Abstract
Cognitive and psychomotor slowing is a complication of epilepsy and is less often a focus of investigation relative to other cognitive domains (e.g., memory). A diversity of tasks has been used to examine psychomotor slowing in epilepsy, but it remains unknown whether the degree of epilepsy-related slowing is fixed or is exacerbated with increasing task demand. Also unknown is to what degree age related slowing is accelerated in epilepsy. Participants with temporal lobe epilepsy (n = 50) were compared to healthy controls (n = 69) across three tasks of psychomotor speed with varied complexity. Performance was examined as a function of group (epilepsy, controls), task complexity (simple, intermediate, complex), and chronological age. The results showed that speed of performance declined across the epilepsy and control participants as a function of task complexity. Epilepsy participants were significantly slower than controls across the three tasks, and there was a significant three way interaction (group by task complexity by age). These results demonstrate that psychomotor slowing is related to task complexity in both epilepsy and healthy control participants, always greater in epilepsy participants, and there is a significant age acceleration of psychomotor slowing in the epilepsy group that is magnified by complex tasks.
Keywords: Temporal lobe epilepsy, psychomotor slowing, cognition, aging, progression
INTRODUCTION
The potential neurocognitive complications of chronic temporal lobe epilepsy are generally well appreciated and may include problems in memory, language, and executive function (Cahn-Weiner et al., 2009; Dodrill, 2004; Helmstaedter and Elger, 2009; Jokeit et al., 2000; Mameniskiene et al., 2006; Riley et al., 2010). While psychomotor speed has been a less frequent focus of attention as a core complication of epilepsy, it appears to be a broadly represented abnormality that has been reported in investigations of neuropsychological function in adults with chronic epilepsy (Piazzini et al., 2006) as well as children with established cryptogenic localization-related (van Mil et al., 2010) and uncomplicated epilepsies (Boelen et al., 2005). Psychomotor slowing has also been reported in children and adults with new onset epilepsies prior to initiation of medications (Oostrom et al., 2003; Prevey et al., 1998; Taylor et al., 2010). Furthermore, especially in adults, psychomotor slowing worsens over time and exceeds the rate of change in many other cognitive abilities (Baker et al., 2011; Hermann et al., 2006). In addition, psychomotor slowing is an appreciated complication of seizure medications (Eddy et al., 2011; Loring et al., 2007; Park and Kwon, 2008), an impact that may be added to the intrinsic slowing that can be observed antecedent to medication initiation in pediatric and adult new onset epilepsies (Oostrom et al., 2003; Taylor et al., 2010).
Normal aging is well known to be associated with slowing of psychomotor speed (Era et al., 2011), an effect that has been attributed to age-related disruption of both cerebral white (Lu et al., 2011) and grey matter (see Seidler et al., 2010 for a review). How normal aging processes may impact already affected cognitive systems in epilepsy remains an area of active investigation, with little work devoted to characterizing the added impact of age to already evident psychomotor slowing.
Complicating the issue further is the diversity of tasks used in this literature. Psychomotor speed has been measured using a wide range of psychomotor tasks that vary in their complexity and relation to real world situations. Available tasks range from simple and uncomplicated unimanual tasks to more complicated bimanual assembly tasks, and include tests of simple and complex reaction time, motor dexterity, coding, assembly and other tasks. For instance, in a sample of 65 adults with temporal lobe epilepsy who participated in a comprehensive neuropsychological evaluation, Wang et al. (2011) found that 86% of the sample had some form of cognitive impairment and of those 69% had deficits in psychomotor speed as measured by the Purdue Pegboard. Exner et al. (2002) examined a small sample of adults with frontal lobe epilepsy (n= 16) and temporal lobe epilepsy (n= 16) and reported psychomotor slowing in both groups when compared to healthy controls on the Trail Making Test (A and B). On the other hand, in another study of individuals with frontal lobe epilepsy (FLE), temporal lobe epilepsy, and controls employing the Trail Making Test of the Delis-Kaplan Executive Function System, McDonald et al. (2005) reported no difference in motor speed between individuals with temporal lobe epilepsy and healthy controls groups, and this finding may be attributed to younger age of the sample. Nevertheless, there has been no systematic investigation of psychomotor slowing as a function of task demand and complexity, the degree to which these task demands may exert differential effects on participants with epilepsy versus healthy controls, and whether normal age-related changes in speeded performances are exacerbated aging individuals with epilepsy.
In this investigation we administered three psychomotor tasks of increasing complexity to individuals with epilepsy and healthy controls, measures that have been widely used to assess psychomotor speed and function in various settings (Grigsby et al., 2008; Sachdev et al., 2005; Wright et al., 2008). Based on the existing research on psychomotor slowing and epilepsy, we hypothesized the following: a) individuals with epilepsy would perform more slowly across all tasks than controls; b) the impairment in the epilepsy group would be magnified as a function of task complexity; and c) age-accelerated progression of this impairment would be observed in the epilepsy group relative to controls.
METHODS
Participants
A total of 119 research participants were the focus of this investigation including 50 individuals with temporal lobe epilepsy and 69 healthy controls. Initial selection criteria for the participants with epilepsy included: a) chronological age between 18 and 63 years, b) WAIS-III IQ >69, c) complex partial seizures of definite or probable temporal lobe origin based on consensus conference review, d) no MRI abnormalities other than atrophy on clinical interpretation, and e) no other neurological disorder. Temporal lobe epilepsy was defined by continuous video/EEG monitoring demonstrating temporal lobe seizure onset of spontaneous seizures, clinical semiology in conjunction with interictal EEGs, clinical neuroimaging, and developmental and clinical history. Initial selection criteria for the controls included: a) chronological age between 18 and 63, b) WAIS-III Full Scale IQ > 69, c) either a friend, relative, or spouse of the participant with epilepsy, d) no current substance abuse, or medical or psychiatric condition that could affect cognitive functioning, and e) no episode of loss of consciousness greater than five minutes, identified developmental learning disorder, or repetition of a grade in school. This project was reviewed and approved by the University of Wisconsin Madison Institutional Review Board, and all participants were informed of the nature and purposes of this investigation, their questions were answered, and signed informed consent was obtained.
Table 1 provides information regarding the baseline characteristics of the epilepsy and control participants. The participants with epilepsy had a significantly lower Full Scale IQ than controls (although still within the average range of performance). Salthouse et al. (2003) suggested that fluid intelligence (i.e., memory and processing speed) begins to reveal major declines around age 45, even as vocabulary continues to increase or stabilize. Given the findings from previous studies and the statistical power calculations, a median split (median = 37 years old) was used to derive groups of younger and older research participants and their characteristics are presented in Table 1. Although not a geriatric sample, individuals in the older group with a mean age of 46 were expected to perform more poorly on the psychomotor tasks than the younger comparison group.
Table 1.
Demographic characteristics of the epilepsy and control subjects
| Epilepsy | Control | |||
|---|---|---|---|---|
|
| ||||
| Younger (n=22) | Older (n=28) | Younger (n=41) | Older (n=28) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 29.36 (5.88) | 46.32 (6.38) | 27.93 (5.75) | 48.93 (7.10) |
| IQ Full Score* | 96.50 (15.08) | 87.61 (16.62) | 112.71 (15.02) | 109.11 (13.67) |
| Education | 13.76 (2.47) | 13.25 (2.01) | 14.46 (2.18) | 13.96 (2.19) |
| Gender | 6M/16F | 12M/16F | 14M/27F | 13M/15F |
| Seizure Duration (yr) | 19.30 (6.51) | 33.01 (11.20) | --- | --- |
| Seizure Frequency (<1 month, >1 month) | 11/11 | 10/14 | --- | --- |
| Antiepileptic Drugs (polytherapy, monotherapy, none) | 13/8/1 | 19/9/0 | --- | --- |
Note.
p < .05;
p < .01
Assessment of Psychomotor Function
Using the Purdue Pegboard (Tiffin and Asher, 1948) and the Grooved Pegboard Test (Klove, 1963), participants completed three motor tasks containing an identical number of stimulus items (n=25) that ranged in complexity from simple (unilaterally placing round pegs in round holes), to intermediate (unilaterally placing slotted keys in grooved but rotated slots), to complex (bimanual 3-piece assembly task). Time to complete all 25 items on each test was recorded and used as the dependent variable in all analyses.
The simple task utilized the Purdue Pegboard (Tiffin and Asher, 1948). Participants were asked to place as many pegs as possible in a 25-item vertical column with their dominant hand only. The Grooved Pegboard Test (Klove, 1963), which presents the participant with a 5 × 5 display of slotted holes positioned in different directions, served as the intermediate complexity motor task. Grooved pegs were provided and participants were asked to place the pegs into the holes using the dominant hand only. Finally, the Purdue Pegboard assembly task (Tiffin and Asher, 1948) was used as the complex task. Four cups containing pegs, washers, and collars were presented to participants. The assembly task required the participants to pick up and place pegs, washers, and collars from different cups with both hands, alternating use of the dominant and nondominant hands. Each item of the task begins with the dominant hand picking up the peg and placing it in the hole on the stimulus board. The second step begins with the nondominant hand picking up a washer and placing it over the peg. The third step begins with the dominant hand picking up the collar and placing it over the peg and on top of the washer, and the task ends with the nondominant hand picking up the final washer and placing it on top of the collar. This series of steps continues until all 25 holes are filled. The time from the start of the task to the end of the task is recorded.
Data Analyses
Psychomotor speed was represented by the time taken to complete each task. In order to investigate the effect of age on motor task performance, the participants were divided into two age groups using median age (37 years) as the cut-off. The data were analyzed using a three-way analysis of variance (ANOVA) with one within-group factor (Task Complexity, simple vs. intermediate vs. complex) and two between-group factors (Group, epilepsy vs. control; Age, younger vs. older). The three-way mixed-design ANOVA was used in which two factors are between-subject variables and the other factor is a within-subject variable. This type of analysis is considered more appropriate for studying psychomotor slowing as a function of age, disability, and task complexity, and their interaction effects. Mean completion times of these speeded psychomotor tasks were used as dependent variables in all analyses. Greenhouse-Geisser corrections were adopted in these analyses, while Bonferroni corrections were applied to all post-hoc comparisons (p < .01).
RESULTS
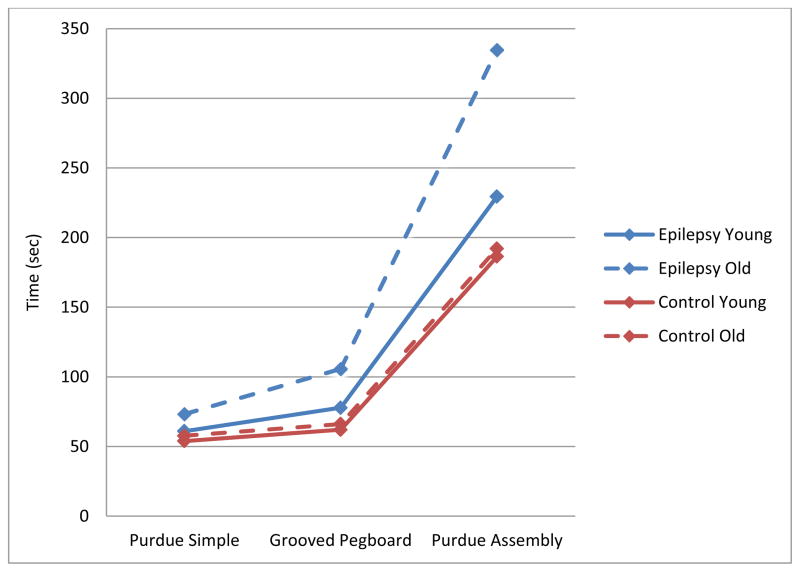
A three-way ANOVA (Group x Age x Task Complexity) revealed significant main effects for Group (F[1,114] = 45.68, p < .001), Age (F[1,114] = 16.60, p < .001), and Task Complexity (F[1.07, 121.79] = 661.54, p < .001). Means and standard deviations are presented in Table 2. Participants with epilepsy were slower than healthy controls, older participants were slower than younger participants, and all participants took longer to complete more complex tasks than to complete less complex ones. These main effects are qualified by significant 2-way (Group x Task Complexity: F[1.07, 121.79] = 33.27, p < .001; Age x Task Complexity: F[1.07, 121.79] = 11.53, p < .001) and 3-way (Group x Age x Task Complexity: F[1.07, 121.79] = 10.62, p < .001) interactions. These interactions are displayed graphically in Figure 1. All main effects and interactions were then decomposed to examine the effects of Group and Age on task completion time across all three levels of Task Complexity (see Table 2).
Table 2.
Means and standard deviations of psychomotor speed
| Epilepsy | Control | |||
|---|---|---|---|---|
|
| ||||
| Younger (n=41) | Older (n=28) | Younger (n=22) | Older (n=28) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Purdue Simplea | 60.95 (16.16) | 73.26 (18.43) | 53.93 (7.94) | 57.68 (9.52) |
| Grooved Pegboarda | 77.86 (25.29) | 105.74 (31.96) | 62.07 (9.52) | 66.18 (9.67) |
| Purdue Assemblya | 229.45 (82.96) | 334.67 (136.27) | 186.39 (29.50) | 192.07 (36.22) |
Note. ME for Group: Epilepsy < Control, F[1,114] = 45.68, p < .001
ME for Age: Younger < Older, F[1,114] = 16.60, p < .001
ME for Task Complexity: F[1.07, 121.79] = 661.54, p < .001
Group x Task Complexity Interaction: F[1.07, 121.79] = 33.27, p < .001
Age x Task Complexity Interaction: F[1.07, 121.79] = 11.53, p < .001
Group x Age x Task Complexity Interaction: F[1.07, 121.79] = 10.62, p < .001
Independent t-test was used with t-value reported for parametric variables include: simple, intermediate, and complex variables and duration of epilepsy.
Mann-Whitney test was with Z-value reported used for non-parametric variables include: frequency of epilepsy and number of AEDs.
Figure 1.
Graphical representation of average total time of completion across epilepsy and control groups for each age group.
For healthy controls, there was no difference in task completion time between older and younger participants. However, for the participants with epilepsy, the results varied across task complexity. There was no difference in simple task completion time between the younger and older participants with epilepsy; only in the intermediate and complex task conditions did age predict task completion time. Older participants with epilepsy were significantly slower than their younger counterparts to complete intermediate and complex tasks (p’s < .01).
Clinical Epilepsy Factors and Psychomotor Speed
It is important to note that there was no difference in seizure frequency or the number of AEDs between older and younger epilepsy participants. Individuals with epilepsy in the older group have a longer duration compared to those individuals in the younger group (p <0.001). However, after controlling for age, there was no significant difference between the groups (p = .640). Using Pearson’s r, IQ scores were negatively correlated with task completion time across tasks of varying complexity (r’s = −.511– −.632, p’s < .001) indicating faster completion time with higher IQ. Number of AEDs was positively correlated with task completion time for the complex task only (r = .450, p < .001). Age and polytherapy were not correlated. We found no relationship between seizure frequency and motor task performance.
DISCUSSION
Three main findings were observed in this investigation of the effects of age and task complexity in psychomotor slowing among research participants with epilepsy and controls.
First, epilepsy participants were slower across all tasks (simple, intermediate, complex) compared to healthy controls—an anticipated finding. Individuals with epilepsy performed more slowly compared to controls across all administered tasks including simple, intermediate, and complex motor tasks, with greater mean completion time in the participants with epilepsy.
Second, across psychomotor tasks of increasing complexity, performance slowed across both the epilepsy and control groups, but the slowing with increasing task difficulty was greater in the epilepsy participants. This was demonstrated by a significant group by task interaction effect, which demonstrated that individuals with epilepsy were differentially affected by psychomotor task complexity. As task difficulty increased the participants with epilepsy exhibited an increasingly greater completion time compared to the controls—most notably on the complex assembly task (Figure 1).
Third, there was a clear age acceleration of this effect in the epilepsy but not the control group. Older participants with epilepsy showed significantly greater age-accelerated slowing on the intermediate and complex task compared to the young epilepsy group and controls. While these findings need to be interpreted cautiously given the cross-sectional nature of the data, the results are consistent with the possibility of increased age-accelerated slowing of the psychomotor system in people with chronic epilepsy. While cognitive and psychomotor slowing is known to be associated with advancing chronological age, this slowing is accelerated in an older epilepsy group that is only 46 years of age on average, indicating that aging effects are occurring earlier in epilepsy participants than control participants.
There are no previous studies that systematically investigated psychomotor slowing as a function of task demand and complexity as conceptualized in this study, and in general, there is an inadequate understanding of epilepsy’s impact on normal age-related changes in cognitive and psychomotor processing speed. A few prospective investigations of adults with chronic epilepsy have included measures of psychomotor speed in the context of a broader test battery. Hermann et al. (2006) followed this sample over a four year interval and included measures of motor/psychomotor speed (Grooved Pegboard, Trail Making Test). Prospective declines in performance (regression based z-scores) on the composite psychomotor speed factor were disproportionate to healthy controls and other ability areas over the same interval. Piazzini et al. (2006) followed 50 adults with temporal lobe epilepsy and 50 healthy controls over a five year interval and evaluated their cognitive performance including attention and psychomotor function. They also reported that epilepsy participants demonstrated psychomotor slowing over a five year interval when compared to healthy controls. Similarly, Taylor and Baker (2010) found evidence for psychomotor slowing in 38% of participants with epilepsy in a five-year follow-up study.
Helmstaedter and Elger (2009) postulated that aging may, in fact, have a greater negative impact on cognition compared to healthy controls in terms of learning and memory. How aging may affect other cognitive domains that are known to be age-sensitive (executive functions, speeded abilities) have been less a focus of investigation, but there are many reasons for concern (Hermann et al., 2008). The existing research has been quite focused on the impact of clinical factors on cognition such as duration of epilepsy (which is highly correlated with age), age of onset of epilepsy, and other related disease-based markers which have been shown to have an adverse impact on cognitive functions (Jokeit and Ebner, 1999; Oyegbile et al., 2004; Pitkanen and Sutula, 2002). More focus on the unique effects of aging may prove valuable.
Antiepileptic drugs are widely appreciated to negatively affect psychomotor speed and other cognitive abilities (Eddy et al., 2011; Loring et al., 2007; Park and Kwon, 2008), but here there was no difference in the number of AEDs in the younger and older groups. Nevertheless, it has been demonstrated that AEDs can have adverse effects on psychomotor performance (Aldenkamp et al., 1994; Dodrill and Temkin, 1989; Loring et al., 2007; Vermeulen and Aldenkamp, 1995). Older adults are particularly vulnerable to AED side effects due to physiological changes associated with aging, reduced metabolic rate for AEDs, and increased risk of polydrug interactions, all or some of which may subsequently affect psychomotor speed. Although Craig and Tallis (1994) found no differences when comparing the effects of AEDs on cognitive functioning in elderly persons with epilepsy, Ramsay et al. (2004) later showed increased sensitivity to the CNS toxic side effects of AEDs. While there is no denying AED impact on speeded and other processes, and the possibility that advancing age may exacerbate adverse AED effects, both Prevey et al. (1998) and Taylor et al.(2010) found significant psychomotor slowing in new onset adult cases prior to AED treatment, indicating the intrinsic impact of epilepsy on speeded processes. This relationship is likely not specific to epilepsy and may be a broader signal associated with other disorders (e.g., schizophrenia) where it has been demonstrated that cognitive and psychomotor slowing is an important core feature of the disorder, even though other cognitive abilities have garnered more research and investigation (Dickinson et al., 2007).
Finally, the “upstream” impact of cognitive and psychomotor slowing on higher cognitive abilities remains to be more systematically investigated. For instance, how cognitive slowing per se might affect information processing and memory efficiency remains to be determined. The underlying neurobiology of psychomotor slowing also remains to be characterized. In the end, it appears that there is an increase in psychomotor slowing as individuals with chronic epilepsy age compared to fewer age related changes in psychomotor speed in healthy controls.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Connie Sung, Email: csung@msu.edu, Department of Counseling, Educational Psychology and Special Education, Michigan State University, Erickson Hall, 620 Farm Lane Room 455, East Lansing, MI 48824, Fax: 517-353-6393.
Jana E. Jones, Email: jejones@neurology.wisc.edu, University of Wisconsin School of Medicine and Public Health, Department of Neurology, 1685 Highland Ave, Madison WI 53705, Fax: 608-263-0412
Daren C. Jackson, Email: jackson@neurology.wisc.edu, University of Wisconsin School of Medicine and Public Health, Department of Neurology, 1685 Highland Ave, Madison WI 53705, Fax: 608-263-0412
Yui Chung Chan, Email: ychan@bsu.edu, Department of Counseling Psychology and Guidance Services, Teachers College, Room 1008, Ball State University, Muncie, IN 47306, Fax: 765-285-5455.
Fong Chan, Email: chan@education.wisc.edu, Department of Rehabilitation Psychology and Special Education, University of Wisconsin, 403 Education Building, 1000 Bascom Mall, Madison, WI, 53706, Fax: 608-262-8108.
Michael Seidenberg, Email: michael.seidenberg@rosalindfranklin.edu, Department of Psychology, Rosalind Franklin School of Medicine and Science, 3333 Green Bay Road, North Chicago, IL 60064, Fax: 847-578-8758.
Bruce P. Hermann, Email: hermann@neurology.wisc.edu, University of Wisconsin School of Medicine and Public Health, Department of Neurology, 1685 Highland Ave, Madison WI 53705, Fax: 608-608-263-0412
References
- Aldenkamp AP, Alpherts WC, Diepman L, van’t Slot B, Overweg J, Vermeulen J. Cognitive side-effects of phenytoin compared with carbamazepine in patients with localization-related epilepsy. Epilepsy Res. 1994;19:37–43. doi: 10.1016/0920-1211(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Baker GA, Taylor J, Aldenkamp AP. Newly diagnosed epilepsy: Cognitive outcome after 12 months. Epilepsia. 2011;52:1084–1091. doi: 10.1111/j.1528-1167.2011.03043.x. [DOI] [PubMed] [Google Scholar]
- Boelen S, Nieuwenhuis S, Steenbeek L, Veldwijk H, van de Ven-Verest M, Tan IY, Aldenkamp AP. Effect of epilepsy on psychomotor function in children with uncomplicated epilepsy. Developmental Medicine & Child Neurology. 2005;47:546–550. doi: 10.1017/s0012162205001064. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Wittenberg D, McDonald C. Everyday cognition in temporal lobe and frontal lobe epilepsy. Epileptic Disorders. 2009;11:222–227. doi: 10.1684/epd.2009.0265. [DOI] [PubMed] [Google Scholar]
- Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35:381–390. doi: 10.1111/j.1528-1157.1994.tb02448.x. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: A meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dodrill CB. Neuropsychological effects of seizures. Epilepsy & Behavior. 2004;5:S21–S24. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Temkin NR. Motor speed is a contaminating factor in evaluating the “cognitive” effects of phenytoin. Epilepsia. 1989;30:453–457. doi: 10.1111/j.1528-1157.1989.tb05325.x. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. 2011;4:385–407. doi: 10.1177/1756285611417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Era P, Sainio P, Koskinen S, Ohlgren J, Harkanen T, Aromaa A. Psychomotor speed in a random sample of 7,979 subjects aged 30 years and over. Aging Clin Exp Res. 2011;23:135–144. doi: 10.1007/BF03351077. [DOI] [PubMed] [Google Scholar]
- Exner C, Boucsein K, Lange C, Winter H, Weniger G, Steinhoff BJ, Irle E. Neuropsychological performance in frontal lobe epilepsy. Seizure. 2002;11:20–32. doi: 10.1053/seiz.2001.0572. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Elger E. Chronic temporal lobe epilepsy: A neurodevelopmental or progressively dementing disease? Brain: A Journal of Neurology. 2009;132:2822–2830. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Jones J. The neurobehavioural comorbidities of epilepsy: can a natural history be developed? Lancet Neurol. 2008;7:151–160. doi: 10.1016/S1474-4422(08)70018-8. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, Bell B. Cognitive Prognosis in Chronic Temporal Lobe Epilepsy. Annals of Neurology. 2006;60:80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: A cross sectional study. Journal of Neurology, Neurosurgery & Psychiatry. 1999;67:44–50. doi: 10.1136/jnnp.67.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokeit H, Luerding R, Ebner A. Cognitive impairment in temporal-lobe epilepsy. Lancet. 2000;355:1018–1019. doi: 10.1016/S0140-6736(05)74765-6. [DOI] [PubMed] [Google Scholar]
- Klove H. Clinical Neuropsychology. Med Clin North Am. 1963;47:1647–1658. [PubMed] [Google Scholar]
- Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychology Review. 2007;17:413–425. doi: 10.1007/s11065-007-9043-9. [DOI] [PubMed] [Google Scholar]
- Lu PH, Lee GJ, Raven EP, Tingus K, Khoo T, Thompson PM, Bartzokis G. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J Clin Exp Neuropsychol. 2011;33:1059–1068. doi: 10.1080/13803395.2011.595397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameniskiene R, Jatuzis D, Kaubrys G, Budrys V. The decay of memory between delayed and long-term recall in patients with temporal lobe epilepsy. Epilepsy & Behavior. 2006;8:278–288. doi: 10.1016/j.yebeh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Tecoma ES, Iragui-Madoz VJ. Is impairment in set-shifting specific to frontal-lobe dysfunction? Evidence from patients with frontal-lobe or temporal-lobe epilepsy. Journal of the International Neuropsychological Society. 2005;11:477–481. [PubMed] [Google Scholar]
- Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, Seidenberg M, Hermann BP. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- Park SP, Kwon SH. Cognitive effects of antiepileptic drugs. J Clin Neurol. 2008;4:99–106. doi: 10.3988/jcn.2008.4.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzini A, Turner K, Chifari R, Morabito A, Canger R, Canevini MP. Attention and psychomotor speed decline in patients with temporal lobe epilepsy: a longitudinal study. Epilepsy Res. 2006;72:89–96. doi: 10.1016/j.eplepsyres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Prevey ML, Delaney RC, Cramer JA, Mattson RH. Complex partial and secondarily generalized seizure patients: cognitive functioning prior to treatment with antiepileptic medication. VA Epilepsy Cooperative Study 264 Group. Epilepsy Res. 1998;30:1–9. doi: 10.1016/s0920-1211(97)00091-0. [DOI] [PubMed] [Google Scholar]
- Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology. 2004;62:S24–29. doi: 10.1212/wnl.62.5_suppl_2.s24. [DOI] [PubMed] [Google Scholar]
- Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, Lin JJ. Altered white matter integrity in temporal lobe epilepsy: Association with cognitive and clinical profiles. Epilepsia. 2010;51:536–545. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry. 2005;76:362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive Functioning as a Potential Mediator of Age-Related Cognitive Decline in Normal Adults. Journal of Experimental Psychology. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Baker GA. Newly diagnosed epilepsy: Cognitive outcome at 5 years. Epilepsy & Behavior. 2010;18:397–403. doi: 10.1016/j.yebeh.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Taylor J, Kolamunnage-Dona R, Marson AG, Smith PEM, Aldenkamp AP, Baker GA. Patients with epilepsy: Cognitively compromised before the start of antiepileptic drug treatment? Epilepsia. 2010;51:48–56. doi: 10.1111/j.1528-1167.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The Purdue Pegboard: norms and studies of reliability and validity. Journal of Applied Psychology. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- van Mil SG, de la Parra NM, Reijs RP, van Hall MH, Aldenkamp AP. Psychomotor and motor functioning in children with cryptogenic localization related epilepsy. NeuroRehabilitation. 2010;26:291–297. doi: 10.3233/NRE-2010-0565. [DOI] [PubMed] [Google Scholar]
- Vermeulen J, Aldenkamp AP. Cognitive side-effects of chronic antiepileptic drug treatment: a review of 25 years of research. Epilepsy Res. 1995;22:65–95. doi: 10.1016/0920-1211(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Wang WH, Liou HH, Chen CC, Chiu MJ, Chen TF, Cheng TW, Hua MS. Neuropsychological performance and seizure-related risk factors in patients with temporal lobe epilepsy: A retrospective cross-sectional study. Epilepsy & Behavior. 2011;22:728–734. doi: 10.1016/j.yebeh.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, DeCarli C, Sacco R, Stern Y. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39:800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]