radiology resident (D.H.) developed a protocol to identify muscles of interest in the axial plane with the aid of anatomical scans in coronal and sagittal dimensions. Superiorly, the thyrohyoid was identified as the deeper of the infrahyoid muscles distal to the hyoid bone; inferiorly, the muscle was identified immediately anterior to the thyroid cartilage. The geniohyoid was carefully differentiated in a position just inferior to the genioglossus muscle between the hyoid and genial tubercles. Only the posterior body of the mylohyoid was measured, as it was deemed more structurally relevant to hyolaryngeal elevation and more reliably identifiable lateral to the sublingual gland and medial to the mandible. The anterior belly of the digastric was readily found inferior and lateral to all muscles in the submental region. Segmentation of the complex created by the posterior belly of the digastric and stylohyoid began within the digastric fossa of the temporal bone; inferiorly, medial to the parotid gland, the posterior digastric is joined by the stylohyoid and continues until just medial to the posterior portion of the submandibular gland.
Inferiorly, the longitudinal pharyngeal muscles blend indistinguishably into the pharyngeal constrictor muscles; as a result, only the proximal portions of these muscles were segmented. The palatopharyngeus lies behind the palatine tonsils, between it and the nasopharyngeal mucosa. Segmentation began where the muscle inserts into the soft palate, adjacent to the uvula, and continued posterolaterally toward the pharyngeal wall until just prior to blending into the pharyngeal constrictor muscles. Stylopharyngeus segmentation began on the medial side of the styloid process, continuing inferiorly until indistinguishable from the pharyngeal constrictor muscles. The sternocleidomastoid and masseter were segmented as control muscles. All muscles were segmented on the right side only.
Both investigators segmented all images, with the radiologist blinded to the test condition of each image series. Segmentations were reconciled between the investigators with agreement established by slice number and location determined by coordinate data and visual inspection. Because muscles change shape from slice to slice and subject to subject, signal intensities were weighted according to the number of voxels selected by the algorithm at each slice level and averaged to determine mean weighted signal intensities for the whole muscle. These mean-weighted signal intensities for the whole muscle at long and short TE times were used to calculate a whole-muscle T2 signal profile using the following formula:
where TE is the echo time and SI is the mean signal intensity (13).
Inter-rater reliability was determined by intraclass correlation coefficients (ICC) of T2 signal profiles for each muscle ranging from an ICC of 0.76-0.94 (Table 1) (14). Results were determined using the mean T2 signal profile from both raters.
Table 1.
Inter-rater reliability determined by interclass correlation coefficients of all whole-muscle T2 signal profiles generated by 2 independent investigators.
| Muscle | ICC | Lower 95% confidence limit | Upper 95% confidence limit | F |
|---|---|---|---|---|
| Thyrohyoid | 0.88 | 0.79 | 0.93 | 15.70 |
| Mylohyoid | 0.79 | 0.64 | 0.88 | 8.60 |
| Geniohyoid | 0.79 | 0.64 | 0.88 | 5.30 |
| Anterior digastric | 0.92 | 0.85 | 0.95 | 22.60 |
| Post-digastric-stylohyoid | 0.91 | 0.84 | 0.95 | 21.30 |
| Palatopharyngeus | 0.76 | 0.59 | 0.87 | 7.40 |
| Stylopharyngeus | 0.94 | 0.88 | 0.97 | 30.60 |
Abbreviation: ICC = intraclass correlation coefficient.
Results
Means and confidence intervals of T2 signal profiles for muscles under each condition and effect size changes of T2 signal profiles of muscles are reported in Table 2. Effect size changes were used to measure relative activation of muscles post-swallowing and under post-exercise conditions, with significance set at a Cohen d value of >0.20 and post hoc corrections to effect sizes made for a sample size of less than 20 (Fig. 3) (15). Hypothesis testing using a 1-tailed paired t test of suprahyoid and longitudinal pharyngeal muscles demonstrated a statistically significant difference between pre- and post-swallowing conditions (n = 11) for the geniohyoid (P = .01) and palatopharyngeus (P = .04). Hypothesis testing using a one-tailed t test for the 2 swallowing exercises (n = 9) demonstrated a statistically significant differences between pre- and post-EPG in targeting the palatopharyngeus (P = .02), stylopharyngeus (P = .05), and mylohyoid (EPG, P = .05) muscles.
Table 2.
Mean values and 95% confidence intervals of whole-muscle T2 signal profiles by condition reported in milliseconds (ms).
| Muscle | Pre-swallowing | Post-swallowing | Post-Mendelsohn maneuver | Post-effortful pitch glide | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Mean | ±95% CI | Mean | ±95% CI | Mean | ±95% CI | Mean | ±95% CI | |
| Thyrohyoid | 46.08 | 1.53 | 47.62 | 2.79 | 47.58 | 3.81 | 46.60 | 2.48 |
| Mylohyoid | 46.13 | 1.27 | 47.13 | 1.59 | 49.64 | 1.47 | 48.03 | 1.80 |
| Geniohyoid | 48.93 | 0.80 | 50.94 | 1.84 | 50.12 | 2.02 | 50.00 | 2.15 |
| Anterior digastric | 47.93 | 1.16 | 48.07 | 2.51 | 50.11 | 2.84 | 48.87 | 1.53 |
| Posterior digastric-stylohyoid | 44.21 | 1.93 | 44.92 | 1.39 | 44.98 | 2.24 | 45.66 | 2.15 |
| Palatopharyngeus | 49.41 | 1.13 | 50.76 | 2.00 | 51.11 | 2.53 | 51.39 | 1.46 |
| Stylopharyngeus | 53.30 | 4.05 | 55.32 | 4.61 | 57.47 | 5.75 | 58.62 | 4.22 |
Fig. 3.
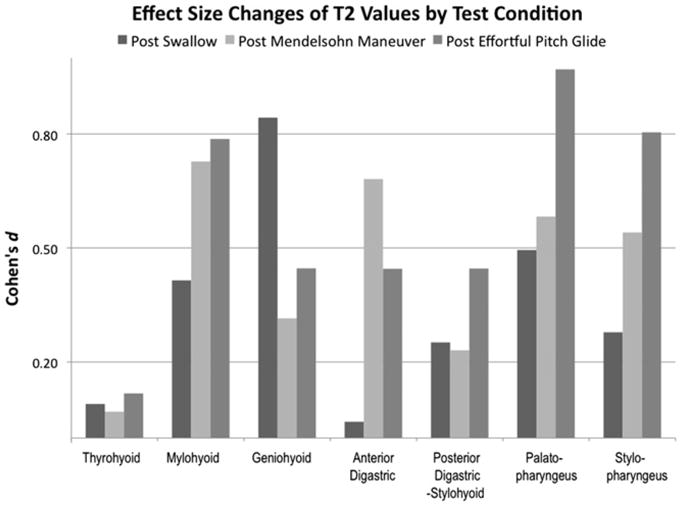
Effect size changes of mean T2 signal profiles under the stated conditions compared to those of the pre-swallow T2 signals. A value greater than 0.20 is considered significant, with >0.50 interpreted as a medium effect size and >0.80 as a large effect size change.
The negative control muscle (sternocleidomastoid) showed no increase in T2 values under pre- vs post-swallowing conditions (Fig. 4a). The positive control muscle (masseter) demonstrated no significant change with swallowing activity but did show significantly higher T2 values after the 80-second bite maneuver using a 2-tailed paired t test (P = .02, n = 2) (Fig. 4b).
Fig. 4.
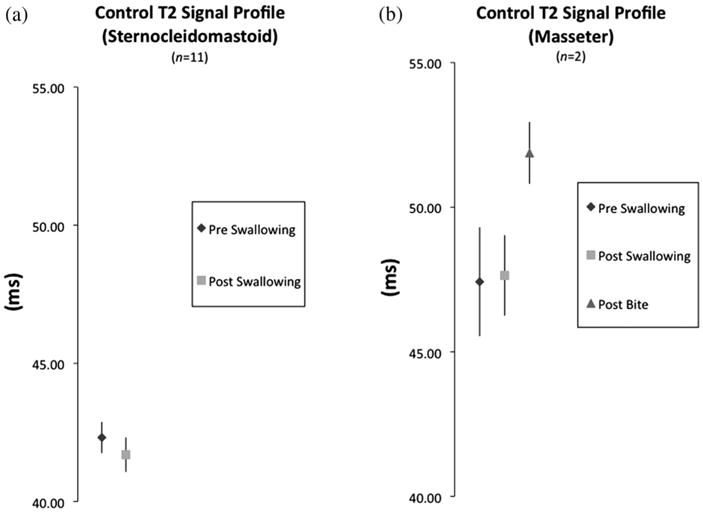
T2 signal profiles of negative and positive control muscles. (a) Comparison of T2 profiles of negative control muscle (sterno-cleidomastoid) from pre- to post-swallowing. (b) Comparison of T2 signal profiles of the masseter after swallowing and after a prolonged bite (80 seconds) in 2 subjects. Post-bite T2 signal profiles show a significant difference (P =. 02) compared to post-swallowing profiles.
Discussion
Reduced elevation of the larynx is a primary predictor of aspiration and is often seen in radiation-induced dysphagia. This study showed that the suprahyoid and longitudinal pharyngeal muscles (except for the anterior digastric) are active in hyolaryngeal elevation and that the Mendelsohn maneuver and EPG exercises effectively target both muscle groups as seen by significant effect size changes in whole-muscle T2 signal profiles (Fig. 3). All muscles, except for the thyrohyoid and anterior digastric, achieved significant effect size changes during swallowing. The long pharyngeal muscles, mylohyoid, posterior digastric, and stylohyoid muscles showed a small effect size, which is desirable for muscles underlying the repetitive and necessary functions of thriving. The geniohyoid here demonstrates a large effort, which may be due to the fact that the geniohyoid is primarily responsible for the anterior movement of the hyoid, exacerbated here by the experimental condition of subjects swallowing in a supine position. These experimental findings share areas of agreement and disagreement with IMRT studies.
Agreement between our findings and those of IMRT studies lies in identifying the longitudinal pharyngeal muscles as important to deglutition and dysphagia. As noted elsewhere (3, 6), IMRT demarcation of the “pharyngeal constrictor muscles” is a conflation of muscle groups. The pharyngeal wall incorporates a double layer of muscles that includes an outer layer of predominately fast-twitch fibers and an inner layer of predominately slow-twitch fibers (16). Therefore, we suggest a more anatomically informative name for this dosage boundary is “pharyngeal wall muscles” vs “pharyngeal constrictor muscles.” The outer layer is composed of the superior, middle, and inferior pharyngeal constrictor muscles. The inner layer is composed of the blended distal fibers of the palatopharyngeus, salpingopharyngeus, and stylopharyngeus, which insert into the thyroid cartilage and pharynx (8). IMRT demarcations for the “pharyngeal constrictor muscles” encompass these distal fibers but exclude the proximal attachment of the stylopharyngeus from the styloid process to the pharyngeal wall and the palatopharyngeus insertion into the palatine aponeurosis (3-6).
Disagreement between this study's findings and IMRT findings lies with the importance of the suprahyoid muscles, which are excluded in current guidelines. The criterion for inclusion in IMRT guidelines was an increase in soft tissue thickness as measured on computed tomography (CT) at 3 months postradiation (3). Eisbruch et al (3) attributed soft tissue thickness to inflammation of the mucosa rather than muscles, tissues that are indistinguishable in CT. In other reports, increase in muscle thickness is assumed to represent increased muscle function (ie, the lack of muscle atrophy) (12). We argue that measuring the impact of radiation on muscle function based solely on the criterion of soft tissue thickness with CT is insufficient to determine whether these muscles should be excluded from IMRT guidelines.
It may be there is a margin for error in the swallowing mechanism such that one muscle group compensates for the loss of another. Such compensation is seen with lateral medullary infarction (Wallenberg syndrome) that impairs the function of muscles of the pharyngeal wall. A prolonged activation of submental muscles (mylohyoid, geniohyoid, and anterior digastric) has been documented in this syndrome, suggesting they are compensating for the acute loss of the longitudinal pharyngeal muscles (17). It is possible that sparing the long pharyngeal muscles with IMRT may prove sufficient to preserve hyolaryngeal elevation and prevent severe dysphagia. However, until this is determined, sparing both muscle groups where possible may result in preservation of hyolaryngeal elevation function during swallowing.
The current study did not address all swallowing events but focused on hyolaryngeal elevation using quantitative measurements of swallowing anatomy and physiology. If the primary incentive for sparing swallowing structures with IMRT is to preserve swallowing function, then future studies should use variables representing structure-to-function relationships. For example, choosing outcome variables that represent the function of the longitudinal pharyngeal muscles (laryngeal elevation and pharyngeal shortening) and the pharyngeal constrictor muscles (pharyngeal narrowing) would provide convincing evidence that the IMRT contour of “pharyngeal constrictor muscles” spares the swallowing function of the pharyngeal wall muscles (18).
An additional contribution of this study is a methodology using mfMRI to test swallowing muscle function. This method could be applied to test the effects of IMRT on specific muscle function in future studies. In this study we also used this method to establish the salience of various exercise therapies targeting functional groups of muscles. We demonstrated that the Mendelsohn maneuver effectively target the suprahyoid muscles and long pharyngeal muscles. We did not control for equivalent expenditures of muscle effort under various test conditions. It may be that adding repetitions to exercise regimens would modify the magnitude of our results.
Limitations of the study include the fact that subjects swallowed in the supine position, which may account for the weak activation of the thyrohyoid muscle. Another limitation is the difficulty establishing a baseline condition for T2 measurement with muscles in constant use, for example, the pharyngeal musculature. For this reason, baseline measurements were taken during a pre-swallowing vs rest condition. Consequently, effect size changes reported here could be underestimated; however, these results more likely represent muscle function in nonexperimental settings where patients also do not rest for prescribed periods of time before swallowing.
Conclusions
In conclusion, we document a previously unreported research methodology (whole-muscle T2 signal profile) for testing swallowing muscle function. We found that suprahyoid and longitudinal pharyngeal muscles elevate the hyolaryngeal complex in swallowing. We used this method to verify the fact that the Mendelsohn maneuver and EPG target the suprahyoid and longitudinal pharyngeal muscles. While IMRT guidelines currently protect many structures critical to swallowing, including the cricopharyngeus muscle, pharyngeal constrictor muscles, longitudinal pharyngeal muscles, and salivary glands, this study shows that guidelines exclude structures important to swallowing function. Whether this exclusion is clinically relevant is unknown. We suggest that studies designed to investigate structure-to-function relationships in swallowing may result in IMRT guidelines that more fully address radiation therapy-related dysphagia and aspiration.
Acknowledgments
The authors acknowledge the expertise of Ron Killiany, Ph.D., and the staff of the Boston University School of Medicine Center for Biomedical Imaging for their assistance in this study.
Supported in part by National Institutes of Health (NIH)/National Institute on Deafness and Other Communication Disorders (NIDCD) grant F31DC011705 (to W.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or NIH.
Footnotes
Conflict of interest: none.
References
- 1.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 2.Pauloski BR, Rademaker AW, Logemann JA, et al. Relationship between swallow motility disorders on videofluorography and oral intake in patients treated for head and neck cancer with radiotherapy with or without chemotherapy. Head Neck. 2006;28:1069–1076. doi: 10.1002/hed.20459. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 4.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110–1118. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Christianen MEMC, Langendijk JA, Westerlaan HE, et al. Delineation of organs at risk involved in swallowing for radiotherapy treatment planning. Radiother Oncol. 2011;101:394–404. doi: 10.1016/j.radonc.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691–707. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson WG, Langmore SE, Yu LB, et al. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia. 2012 doi: 10.1007/s00455-011-9392-7. http://dx.doi.org/10.1007/s00455-011-9392-7. In press. [DOI] [PMC free article] [PubMed]
- 9.Cook IJ, Dodds WJ, Dantas RO, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 1989;257:748–759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 10.Van Daele DJ, McCulloch TM, Palmer PM, et al. Timing of glottic closure during swallowing: a combined electromyographic and endoscopic analysis. Ann Otol Rhinol Laryngol. 2005;114:478–487. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- 11.Segal RL. Use of imaging to assess normal and adaptive muscle function. Phys Ther. 2007;87:704–718. doi: 10.2522/ptj.20060169. [DOI] [PubMed] [Google Scholar]
- 12.Carnaby-Mann G, Crary MA, Schmalfuss I, et al. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:210–219. doi: 10.1016/j.ijrobp.2011.06.1954. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi S, Itoh S, Watanabe Y, et al. Quantitative analysis of masticatory activity during unilateral mastication using muscle fMRI. Oral Dis. 2011:1704–1713. doi: 10.1111/j.1601-0825.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 16.Mu L, Sanders I. Neuromuscular compartments and fiber-type regionalization in the human inferior pharyngeal constrictor muscle. Anat Rec. 2001;264:367–377. doi: 10.1002/ar.10020. [DOI] [PubMed] [Google Scholar]
- 17.Aydogdu I, Ertekin C, Tarlaci S, et al. Dysphagia in lateral medullary infarction (Wallenberg's syndrome): an acute disconnection syndrome in premotor neurons related to swallowing activity? Stroke. 2001;32:2081–2087. doi: 10.1161/hs0901.094278. [DOI] [PubMed] [Google Scholar]
- 18.Leonard R, Rees CJ, Belafsky P, et al. Fluoroscopic surrogate for pharyngeal strength: the pharyngeal constriction ratio (PCR) Dysphagia. 2011;26:13–17. doi: 10.1007/s00455-009-9258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


